Black tea: chemical analysis and stability
Shiming
Li
*a,
Chih-Yu
Lo
*b,
Min-Hsiung
Pan
c,
Ching-Shu
Lai
c and
Chi-Tang
Ho
a
aDepartment of Food Science, Rutgers University, 65 Dudley Road, New Brunswick, NJ 08901, USA. E-mail: shiming3702@yahoo.com; Fax: +1 732 932 6776; Tel: +1 732 932 9611
bDepartment of Food Science, National Chiayi University, Chiayi City 60004, Taiwan. E-mail: chihyulo@mail.ncyu.edu.tw
cDepartment of Seafood Science, National Kaohsiung Marine University, Kaohsiung 811, Taiwan
First published on 5th September 2012
Abstract
Tea is the most popular flavored and functional drink worldwide. The nutritional value of tea is mostly from the tea polyphenols that are reported to possess a broad spectrum of biological activities, including anti-oxidant properties, reduction of various cancers, inhibition of inflammation, and protective effects against diabetes, hyperlipidemia and obesity. Tea polyphenols include catechins and gallic acid in green and white teas, and theaflavins and thearubigins as well as other catechin polymers in black and oolong teas. Accurate analysis of black tea polyphenols plays a significant role in the identification of black tea contents, quality control of commercial tea beverages and extracts, differentiation of various contents of theaflavins and catechins and correlations of black tea identity and quality with biological activity, and most importantly, the establishment of the relationship between quantitative tea polyphenol content and its efficacy in animal or human studies. Global research in tea polyphenols has generated much in vitro and in vivo data rationally correlating tea polyphenols with their preventive and therapeutic properties in human diseases such as cancer, and metabolic and cardiovascular diseases etc. Based on these scientific findings, numerous tea products have been developed including flavored tea drinks, tea-based functional drinks, tea extracts and concentrates, and dietary supplements and food ingredients, demonstrating the broad applications of tea and its extracts, particularly in the field of functional food.
1. Introduction
Tea (Camellia sinensis) is cultivated worldwide, particularly in China, India and some other Asian countries. The origin of tea has been traced back to the southern part of Yunnan Province, located in the southwest of China. There are two major varieties of tea plants: Camellia sinensis var. sinensis which is characterized by its small leaves and bush like plants, originating in China and growing in some countries in Asia with mild cold climates, and Camellia sinensis var. assamica which is a large leaved tree discovered in the southwest region of China and India and exported to several other countries with semitropical climates. Owing to its unique flavor and taste, the sinensis tea dominates green tea production, whereas the assamica tea is mainly used in black tea production because of its high content of catechins and tannins.Tea is the most consumed flavored functional beverage in the world. As a result of tea plant variety and different delicate manufacturing processes, there are numerous tea products commercially available in the global market. However, based on handling methods and prompt processing after tea picking, they generally comprise three major types: green tea, oolong tea and black tea. Green and black tea account for about 20% and 78% of worldwide tea consumption respectively, whereas approximately 2% is consumed as oolong tea. In regional preferences, green, white and oolong tea are dominant in China and Japan, and black tea occupies the majority of the market in western countries.
Although originally tea consumption was mainly for its central nerve stimulating and soothing effects, tea drinking has been linked to health promoting effects for centuries. The health benefits of tea consumption are reported to include anti-oxidant,1 anti-inflammation,1 cancer prevention,1,2 reduced occurrence of heart disease3etc. A myriad of scientific data has shown that the beneficial health effects of tea are mainly attributed to its polyphenolic compounds, which are illustrated in Table 1. Tea contains different polyphenols in terms of percentage content and the variety of polyphenols (Table 2). Green tea polyphenols, i.e. catechins, are the most abundant polyphenols in green, white and oolong tea. In general, tea polyphenols are either categorically classified as catechins or equivocally unspecified. Polyphenols in black tea include theaflavins, thearubigins and other catechin polymeric pigments in addition to a certain amount of catechins. Unless a special chromatographic separation is performed, catechins are consistently present in black tea and its extracts. This is due to the natural imbalance of catechins which always yields incomplete conversion of green tea catechins to black tea polyphenols. Tea polyphenols in oolong tea chiefly consist of green tea catechins and a small proportion of black tea theaflavins and thearubigins due to its partial fermentation process. The polyphenolic composition of pu-erh tea, or raw pu-erh tea, is reminiscent of oolong tea, whereas the fully fermented pu-erh tea contains mostly gallic acid while it lacks both green and black tea polyphenols.
| Polyphenol | Structure | No. | Name | R | R′ | Acronym |
|---|---|---|---|---|---|---|
| Catechins |
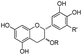
|
I | Epicatechin | H | H | EC |
| II | Epigallocatechin | H | OH | EGC | ||
| III | Epicatechin gallate | Galloyl | H | ECG | ||
| IV | Epigallocatechin gallate | Galloyl | OH | EGCG | ||
| Theaflavins |
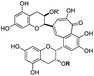
|
V | Theaflavin | H | H | TF1 |
| VI | Theaflavin-3-monogallate | Galloyl | H | TF2a | ||
| VII | Theaflavin-3′-monogallate | H | Galloyl | TF2b | ||
| VIII | Theaflavin-3,3′-digallate | Galloyl | Galloyl | TF3 |
2. Black tea chemistry
2.1. Origin of black tea and its polyphenols
Traditionally, the manufacturing process of black tea is regarded as the fermentation of green tea. However, it is a different process from the microbial fermentation in the production of alcoholic beverages and vinegar etc. The formation of black tea polyphenols involves two steps: oxidation and polymerization.4In the first reaction step as illustrated in Scheme 1, green tea catechins such as epigallocatechin (EGC) and epigallocatechin gallate (EGCG) are partially oxidized to quinones under the enzymatic catalysis of polyphenol oxidase (PPO, EC1.14.18.1) or peroxidase (POD, EC1.11.1.7). Both PPO and POD exist naturally in fresh tea leaves, fruits and vegetables.
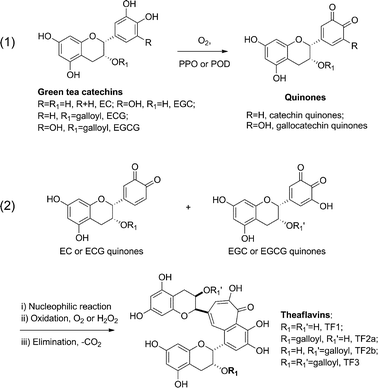 | ||
| Scheme 1 Enzymatic catalyzed process of theaflavin formation. | ||
The second step is often labeled as polymerization, which involves a nucleophilic addition reaction of the resulting gallocatechin quinones to catechin quinones, further oxidation by oxygen or hydrogen peroxide, elimination of a carbon dioxide molecule and rearrangement4 to complete the synthesis of the core of black tea polyphenols – benzotropolone. It can be rationalized that the driving force of this enzyme catalyzed reaction is the release of small molecules of CO2 and water and also the formation of a stable aromatic bicyclic ring termed as benzotropolone.
2.2. Major components of black tea
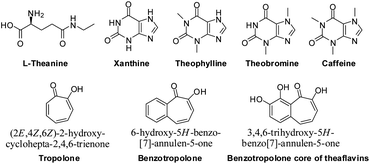 | ||
| Fig. 1 Amino acid, xanthines and benzotropolone skeletons in black tea. | ||
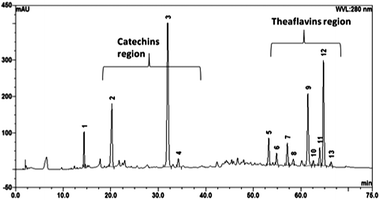 | ||
| Fig. 2 HPLC profile of commercially available BTE (analysis conditions: C18 column, 4.6 × 150 mm, 3 μm, 100 Å, UV 280 nm, mobile phase: water (0.1% HOAc) and acetonitrile). | ||
In contrast to the theoretical eight or more theaflavins that can be produced proportionally, usually there are four major theaflavins formed (Scheme 2), which are, theaflavin (TF1), theaflavin-3-O-gallate (TF2a), theaflavin-3′-O-gallate (TF2b) and theaflavin-3,3′-O,O-digallate (TF3). The enzymatic catalyzed formation mechanism of theaflavins is illustrated in Scheme 1 and detailed specific theaflavin formation in Scheme 2. The major theaflavins are formed from an epicatechin and an epigallocatechin. For example, as shown in Scheme 2, theaflavin (TF1) is formed from EC and EGC; theaflavin-3-O-monogallate (TF2a) from ECG and EGC; theaflavin-3′-O-monogallate (TF2b) from EC and EGCG; and ECG and EGCG form theaflavin-3,3′-O,O-digallate (TF3).
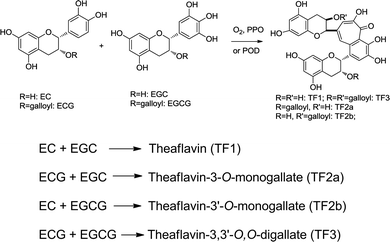 | ||
| Scheme 2 Major specific theaflavin formation from catechins. | ||
In essence, without considering the side chains of catechins, the generation of the theaflavin core – benzotropolone – is from the reaction of a catechol and a pyrogallol. Any molecule bearing a catechol moiety can react with a pyrogallolyl embedded substance to form benzotropolone derivatives under enzymatic catalysis. The reaction between a catechol and a pyrogallolyl group on a gallate occurs at a slow rate and yields only small amounts of other theaflavin isomers. The other isolated and characterized benzotropolone derivatives are summarized in the following sections.
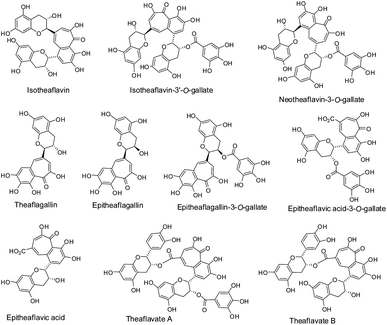 | ||
| Fig. 3 Isomers of black tea theaflavins. | ||
Theaflavic acids and theaflavates have been found in some black tea infusions or extracts. In a model study of tea fermentation, epitheaflavic acid and epitheaflavic acid-3-O-gallate (Fig. 3) were formed and identified,9 which shows the possibility of their presence in industrial black tea manufacturing, because their precursors are epicatechin and gallic acid that exist in green tea leaves or from cleavage of gallate esters. Fig. 3 also shows the structures of theaflavate A and B.7,10 Theaflavate A was detected in black tea infusions and it is formed from ECG. Theaflavate B was detected in black tea leaves and it is the product of EC and ECG.
However, these theaflavin isomers, namely isotheaflavins, neotheaflavins, theaflagallins, theaflavates and theaflavic acids etc., are usually minor components or hardly detectable in black tea or its extracts compared with the four major theaflavins illustrated in Scheme 2 and Table 1. The biological properties of these theaflavin isomers have been scarcely evaluated due to their limited availability and negligible contribution.
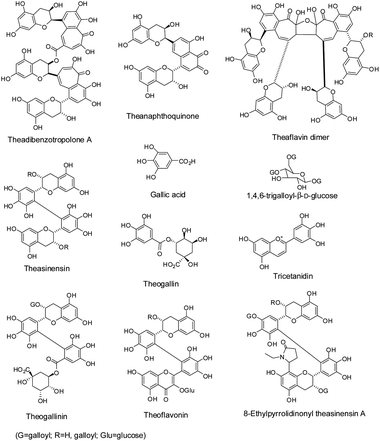 | ||
| Fig. 4 Structures of tea polyphenol derivatives in black tea. | ||
Gallic acid, its quinic acid ester – theogallin – and glucose ester (Fig. 4), are the major simple polyphenols identified in black tea.16 The amount of gallic acid in black tea is significantly increased due to the ester hydrolysis of the 3-galloyl substituted catechins either by native esterase or oxidative degallation during the fermentation. An anthocyanidin pigment found in black tea is tricetanidin, a 3-deoxyanthocyanidin (Fig. 4).6 Other tea polyphenol related compounds isolated from black tea are also illustrated in Fig. 4, which include theogallinin (biaryl molecule of EGCG and theogallin) and theaflavonin (biaryl molecules of catechins and 3-glycosylated myricetin). An example of a nitrogen containing compound isolated from black tea is 8-(N-ethyl-pyrrolidinon-5-yl) theasinensin A (Fig. 4). In addition to the compounds mentioned above, more tea polyphenol related substances have been identified in black tea, such as theacitrins from Assam black tea.6
3. Analysis and stability of black tea and its extracts
To date, numerous studies of black tea in various research institutions around the world have been performed for many decades and a myriad of reports, publications and conference communications carried messages to every corner of the media educating the public that black tea has beneficial effects for human health. However, the information given to the public is not explicitly described or is vague regarding (i) the composition of black tea both qualitatively and quantitatively and (ii) the correlation or SAR (structure–activity relationship) between the characterized black tea and its biological activity. With the aid of modern advanced instrumental and data processing technology, detailed analysis and well-performed characterization of black tea and its related products are feasible and should become routine in studying and reporting the efficacious activity of a particular black tea or its products, which will facilitate the discovery of the SAR between the black tea and its bioactivity, expedite the development of black tea products that have functionality, and consequently, improve the overall health of the general public.The standardization and quality control of natural products such as tea might have been extremely difficult in part due to large variations in genus, growth environment, process and storage conditions etc. However, biological activity data of natural products without referencing composition or major components provide little information to other researchers and are not practicably useful references.
3.1. Analysis and characterization
In the analysis and characterization of the polyphenol composition of black tea, we have chosen a self-made black tea infusion, commercial black tea beverages and black tea extracts sold on the markets to compare the contents of major components such as caffeine, catechins (EC, EGC, EGCG, ECG) and theaflavins (TF1, TF2a, TF2b and TF3). The analysis results show that the contents vary dramatically from one product to another even within a category.As illustrated in Fig. 5, in solid tea leaves, there are large amounts of caffeine and tea catechins (EGCG, ECG), and also gallic acid and EC (Fig. 5A). In tea bags, the dominant component is caffeine whereas tea polyphenols are minor components including EGCG, ECG, and theaflavins (Fig. 5B). In both cases, a small amount of black tea theaflavins has been detected, but not within the range of the quantification limit. The message from these identity analyses is that the black tea leaves and tea bags are not good sources of theaflavins and other black tea polyphenols, but black tea leaves can provide sufficient green tea catechins.
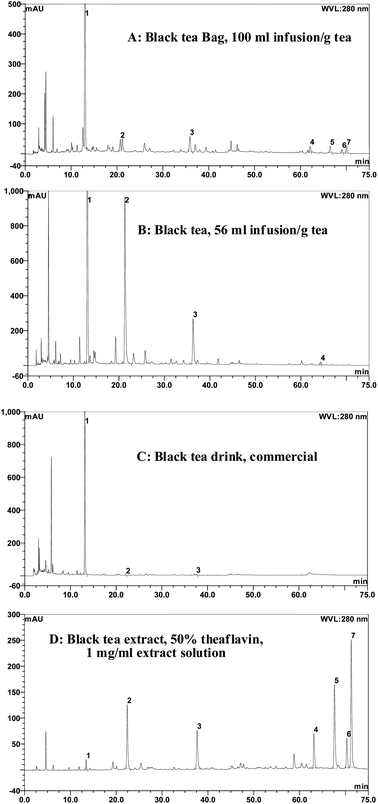 | ||
| Fig. 5 HPLC profiles of different forms of black tea (A: black tea bag, 100 ml infusion per g tea; B: black tea leaves infusion, 56 ml infusion per g tea; C: commercial black tea drink, original form; D: black tea extract, 50% theaflavin, 1 mg per ml solution. Peak 1: caffeine; Peak 2: EGCG; Peak 3: ECG; Peak 4: TF1; Peak 5: TF2a; Peak 6: TF2b; Peak 7: TF3. HPLC conditions were same as those in Fig. 2). | ||
Fig. 5C shows an HPLC chromatograph of a commercial beverage labeled as “black tea”. We have noticed that the dominant compound in this tea beverage is caffeine, peak 1 at a retention time of 13.15 min in Fig. 5C. The amounts of both green tea catechins and black tea theaflavins are extremely low and barely detectable. Other than the labeling of “black tea”, this marketed beverage does not serve any function or health beneficial effects of black tea. Besides, there are not many black tea beverages on the market. The possible reasons include: (i) cost effectiveness; black tea, particularly with a high content of theaflavins, is much more expensive to produce than green tea; (ii) taste formulation; black tea is known for its astringency and bitterness, which is difficult to mask when satisfying consumer demands in terms of taste; (iii) the stability of black tea polyphenols; consideration in formulation must be given because theaflavins are easily oxidized to form quinones and other byproducts.
The other form of goods for consumption is black tea extracts, which are the purposely extracted and concentrated form of black tea polyphenols with the removal of caffeine. Black tea extracts (BTE) are commercially available as several types and are classified based on the content of theaflavins, from 20% theaflavins BTE to 30% theaflavins BTE to 40% theaflavins BTE to newly developed 50% and 60% theaflavins BTE by HPLC analysis. For example, the content of total theaflavins in BTE in Fig. 2 is 40% and in Fig. 5D, 50% of total theaflavins in the BTE extracts. Moreover, BTE products with an ultra high content of theaflavins have been developed and marketed as dietary supplements based on clinical studies mainly for their anti-inflammation activity. There is great potential for more and more products to be developed and commercialized based on high theaflavin content BTE and its perceived biological functionality, without observed toxicity during previous long term human trial studies.
3.2. Black tea extract production
The production of black tea extracts can be generally summarized as the fermentation of green tea leaves and extraction of tea polyphenols and other soluble substances. In the manufacturing process of black tea extracts, however, the process could vary dramatically for good quality and high efficiency to earn market share and maximum profit. Fig. 6 shows an example of the manufacturing process used to produce high percentage and high quality black tea extract used as food ingredients for potentially various beneficial health claims.17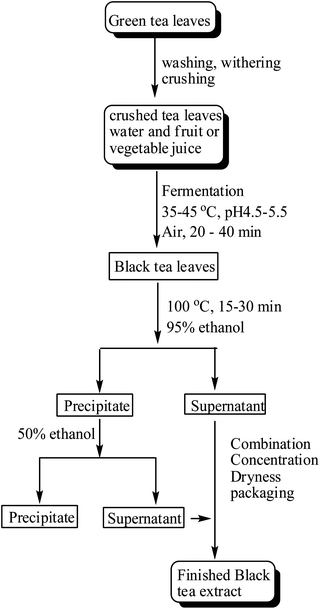 | ||
| Fig. 6 A production process of black tea extract. | ||
3.3. Stability studies
The stability of tea polyphenols has been an unresolved issue for many decades. The research in accurately monitoring and measuring the change of catechins and theaflavins has been hindered by the unavailability of standard compounds, i.e. the majority of tea polyphenols in black tea and the relative complexity of the separation methods for these polyphenols plus other components such as caffeine, gallic acid, and alkaloids etc. in tea.As stated in our published procedures,18 we have prepared the pure standards of theaflavin (TF1), theaflavin monogallate (TF2a and TF2b), theaflavin digallate (TF3) and epigallocatechin (EGC) that were either commercially unavailable or available but with unknown impurities.
In the preparation of caffeine, catechin and theaflavin standard compounds and black tea samples, it is crucial to have a complete dissolution of caffeine, catechins, theaflavins and black tea extracts to make aqueous ethanol stock solutions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) which contain 0.1% ascorbic acid. Fresh samples were prepared prior to sample analysis. A series of standard compounds with various concentrations were determined and quantified by HPLC. The quantification of black tea samples was based on the standard curves (external method) of individual tea polyphenols and caffeine.
1 v/v) which contain 0.1% ascorbic acid. Fresh samples were prepared prior to sample analysis. A series of standard compounds with various concentrations were determined and quantified by HPLC. The quantification of black tea samples was based on the standard curves (external method) of individual tea polyphenols and caffeine.
In establishing the stability profile of individual tea polyphenols and black tea extracts, we have performed studies both at ambient temperature for long term storage and at higher temperature for extended storage by monitoring the concentration change of tea polyphenols. The concentrations of total theaflavins were measured at 0, 3, 7, 10 and 14 days for BTE samples stored at temperatures of 37 °C, 60 °C and 75 °C (Fig. 7). For example, at 37 °C, the measured concentrations of BTE theaflavins at 0, 3, 7, 10 and 14 days, were between 24.1% and 25.5%. The obtained concentration of BTE theaflavins for the five days was within the measuring variation range. At 60 °C, the concentrations of BTE theaflavins varied from 24.7% at day 0, to 24.3% at day 3, to 25.0% at day 7, to 24.6% at day 10, and to 23.5% at day 14. By comparing the concentrations of total theaflavins among individual samples, we found that the total concentration of theaflavins in BTE samples remained unchanged and thus we have concluded that the storage of BTE samples at these conditions does not alter the content of theaflavins, even at 75 °C for 14 days, meaning that the theaflavins in BTE solid form are exceptionally stable at high temperatures and during long term storage. This result is contradictory to the general concept that black tea polyphenols are unstable. It is necessary to note that the BTE samples we have tested and analyzed were in dry solid form, which has a different environment from the solution form of black tea.
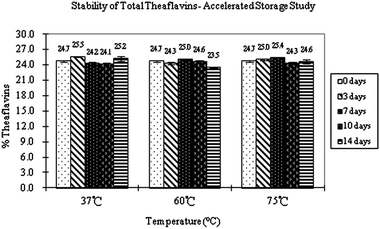 | ||
| Fig. 7 Stability of total theaflavins – extended storage study. | ||
Furthermore, we have examined the stability of individual tea polyphenols present in black tea and BTE, including four green tea catechins – EC, EGC and EGCG – and four black tea theaflavins – TF1, TF2a, TF2b and TF3 – with various temperatures, humidity and times of storage (Fig. 8). Results from this stability study of individual tea polyphenols have shown that they were remarkably stable even under some harsh conditions such as high temperature and high humidity for prolonged periods of time.
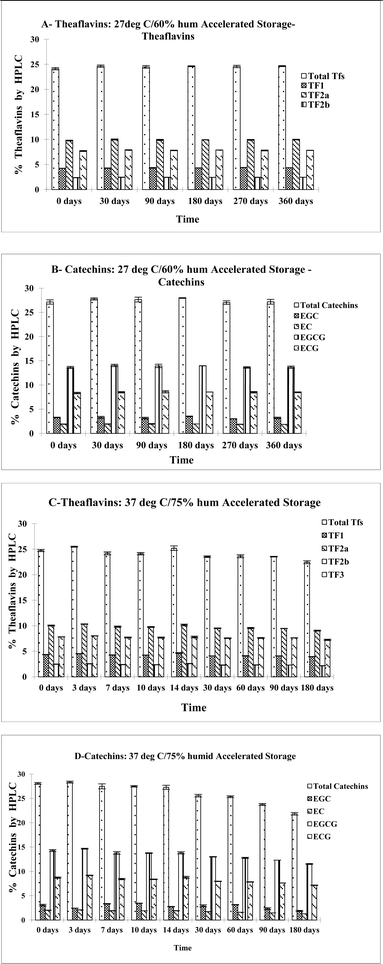 | ||
| Fig. 8 Stability of individual catechins over time at two elevated temperatures (27 °C and 37 °C) and two humidity conditions. | ||
The summation of these experimental findings is illustrated in Fig. 8A–D, which show the concentration changes of eight individual tea polyphenols, total catechins and total theaflavins, over time under various temperature and humidity conditions. As shown in Fig. 8A and B, during a 360 day storage at conditions of 27 °C and 60% humidity, there was no observed change in concentration of all four catechins and four theaflavins, which can be interpreted as there is no content variation of catechins and theaflavins within a year of storage under normal living conditions such as room temperature and regular humidity. A noticeable decline in tea polyphenols was observed at 37 °C and 75% humidity (Fig. 8C and D). The decline in total catechin concentration (Fig. 8D) started at 30 days and remained a declining trend at 90 days. After six months (180 days) of storage at 37 °C and 75% humidity, a mimic of a tropical environment, total unchanged catechins were about 90%, strong evidence that these compounds are relatively stable even under harsh conditions. However, a change in theaflavins was not observed even after a six month period of storage in the simulated tropical environment. Under all other storage conditions tested, 65 °C and 75 °C for 14 days (figure not shown), both catechins and theaflavins were not observed to decline, meaning the tea polyphenols can tolerate high temperatures and prolonged storage conditions.
In essence, the researchers found that in solution, both green tea catechins and black tea theaflavins are unstable and have a tendency for rapid degradation, especially at high temperature or basic pH.19
The thermal stability of catechins is better than that of theaflavins.19 For example, after a 3 hour open-air storage in a water solution, the amount of catechins and theaflavins was not found to have decreased at 24 °C, whereas catechins had a 25% reduction at 70 °C and a 29% decrease at 100 °C; theaflavins on the other hand had a 56% decrease at 70 °C and amounts were undetectable at 100 °C after 3 hours. The discrepancy in the aqueous stabilities of catechins and theaflavins can be postulated to be due to the large difference in chemical reactivity between the two groups of compounds: relative inertness and less steric hindrance of catechins vs. higher reactivity, especially anti-oxidant activity because of the ring-fusing, increased number of hydroxyl groups and steric hindrance surrounding the benzotropolone core.
Chen's group also found that the pH of the tea solution plays a pivotal role in the stability of the aqueous tea sample.19 It has been found that tea polyphenols are stable at lower pH, meaning a weak acid environment. For instance, both catechins and theaflavins showed no sign of degradation at pH 5 in sodium phosphate buffer over 16 hours. However, the degradation rate was accelerated with an increase in buffer pH. As an example, 95% of theaflavins and 65% of catechins had disappeared respectively at pH 7.4 after six hours. Again, this has shown that the stability of catechins is better than that of theaflavins in a certain pH solution of tea. Chen et al. also investigated other solution conditions such as more acidic (pH 5.0, 6.0 and 6.5),19 the environment of soft drinks (pH 2.6, 3.2, 4.0, 4.5), and long term storage etc. and found that tea polyphenols were also susceptible to degradation in non-weak acidic conditions, which translates to extreme instability in soft drink pH conditions.20
In summary, black tea extracts are very stable in solid form. Hence, they could be stored for years in dry conditions. But tea polyphenols are susceptible to degradation readily and rapidly when black tea is in aqueous solution or black tea extracts in very humid storage conditions.
4. Conclusion
In summary, black tea is the most consumed functional beverage in the world. Besides alkaloid content represented by caffeine, it contains large amounts of polyphenols including fermentation yielded black tea polyphenols – theaflavins and thearubigins – unchanged green tea catechins particularly EGCG and ECG, and other minor components such as gallic acid and tea amino acid – theanine etc. Much scientific evidence, especially the application studies of black tea and its constituent polyphenols in animal disease models and some human clinical studies, has shown that black tea or its extracts are efficacious and have potent biological activities associated with beneficial health effects such as cancer prevention and anti-inflammatory properties etc.We also conclude the necessity in black tea research of a well-characterized chemical profile, and its related biological activity, of black tea and black tea extracts, particularly the quantification of tea polyphenols.
Notes and references
- E. G. de Majia, M. V. Ramirez-Mares and S. Puangpraphant, Brain, Behav., Immun., 2009, 23, 721–731 Search PubMed.
- C.-S. Yang, J. D. Lambert and S. Sang, Arch. Toxicol., 2009, 83, 11–21 CrossRef CAS.
- V. Stangl, H. Dreger, K. Stangl and M. Lorenz, Cardiovasc. Res., 2007, 73, 348–358 Search PubMed.
- Y. Matsuo, T. Tanaka and I. Kouno, Tetrahedron Lett., 2009, 50, 1348–1351 Search PubMed.
- A. Gauslau, S. Li, K.-Y. Chen, C.-T. Ho and N. Rawson, Mol. Nutr. Food Res., 2011, 55, 74 Search PubMed.
- J. W. Drynan, M. N. Clifford, J. Obuchowicz and N. Kuhnert, Nat. Prod. Rep., 2010, 27, 417–462 RSC.
- J. R. Lewis, A. L. Davis, Y. Cai, A. P. Davies, J. P. G. Wilkins and M. Pennington, Phytochemistry, 1998, 49(8), 2511–2519 CrossRef CAS.
- G. Nanaka, F. Hashimoto and I. Nishioka, Chem. Pharm. Bull., 1986, 34, 61–65.
- J. E. Berkowitz, P. Coggon and G. W. Sanderson, Phytochemistry, 1971, 10, 2271–2278 CrossRef CAS.
- N. Kuhnert, M. N. Clifford and A. Muller, Food Funct., 2010, 1, 180–199 RSC.
- S. Sang, J. D. Lambert, S. Tian, J. Hong, Z. Hou, J.-H. Ryu, R. E. Stark, R. T. Rosen, M.-T. Huang, C. S. Yang and C.-T. Ho, Bioorg. Med. Chem., 2004, 12, 459–467 CrossRef CAS.
- S. Sang, S. Tian, X. Meng, R. E. Stark, R. T. Rosen, C. S. Yang and C.-T. Ho, Tetrahedron Lett., 2002, 43, 7129–7133 CrossRef CAS.
- T. Tanaka, Y. Betsumiya, C. Mine and I. Kouno, Chem. Commun., 2000, 1365–1366 RSC.
- T. Tanaka, K. Inoue, Y. Betsumiya, C. Mine and I. Kouno, J. Agric. Food Chem., 2001, 49, 5785–5789 CrossRef CAS.
- T. Shil, M. Miyamoto, Y. Matsuo, T. Tanaka and I. Kouno, Chem. Pharm. Bull., 2011, 59, 1183–1185 Search PubMed.
- F. Hashimoto, G. Nonaka and I. Nishioka, Chem. Pharm. Bull., 1992, 40, 1383–1389 CAS.
- X. Shi, X. Ren, J. Liu, H. Cao, W. Ren and Y. Cao, US Pat., US2011/0082196, 2009.
- C.-Y. Lo, S. Li, Y. Wang, D. Tan, M.-H. Pan, S. Sang and C.-T. Ho, Food Chem., 2008, 107, 1099–1105 CrossRef CAS.
- Y. L. Su, L. K. Leung, Y. Huang and Z.-Y. Chen, Food Chem., 2003, 83, 189–195 Search PubMed.
- Z.-Y. Chen, Q. Y. Zhu, D. Tsang and Y. Huang, J. Agric. Food Chem., 2001, 49, 477–482 Search PubMed.
- Q. Y. Zhu, A. Zhang, D. Tsang, Y. Huang and Z.-Y. Chen, J. Agric. Food Chem., 1997, 45, 4624–4628 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
