Modeling the flows of engineered nanomaterials during waste handling
Nicole C.
Mueller
a,
Jelena
Buha
bc,
Jing
Wang
bc,
Andrea
Ulrich
b and
Bernd
Nowack
*a
aEmpa – Swiss Federal Laboratories for Materials Science and Technology, Technology and Society Laboratory, CH-9014 St Gallen, Switzerland. E-mail: nowack@empa.ch
bEmpa – Swiss Federal Laboratories for Materials Science and Technology, Laboratory for Analytical Chemistry, CH-8600 Dübendorf, Switzerland
cETH Zurich, Institute of Environmental Engineering, CH-8093 Zurich, Switzerland
First published on 5th December 2012
Abstract
Little is known about the behavior of engineered nanomaterials (ENM) at the interface from the technosphere to the ecosphere. Previous modeling of ENM flows to the environment revealed that significant amounts of ENM enter the waste stream and therefore waste incineration plants and landfills. It is the aim of this study to model the flows of ENM during waste incineration and landfilling in greater depth by including a more detailed description of the different processes and considering ENM-specific transformation reactions. Four substances were modeled: nano-TiO2, nano-ZnO, nano-Ag and carbon nanotube (CNT). These ENM are representative for commonly used materials and products, illustrating a variety of ENM with different behavior. The modeling was performed for Switzerland where almost 100% of the municipal waste and sewage sludge are burned. The mass-based modeling showed that – despite several differences among the models for nano-TiO2, nano-ZnO and nano-Ag (e.g. partial dissolution of nano-ZnO in acid washing of exhaust air or fly ash) – the major ENM flows go from the waste incineration plant to the landfill as bottom ash. All other flows within the system boundary (e.g. with the fly ash) were predicted to be about one magnitude smaller than the bottom ash flow. A different ENM distribution was found for CNTs that are expected to burn to a large extent (94%) so that only insignificant amounts remain in the system. The results of the modeling show that waste incineration can have a strong influence on some ENM but that still the majority of the ENM-mass is expected to end up in landfills.
Environmental impactProducts containing engineered nanomaterials (ENM) are already on the market and are used by consumers and industry. Almost nothing is known about their fate during end-of-life treatments. However, in order to predict possible exposure of workers and the environment, it is important to quantify in more detail the flows of ENM after the use of ENM-containing products. Previous modeling has shown that major flows of ENM end up in waste incineration and landfills. This work extends this modeling and considers in more detail the various process steps. |
Introduction
Numerous products containing engineered nanomaterials (ENM) are already on the market. Even though the number of products is still relatively low (currently estimated to be less than about 1% of all products1), the trend is increasing. Release studies are still rare2–5 and analyses especially in complex matrices or the environment are challenging.6,7 The increasing interest in nanotechnology and nano-enhanced products has raised concerns about the safe handling as well as human and environmental exposure.8 Researchers are thus investigating not only the potential toxicity of ENM but also their distribution in the environment. However, little is known about the behavior of ENM at the interface from the technosphere to the ecosphere. The modeling of ENM in the environment by Mueller and Nowack9 and Gottschalk et al.10,11 revealed a significant flow of ENM to landfills either via wastewater and sludge incineration and the subsequent deposition of bottom and fly ash or via direct dumping of construction waste.Only little knowledge is available so far on end-of-life treatment of nanomaterials, i.e. waste treatment like incineration,12 deposition on landfills or fate during recycling. Only a few studies exist on the fate in end-of life processes of ENM.13–15 One experimental study is available about the behavior of ENM in a waste incineration plant (WIP).16 The behavior of nano-CeO2 added to domestic waste was followed during the combustion steps in a Swiss waste incineration plant. Out of the total nano-CeO2 recovered, 81% were found in the slag, 19% in the fly ash and 0.02% in the quench water. Nano-CeO2 in the exhaust air was below the detection limit. Compared to the general weight distribution of bottom ash/fly ash which is on average about 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the CeO2 seemed to be slightly enriched in the fly ash according to the measurements of Walser et al.16 This may be due to the small particle size, which favors suspension in the flue gas. Moreover, the solubility of CeO2 is low especially at neutral or alkaline pHs. However, it partially dissolves in an acidic environment such as during acid washing of the flue gas and fly ash. Since the measurements by Walser et al.16 are based on chemical analysis and do not consider the morphology of the particles, it is possible that the Ce measured in the quench water is not exclusively nanoparticulate but partially dissolved.
1, the CeO2 seemed to be slightly enriched in the fly ash according to the measurements of Walser et al.16 This may be due to the small particle size, which favors suspension in the flue gas. Moreover, the solubility of CeO2 is low especially at neutral or alkaline pHs. However, it partially dissolves in an acidic environment such as during acid washing of the flue gas and fly ash. Since the measurements by Walser et al.16 are based on chemical analysis and do not consider the morphology of the particles, it is possible that the Ce measured in the quench water is not exclusively nanoparticulate but partially dissolved.
Roes et al.12 calculated in a desk-top study that by 2020 about 0.5 kg of ENM in plastics are incinerated per ton of waste which would sum up to 1880 t/a of ENM entering waste incineration in Switzerland as nano-composites. Taking into account the density and the volume of the ENM they found that 100–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times higher concentrations of nano-objects will be found in the flue gas of nanocomposite containing waste than produced by conventional waste. The basis for this comparison is the measured particle concentration in the exhaust (after a high efficiency cyclone) of an experimental waste incinerator using a “refuse derived fuel”. However, Roes et al.12 assume that no ENM are destroyed and that all ENM end up in the flue gas.
000 times higher concentrations of nano-objects will be found in the flue gas of nanocomposite containing waste than produced by conventional waste. The basis for this comparison is the measured particle concentration in the exhaust (after a high efficiency cyclone) of an experimental waste incinerator using a “refuse derived fuel”. However, Roes et al.12 assume that no ENM are destroyed and that all ENM end up in the flue gas.
Theoretical considerations show that the fate of ENM in waste incineration depends mainly on two factors: if the ENM are free or released easily from their substrate, they can escape with the flue gas because of their small size, and finally be caught in the flue gas filter (bag filter or electrostatic filter). If the ENM are enclosed in other materials, they may be fused-in in the melts of the surrounding material and hence remain in the bottom ash. If the ENM have a boiling point lower or equal to the temperature in the incineration furnace, they will vaporize based on their small size and hence enter the flue gas stream as a gaseous element. As the flue gas cools down, these elements condensate, however, they are not considered as ENM anymore. Analogously, melted ENM are unlikely to reform a nanoparticle with original composition. Very stable ENM (like TiO2 or CeO2) may remain particulate, other ENM such as carbonaceous materials are oxidized (based on the conditions in the furnace).
It is the aim of this paper to use the knowledge available so far and predict the flows of ENM during waste incineration and landfilling in Switzerland. ENM are defined as intentionally produced particles with at least one dimension between 1 and 100 nm (ISO TS 80004-117). This modeling represents an update of the modeling of Mueller and Nowack9 and Gottschalk et al.11 who did not distinguish between the different processes during waste incineration but used a more simple and generic approach. Four substances are modeled: nano-TiO2, nano-ZnO, nano-Ag and CNT. These ENM are representatives for commonly used materials and products, illustrating a variety of ENM with different behaviors. Nano-TiO2 is very stable (high melting point and low solubility), nano-Ag is semi-stable and can be partially destroyed at high temperatures and in acid washing, nano-ZnO is soluble in acid washing and CNT can be oxidized at high temperature.
Methods
Model description
The model developed in this study focuses on waste incineration in Switzerland with the aim to quantify the input of ENM into the different types of landfills (Fig. 1). The current regulation on the treatment of waste in Switzerland demands that all combustible waste has to be burned before deposition. This is also the case for sewage sludge, which is not allowed to be used as a fertilizer in agriculture to avoid soil contamination with heavy metals. Most ENM flows thus congregate in waste incineration. Based on the very high cleaning efficiency of modern filter systems in WIP, these ENM eventually end up in bottom or fly ash and hence in landfills.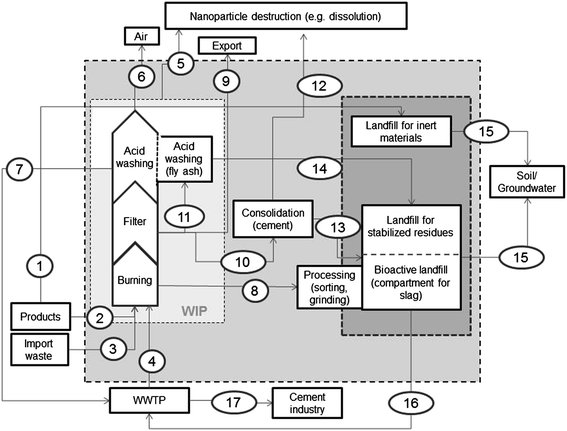 | ||
| Fig. 1 Generic model describing the waste disposal system in Switzerland focusing on waste incineration and landfills. WWTP: wastewater treatment plant, WIP: waste incineration plant. The numbers refer to the flows described in the text (Model description). | ||
The total amount of burnable waste in Switzerland is about 3.65 million tons (year 2006, incl. imported waste) of which municipal waste is the largest fraction (∼2.59 million tons) followed by burnable building and construction waste (∼0.38 million tons).18,19 Municipal waste in Switzerland is estimated to be about 709 kg per person and year of which about 242 kg is recycled, 118 kg composted and the rest is burned in incineration plants.20 From the incineration residues iron as well as different non-ferrous metals are recovered. About one-third of the waste incineration plants (WIP) have a system for acid washing of fly ash installed. Target compounds are Hg (recovery rate: about 90%), Zn (60–83%), Pb (40–70%), (Cu 20–45%) and Cd (85–93%).21
The model quantifies the mass flows of ENM within the system boundaries for the waste handling typical in Switzerland. Fig. 1 illustrates the model developed for this study. There are four input flows feeding into the system (flows 1 to 4). The first flow (1) describes the direct deposition of construction waste in landfills for inert materials. Flow 2 represents the disposal of consumer products (in Switzerland all domestic waste is burned). Flow 3 quantifies the import of waste from neighboring countries. Flow 4 represents the combustion of sewage sludge. The flows 2–4 enter the waste incineration process consisting of (A) burning under oxidizing conditions at a temperature of around 1000 °C, (B) the flue gas filter (either electrostatic precipitator or bag house filter depending on the incineration plant), (C) the flue gas scrubber and (D) wastewater treatment facility (internal or external) for the wastewater originating from the cooling processes of the bottom ash, the wastewater from the scrubber and possibly from the acid washing of the fly ash (E).
Within the waste incineration system ENM may be destroyed by oxidation, melting or volatilization in the furnace or by dissolution/precipitation in the wastewater treatment plant or in the scrubber (5). ENM that survive the waste incineration process are found in the bottom (8) as well as in fly ash (9–11) and they are released into the air (6) or into the quench water (7) wherewith they leave the system. In Switzerland the bottom ash is processed on the landfill site (removal of ferrous and non-ferrous metals, grinding) before deposition in a separate compartment of the bioactive landfill (8). During grinding ENM may be released into the air. However, this potential ENM-flow is not quantified because no data are available. ENM in filter ash are either exported (9, leaving the system), consolidated with cement (10) or undergo acid washing (11). During both processes (consolidation, acid washing) ENM may be destroyed (1 and 12). If not, they are deposited on a landfill for stabilized residues or on a bioactive landfill (13 and 14). In these landfills ENM accumulate and/or leach out to the environment (15) and/or the wastewater treatment plant (16). Flow 17 describes the sludge used in cement industry.
There are currently three types of landfills in Switzerland: landfills for inert materials such as uncontaminated excavation and inert construction waste, landfills for stable materials such as consolidated fly ash, and landfills for reactive materials where bottom ash from waste incineration is deposited in a separate compartment. The flows to the landfills for inert materials are modeled separately from the flows to the other two landfills (which are often operated side-by-side).
The modeling represents a typical material-flow model22 and is executed in Excel. ENM mass flows from one box to the other are calculated using transfer coefficients and conversion of mass. In order to cope with the high uncertainty of many of the input parameters three scenarios were modeled: a realistic scenario, the “min-scenario” resulting in the highest removal or degradation and the worst-case scenario resulting in the lowest removal.
Results
Model parameters (input parameters, transfer coefficients)
The models for the four considered ENM differ significantly depending on the ENM-type based on the physico-chemical properties of the respective ENM. The fate of these most common ENM is expected as follows. Nano-TiO2 (boiling point: 2900 °C) will most probably remain particulate at the temperature during incineration of about 1000 °C while the majority of CNTs are burnt. Nano-Ag (boiling point: 2162 °C) will only volatilize to a small extent. However, nano-Ag particles are likely to melt (melting point: 962 °C). The fate of nano-ZnO is difficult to predict. 37–86 wt% of Zn was found remaining in bottom ash.23 This high variability depended on the redox-conditions in the furnace. In thermal waste incineration Zn starts vaporizing at a temperature of 905 °C and at 1150°C more than 90% is gaseous under reducing conditions.24 However, under oxidizing conditions ZnO remains solid as ZnO up to a temperature of 1500 °C.24 In waste incineration we are expecting oxidizing conditions and therefore nano-ZnO should not vaporize to a significant extent.The input data for the flows feeding into the model were taken from Gottschalk et al.25 (Table 1): These are the flows from production and the use of ENM into the waste stream. Gottschalk et al.25 used only a very simple description of the waste handling system (a one-box model with one transfer factor) and the current paper aims to provide a much more detailed description of the processes during waste incineration and landfilling. Analogous average ENM-concentrations were assumed in both Swiss and imported waste. Based on the high data uncertainty three scenarios are modeled. The mode-scenario describes the values that have the highest probability (mode values taken from Gottschalk et al.25). The low exposure (min) and maximum exposure (max) scenario indicate the range within which the values are to be expected (15 and 85 percentile, respectively, taken from Gottschalk et al.25). The input data and coefficients used in the min-scenario lead to the lowest realistic concentration of ENM in landfills, while the input data and coefficients used in the max-scenario lead to the highest realistic concentration of ENM in landfills.
| Input from products to WIP (t/a) | Input from products to landfill (t/a) | Input from sewage sludge to WIP (t/a) | Input from imported wastea (t/a) | ||
|---|---|---|---|---|---|
a Total imported waste to Switzerland18 amounts to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t/a. This corresponds to 2.9% of the total waste from Switzerland (not including sewage sludge). Assuming analogous ENM-concentration in the waste import the amount of ENM in imported waste is 2.9% of the ENM-amount in Swiss waste. 000 t/a. This corresponds to 2.9% of the total waste from Switzerland (not including sewage sludge). Assuming analogous ENM-concentration in the waste import the amount of ENM in imported waste is 2.9% of the ENM-amount in Swiss waste.
|
|||||
| TiO2 | Mode | 76.8 | 38.0 | 47.6 | 2.23 |
| Min | 46.3 | 28.4 | 28.9 | 1.34 | |
| Max | 271 | 166 | 169 | 7.85 | |
| ZnO | Mode | 2.57 | 1.30 | 2.00 | 0.075 |
| Min | 2.38 | 1.18 | 1.84 | 0.069 | |
| Max | 16.4 | 10.1 | 14.6 | 0.476 | |
| Ag | Mode | 0.510 | 0.340 | 0.389 | 0.015 |
| Min | 0.300 | 0.273 | 0.173 | 0.009 | |
| Max | 1.80 | 1.34 | 1.35 | 0.052 | |
| CNT | Mode | 1.26 | 0.061 | 0.007 | 0.036 |
| Min | 0.875 | 0.164 | 0.008 | 0.025 | |
| Max | 2.65 | 0.545 | 0.027 | 0.077 | |
There are two types of flow coefficients used in the modeling: substance-specific parameters and model-specific parameters. The first are parameters that depend on substance characteristics such as physico-chemical properties while the latter are parameters determined by the waste incineration system and assumed to be equal for all types of considered ENM. Model-specific-parameters are:
• The filter efficiency of the bag house filter or the electrostatic precipitator (ESP) in the WIP: the filter efficiency is particle-size dependent but not substance-specific. According to Walser et al.16 the removal efficiency of ESP is around 99.995% which corresponds quite well to the data by Burtscher et al.26 where the efficiency is also >99.5%. We used for our modeling a value of 99.995%.
• The filter efficiency of the wet scrubber for insoluble particles: according to Walser et al.16 the efficiency is >99.9% which is again comparable to the data by Burtscher et al.26
• The disposal of filter ash (9–11): filter ash from Swiss WIP ends to 39% in the acid washing process (11) and to 39% in consolidation (10) while the remaining 22% are exported (9).27 In consolidation fly ashes are mixed with cement.28
• The use of sludge in the cement industry (17): 22% of the sludge from wastewater treatment is used in cement production.18 The remaining 78% are burnt in WIP or separately in sludge incineration plants.
There are no substance-specific parameters reported in literature that could be used to describe the behavior of ENM during waste incineration. Hence extrapolations from similar data had to be made and these substance-specific parameters are summarized in Table 2. The following paragraphs explain the respective coefficients (described in Table 2).
| Flow in Fig. 1 | TiO2 | ZnO | Ag | CNT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mode | Min | Max | Mode | Min | Max | Mode | Min | Max | Mode | Min | Max | |||
| 1 | Destruction by burning/volatilization/melting | 5 | 0 | 0 | 0 | 0 | 20 | 0 | 0 | 20 | 0 | 98 | 100 | 75 |
| 2 | Destruction by dissolution in acid washing | 5 | 0 | 0 | 0 | 100 | 100 | 100 | 2.5 | 10 | 0 | 0 | 0 | 0 |
| 3 | Destruction by consolidation with cement | 12 | 0 | 0 | 0 | 100 | 100 | 100 | n.q. | n.q. | n.q. | 0 | 0 | 0 |
| 4 | Transfer to bottom ash | 8 | 81 | 81 | 81 | 81 | 81 | 81 | 81 | 81 | 81 | 81 | 81 | 81 |
| 5 | Transfer to fly ash | 11 | 19 | 19 | 19 | 19 | 19 | 19 | 19 | 19 | 19 | 19 | 19 | 19 |
| 6 | Transfer to quench water | 7 | 0.02 | 0.02 | 0.02 | 0 | 0 | 0 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
(1) The temperature in the WIP furnace reaches around 1000 °C. This is significantly lower than the boiling/melting point of TiO2. Nano-TiO2 is thus not affected by the incineration process. In contrast CNTs – as carbon-based material – are supposed to burn completely under the oxidizing conditions in the furnace. However, a few CNTs may still survive in enclosed compartments. Hence, the coefficients are set at 98% elimination11,22 for the most realistic scenario, 100% elimination for the min-scenario and 75% for the worst case scenario. For nano-Ag and nano-ZnO the coefficients are derived from the physico-chemical properties of the bulk substance. Silver has a high boiling point (2162 °C), but a melting point around the predominant temperature in the furnace (962 °C). It is thus possible that nano-Ag particles melt and hence are not nanoparticulate anymore. Based on the oxidizing conditions it is furthermore realistic that the surface of the nano-Ag oxidizes. Since there are no data on the behavior of nano-Ag in waste incineration, we assume that no ENM are destroyed in the mode- and max-scenario and 20% destruction in the min-scenario. ZnO remains solid up to a temperature of 1500 °C under oxidizing conditions24 which are to be expected in the WIP furnace. However, locally reducing conditions may cause nano-ZnO to react and volatilize already at temperature around 900 °C.24 Given the lack of data on the behavior of nano-ZnO in waste incineration, we assume that no ENM are destroyed in the mode- and max-scenario and 20% destruction in the min-scenario as a first approximation.
(2) The scrubber contains the acids HCl (3–5%) and HF (<2%) at a pH of <1 (starting pH)–4.5 (pH after neutralization). The same acids are used in the acid washing of fly ash and thus the coefficients defined hereafter are used for both processes. TiO2 and CNT are almost inert against the attack of these acids and hence do not react in significant amounts with these acids. Elementary silver reacts in oxidizing acids (nitric acid) only. However, nano-Ag may be (partially) oxidized during burning and thus dissolve in the acid. Considering the lack of data, we assume that about 2.5% of the nano-Ag is destroyed for the mode-scenario, 10% for the min-scenario and 0% for the max-scenario. ZnO is highly soluble in acids. It is one of the target substances to be removed in the acid washing for recycling purposes. It can be expected that 100% of the nano-ZnO is dissolved in the acid.
(3) In the consolidation of fly ash with cement very alkaline conditions prevail which most probably lead to the complete dissolution of nano-ZnO. TiO2 and CNT are stable also in an alkaline environment (pH > 7) with no destruction of the ENM. For silver, the reactions in cement are unknown. As a first approximation to allow modeling, the same coefficients as for the acid washing are used (2.5% for the mode-scenario, 10% for the min-scenario and 0% for the max-scenario).
(4–6) The partition of ENM between bottom ash and fly ash is supposed to be substance-specific for chemical compounds. However, since we focus on the morphological units “ENM”, the parameter is regarded as constant at 81% to bottom ash, 19% to fly ash and 0.02% in the quench water (from the wet scrubber).16 These coefficients are only applied to the unburned fraction of the ENM. For nano-ZnO, which is sensitive to acids, no ZnO is expected in the particulate form in the quench water. Based on the general weight distribution of 90% bottom ash and 10% fly ash, ENM seem to be slightly enriched in fly ash.
Model output
In the nano-TiO2 model, no ENM loss is predicted during incineration, acid washing or other processes. Hence almost all particles enter the landfills. Accordingly, the most significant flow in the nano-TiO2-model is the bottom ash flow from the waste incinerator to the bioactive landfill (Fig. 2a). Other relevant flows describe the input of ENM into the WIP from products and from the WWTP as well as the direct disposal of construction waste in the landfill for inert materials.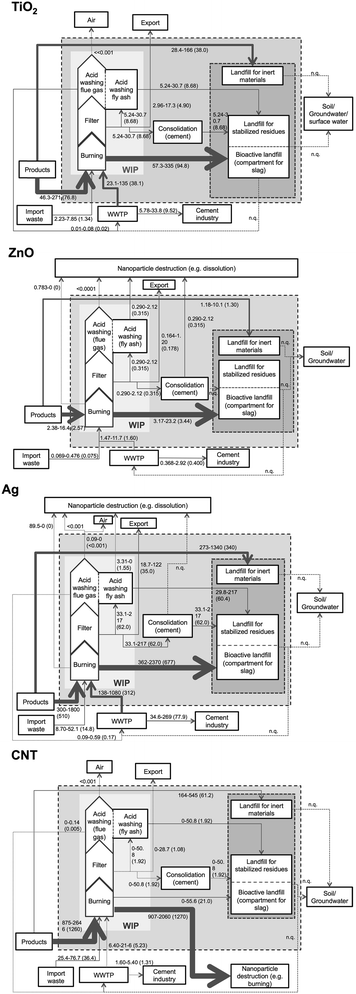 | ||
| Fig. 2 ENM flows to waste incineration and landfills in Switzerland. The numbers indicate a realistic range (15–85 percentile), in parentheses the mode value is shown. The flows for TiO2 and ZnO are given in t/a, for Ag and CNT in kg per annum, all rounded to 3 significant digits but maximum 2 decimals. The strength of the arrow is proportional to the respective ENM flow (mode-value). Thick, dotted lines are flows that could not be quantified. Fine, dotted lines are flows that are less than 2 orders of magnitude smaller than the largest flow. | ||
Nano-ZnO is easily dissolved in acids wherewith it leaves the system. It is also assumed that nano-ZnO is dissolved when mixed with cement. Hence, nano-ZnO enters landfills only when directly deposited on the landfill for inert materials or with the bottom ash. As shown in Fig. 2b, significant flows are predicted from the WIP to the bioactive landfill in the slag, from products to the WIP and to the landfill for inert materials.
Nano-Ag may be (partially) oxidized during incineration and then dissolved in the acid washing. Due to its relatively low melting temperature, it is also likely to melt in the furnace. Since there are no data on the behavior of nano-Ag in waste incineration available, these processes are difficult to model. Flows to any compartment are possible. The coefficients used (as described in the Methods section) are thus approximations with high uncertainties. Still it is shown in Fig. 2c, that – analogously to TiO2 and ZnO – the most significant flow is the bottom ash flow from WIP to the landfill for bioactive materials.
CNTs as carbon-based materials are assumed to almost completely burn under the oxidative conditions in the furnace. However, it is still possible that part of the CNTs survive the incineration in enclosed compartments. The largest flow of CNTs is thus their combustion wherewith they leave the system (Fig. 2d). The remaining CNTs are indeed stable in acid, but the amount entering the landfills is nevertheless insignificant.
Due to the different magnitudes of the input into the waste system – caused by different production amounts – the actual flows of the four ENM are quite different. The highest flows, up to a few hundred tons per annum, are observed for nano-TiO2, up to a few tons per annum for nano-ZnO and up to a few hundred kg per annum for nano-Ag and CNT.
In Fig. 3 the relative flows in and out of the system are depicted for the mode-scenario. It shows that for nano-TiO2, nano-ZnO and nano-Ag the flow with bottom ash to the landfill is with 58–62% the most important flow. Also direct deposition on landfills, mainly with construction waste, is predicted between 23 and 29%. All the other flows, e.g. export of fly ash, landfilling of fly ash are much less important. The emissions into water and air are almost not existent. For CNT the flows are very different with about 94% burned and therefore destroyed. All other flows play only a minimal role.
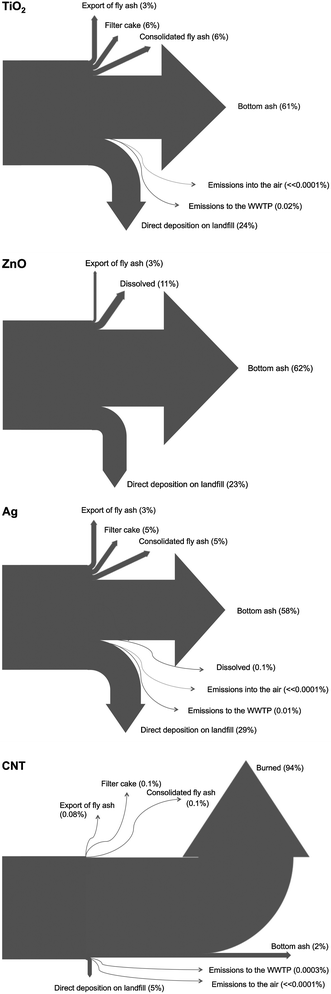 | ||
| Fig. 3 Waste disposal as the input–output system for the ENM. Flows are shown in % of the total flow that enters the waste incineration/landfill system. | ||
Discussion
Despite the differences between the models for the four investigated ENM, we have shown that the major ENM-flows for TiO2, ZnO and Ag go from the WIP to the landfill as bottom ash. All other flows within the system boundary are about one magnitude smaller than the bottom ash flow. However, it is not known in what form the ENM are present in the bottom ash. If the ENM are enclosed into larger (vitrified) fragments of bottom ash, they may not be released any more. A release of substances from vitrified waste is only possible at a pH above 10 or below 2. In this case landfills could be regarded as final sinks. However, Walser et al.16 found that the nano-CeO2 added to the waste was still present as ENM loosely attached to larger particles after incineration and therefore mobilization of ENM in landfills might be possible. Due to the lack of data about mobilization of ENM from waste materials or ashes, we refrained from modeling the transfer of ENM to leachates from landfills. Both the natural nanoparticulate fraction in the leachate as well as the mobilization of ENM from ashes and slags should therefore be investigated in the future. The second most significant input of ENM into landfills is the direct disposal of construction waste in landfills. From these materials that may be crushed and compacted on-site release of ENM might be possible. Release of nano-Ag and TiO2 from paints by weathering has been investigated and it was found that both single nanoparticles and materials still embedded in paint fragments were released.4,29A different ENM distribution was found for CNT. CNTs as carbon-based materials are burned to a large extent so that only insignificant amounts remain in the system. However, in other countries where landfilling without prior incineration is still common the possible release of CNT from landfills cannot be neglected. A recent paper has, for example, studied the stability of single-walled CNT under conditions representative for landfill leachates.30
The input data are mainly based on the study by Gottschalk et al.25 who did not take into account any dissolution of nano-Ag and ZnO during wastewater treatment. It can thus be expected that the flows from WWTP to the WIP are smaller than indicated in the model. It has also been shown that the major Ag-form in sludge is nanoparticulate Ag2S, which is formed during wastewater treatment both from dissolved Ag as well as from nano-Ag.29,31 The metallic nano-Ag used in products is therefore already transformed into another phase before it reaches the WIP. This phase is no longer separable from the mineral phase formed when dissolved Ag enters the WWTP. The further fate of Ag2S during incineration (e.g. oxidation, volatilization) is unknown. Also ZnO was found to be transformed into ZnS during anaerobic digestion of sewage sludge32 and thus ZnS and not ZnO will be the form reaching the WIP burning sludge.
Our modeling treated the ENM as “generic ENM”, not considering that, for example, “nano-TiO2” stands for a wide variety of different forms, morphologies, surface functionalizations or coatings. These different forms will undergo different changes during the incineration process (e.g. organic functionalizations will oxidize) and thus the properties of the ENM in the slag and filter ash will be different from the properties of the initial ENM. Further work needs to investigate the changes that the different ENM undergo during incineration and how this affects their properties. Our model provides a modeling of the ENM mass-flows and is thus able to highlight important flows and compartments that need to be further investigated experimentally.
While most of the model-specific parameters could be taken from the literature or statistical data, there were no substance-specific coefficients on ENM reported in the literature. To date, it is not known to what extent ENM are affected by waste incineration and acid washing. Extrapolations had to be made based on the physico-chemical characteristics of the respective bulk-material. Generally, it can be distinguished between combustible materials, soluble substances and stable compounds. Depending on the properties, the material may oxidize or dissolve and thus leave the system boundary “ENM”. Stable materials such as TiO2 are not lost in any process and they will accumulate either in the technosphere (e.g. landfill) or in the environment (e.g. sediments).
The situation with 0% direct landfilling of municipal waste in Switzerland is somewhat special, however, the tendency goes in most countries towards more incineration and less direct landfilling. Direct landfilling accounted for less than 5% of municipal solid waste management in 2008 in Germany, the Netherlands, Sweden, Denmark, and Austria.33 Also Japan has high incineration rates due to scarcity of land. Incineration residues accounted for 54% of the total landfilled material in 2000.34 In the US only 12% of the waste is incinerated, however, with an increasing trend.35 As shown by our modeling, incineration does not result (with the exception of CNT) in large alterations of the flows to the landfills, however, residues from incineration are normally placed in a different kind of landfill compared to direct landfilling of municipal solid waste.
The model presented a generic system of waste incineration and deposition in Switzerland. However, waste disposal in Switzerland lies within the responsibility of the cantonal (state) authorities, which leads to a different management and handling of certain waste streams depending on the location of the WIP and the landfills. For example, the filter cake from acid washing of the fly ash is in some cantons mixed with the bottom ash and deposited in the landfill for stabilized residues. In other cantons a separate deposition of the filter cake is required. It is also allowed to re-incinerate the filter cake from acid washing. The model is thus a simplification of the different processes, encompassing a synthesis of different practices which may in reality differ from the generic model. Besides the cantonal differences in the implementation of the legislation, the 29 WIP in Switzerland also differ in their equipment (internal or external wastewater treatment, filter types, acid washing of fly ash, dry discharge of bottom ash), size and the type of waste. Significant differences such as the acid washing of the filter ash, which is the state of the art in about one-third of the WIP in Switzerland, are reflected in the transfer coefficients used in the model.
The model combines WIP and sludge incineration. At a time there are WIP that burn exclusively waste, WIP that incinerate also sewage sludge, mono-incinerators that burn exclusively sewage sludge (SIP) and mixed systems (usually small scale) where sludge is burned together with other highly carbonaceous materials such as used wood or residues from the paper industry. While WIP produce a very heterogeneous slag, the only residue from SIP is fly ash that has a relatively high phosphorus content. This fly ash is in some landfills deposited in a separate compartment to allow for phosphorus recovery once a cost-effective technique is available. To foster the P-recycling from sewage sludge fly ash, Switzerland aims at a strict separation of waste and sludge incineration for the year 2030.
The slags from the WIP may be further treated on the landfill site to extract metals, e.g. iron. They may be crushed and undergo other processes that may result in generation of dust and release of ENM to the atmosphere. Due to the complete lack of data about these processes and the generation of nanomaterials, they were not considered in this work but need to be investigated in further studies.
All models proposed in this study are mass-based evaluations. For ENM, an additional number based model would be highly interesting especially if emission flows or toxicity should be assessed as well. The contribution of ENM to the total mass fraction is usually negligible whereas the number concentration can have a significant impact. Thus, the interest in number-based approaches increases and is under constant discussion e.g. in emission control or for the evaluation of ENM. For an improved number-based model, new particle number based data would be needed, which are currently not available. Most of the currently available data are still mass based. However, first models for ENM based on the particle number (particle flow analysis) are available.36,37
Conclusions
The amount of ENM predicted by the mass-based models of this study to be entering Swiss landfills every year is insignificant for CNT (<100 kg per annum) and very small for nano-Ag and nano-ZnO (less than 5 t/a). However, for nano-TiO2, which is quite a widespread yet inert material, the input into Swiss landfills sums up to around 150 t/a. The major flows for each ENM are expected to be transferred with the slag from waste incineration to the landfills – all other flows are at least one order of magnitude smaller (with the exception of the direct input to landfills for inert materials for construction waste). If the slag is processed before landfilling (e.g. crushed or treated for metal recovery), the potential for release of ENM exists. There are 793![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of slag produced per year in Switzerland18 and the concentration of the ENM is therefore about 190 mg kg−1 for TiO2, 6 mg kg−1 for ZnO, 1 mg kg−1 for Ag and 0.1 mg kg−1 for CNT. Compared to the total concentrations of the elements in slag (about 10 g kg−1 for Ti and 4–5 g kg−1 for Zn),38 the ENM constitute only a minor fraction of the respective elements in slag. However, a detailed characterization of the nanoscaled particles (both engineered and of natural origin) in slag and filter ash are needed in order to fully evaluate their significance.
000 tons of slag produced per year in Switzerland18 and the concentration of the ENM is therefore about 190 mg kg−1 for TiO2, 6 mg kg−1 for ZnO, 1 mg kg−1 for Ag and 0.1 mg kg−1 for CNT. Compared to the total concentrations of the elements in slag (about 10 g kg−1 for Ti and 4–5 g kg−1 for Zn),38 the ENM constitute only a minor fraction of the respective elements in slag. However, a detailed characterization of the nanoscaled particles (both engineered and of natural origin) in slag and filter ash are needed in order to fully evaluate their significance.
Acknowledgements
This work was supported by the Swiss Federal Office for the Environment (FOEN).References
- K. Schmid, B. Danuser and M. Riediker, J. Occup. Environ. Hyg., 2010, 7, 224–232 CrossRef.
- L. Geranio, M. Heuberger and B. Nowack, Environ. Sci. Technol., 2009, 43, 8113–8118 CrossRef CAS.
- H. Hagendorfer, C. Lorenz, R. Kaegi, B. Sinnet, R. Gehrig, N. V. Goetz, M. Scheringer, C. Ludwig and A. Ulrich, J. Nanopart. Res., 2010, 12, 2481–2494 CrossRef CAS.
- R. Kaegi, A. Ulrich, B. Sinnet, R. Vonbank, A. Wichser, S. Zuleeg, H. Simmler, S. Brunner, H. Vonmont, M. Burkhardt and M. Boller, Environ. Pollut., 2008, 156, 233–239 CrossRef CAS.
- L. Schlagenhauf, B. T. T. Chu, J. Buha, F. Nuesch and J. Wang, Environ. Sci. Technol., 2012, 46, 7366–7372 CrossRef CAS.
- F. von der Kammer, P. L. Ferguson, P. A. Holden, A. Masion, K. R. Rogers, S. J. Klaine, A. A. Koelmans, N. Horne and J. M. Unrine, Environ. Toxicol. Chem., 2012, 31, 32–49 CrossRef CAS.
- A. Ulrich, S. Losert, N. Bendixen, A. Al-Kattan, H. Hagendorfer, B. Nowack, C. Adlhart, J. Ebert, M. Lattuada and K. Hungerbuhler, J. Anal. At. Spectrom., 2012, 27, 1120–1130 RSC.
- S. J. Klaine, A. A. Koelmans, N. Horne, S. Carley, R. D. Handy, L. Kapustka, B. Nowack and F. von der Kammer, Environ. Toxicol. Chem., 2012, 31, 3–14 CrossRef CAS.
- N. C. Mueller and B. Nowack, Environ. Sci. Technol., 2008, 42, 4447–4453 CrossRef CAS.
- F. Gottschalk, R. W. Scholz and B. Nowack, Environ. Model. Software, 2010, 25, 320–332 CrossRef.
- F. Gottschalk, T. Sonderer, R. Scholz and B. Nowack, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS.
- L. Roes, M. K. Patel, E. Worrell and C. Ludwig, Sci. Total Environ., 2012, 417–418, 76–86 CrossRef CAS.
- E. Asmatulu, J. Twomey and M. Overcash, J. Nanopart. Res., 2012, 14, 720 CrossRef.
- G. Bystrzejewska-Piotrowska, J. Golimowski and P. L. Urban, Waste Manag., 2009, 29, 2587–2595 CrossRef CAS.
- N. Musee, Environ. Int., 2011, 37, 112–128 CrossRef CAS.
- T. Walser, L. K. Limbach, R. Brogioli, E. Erismann, L. Flamigni, B. Hattendorf, M. Juchli, F. Krumeich, C. Ludwig, K. Prikopsky, M. Rossier, D. Saner, A. Sigg, S. Hellweg, D. Gunther and W. J. Stark, Nat. Nanotechnol., 2012, 7, 520–524 CrossRef CAS.
- ISO, in Core Terms, International Organisation for Standardisation (ISO), Geneva, 2010, ISO/TS 80004-1 Search PubMed.
- M. Hügi, P. Gerber, A. Hauser, A. Laube, R. Quartier, K. Schenk and M. Wysser, Zahlen und Entwicklungen der schweizerischen Abfallwirtschaft 2005–2007, Abfallwirtschaftsbericht 2008, Umwelt-Zustand Nr. 0830, Bern, 2008 Search PubMed.
- C. Hanser, J. Kuster, R. Gessler, M. Ehrler, U. Dietler, H. Zimmermann, R. Speck, K. Schenk and R. Kettler, Evaluation der Abfallpolitik des Bundes, 2005 Search PubMed.
- NZZ-Folio, NZZ Beilage, 2006.
- A. Bühler and S. Schlumberger, Schwermetalle aus der Flugasche zurückgewinnen «Saure Flugaschewäsche – FLUWA-Verfahren» ein zukunftsweisendes Verfahren in der Abfallverbrennung, http://www.bsh.ch/upload/public/0/71/BSH-Umweltservice-AG-Saure-Flugaschenwaesche-FLUWA-Verfahren.pdf.
- P. Baccini and P. H. Brunner, Metabolism of the Anthroposphere, Springer-Verlag, Berlin, Heidelberg, New York, 1991 Search PubMed.
- L. Sørum, M. Fossum, E. Evensen and J. Hustad, Fifth Annual North American Waste-to-Energy Conference, 1997 Search PubMed.
- L. Sorum, F. J. Frandsen and J. E. Hustad, Fuel, 2003, 82, 2273–2283 CrossRef CAS.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Environ. Toxicol. Chem., 2010, 29, 1036–1048 CAS.
- H. Burtscher, M. Zürcher, A. Kasper and M. Brunner, in Proc. Int. ETH Conf. on Nanoparticle Measurement, ed. A. Mayer, BUWAL, 2002, vol. 52 Search PubMed.
- B. Frey, Total Revision TVA, 2010, http://kommunale-infrastruktur.ch/cmsfiles/bafu_frey.pdf Search PubMed.
- C. Ludwig, C. A. Johnson, M. Kappeli, A. Ulrich and S. Riediker, J. Contam. Hydrol., 2000, 42, 253–272 CrossRef CAS.
- R. Kaegi, A. Voegelin, B. Sinnet, S. Zuleeg, H. Hagendorfer, M. Burkhardt and H. Siegrist, Environ. Sci. Technol., 2011, 45, 3902–3908 CrossRef CAS.
- P. Lozano and N. D. Berge, Waste Manag., 2012, 32, 1699–1711 CrossRef CAS.
- B. Kim, C. S. Park, M. Murayama and M. F. Hochella, Environ. Sci. Technol., 2010, 44, 7509–7514 CrossRef CAS.
- E. Lombi, E. Donner, E. Tavakkoli, T. W. Turney, R. Naidu, B. W. Miller and K. G. Scheckel, Environ. Sci. Technol., 2012, 46, 9089–9096 CrossRef CAS.
- European Commission, Europe in Figures. Eurostat Yearbook 2010, Publications Office of the European Union, Luxembourg, 2010, DOI:10.2785/40830.
- N. Tanaka, Y. Tojo and T. Matsuto, J. Mater. Cycles Waste Manage., 2005, 7, 104–111 CrossRef CAS.
- US EPA, Municipal Solid Waste Generation, Recycling, and Disposal in the United States: Facts and Figures for 2010, United States Environmental Protection Agency Solid Waste and Emergency Response (5306P), Washington, DC 20460, 2011 Search PubMed.
- R. Arvidsson, S. Molander and B. A. Sandén, J. Ind. Ecol., 2011, 15, 844–854 CrossRef CAS.
- R. Arvidsson, S. Molander and B. A. Sandén, J. Ind. Ecol., 2012, 16, 343–351 CrossRef CAS.
- K. Schenk, KVA-Rückstände in der Schweiz. Der Rohstoff mit Mehrwert, Bundesamt für Umwelt, Bern, 230 S., 2010 Search PubMed.
| This journal is © The Royal Society of Chemistry 2013 |
