Extracellular conversion of silver ions into silver nanoparticles by protozoan Tetrahymena thermophila†
Katre Jugansonab,
Monika Mortimer*a,
Angela Ivaska,
Kaja Kasemetsa and
Anne Kahrua
aLaboratory of Environmental Toxicology, National Institute of Chemical Physics and Biophysics, Akadeemia tee 23, Tallinn 12618, Estonia. E-mail: monika.mortimer@kbfi.ee; Fax: +372 639 8382; Tel: +372 639 8382
bDepartment of Chemistry, Tallinn University of Technology, Akadeemia tee 15, Tallinn, 12618, Estonia
First published on 11th December 2012
Abstract
In the current study, cell-free exudates of the ciliated protozoan Tetrahymena thermophila were shown to progressively convert silver nitrate to silver nanoparticles (Ag NPs) under illumination at ambient temperature. The formation of Ag NPs in the reaction mixture was evidenced by gradual colour changes, appearance of a specific absorbance peak (420–450 nm) and visualization using scanning electron microscopy coupled to an energy-dispersive X-ray spectrometer. After 2 h of incubation the mean hydrodynamic size of the Ag NPs was 70 nm. Seven days of incubation resulted in larger agglomerates and a significant decrease in silver toxicity to T. thermophila, accompanied by about 100-fold reduction in the silver ion concentration. Protein analysis indicated an extensive extracellular protein binding by the Ag NPs formed in the protozoan exudates. As protozoa are important components in wastewater treatment, their ability to sequester silver ions into a less bioavailable and less toxic form of silver (e.g. NPs) may be one of the adaption mechanisms of ciliate survival in contaminated environments.
Environmental impactThe use of silver nanoparticles (Ag NPs) in various consumer products has remarkably increased during the last few decades. Thus, it is more likely that such materials are released into the environment and the silver ion (Ag+) concentrations will increase due to dissolution of Ag NPs. It is therefore imperative to assess their potential adverse effects on different trophic levels. The current work describes the possible adaptation mechanisms of the fresh-water ciliated protozoan Tetrahymena thermophila to elevated silver concentrations in the environment. It was shown that the protozoan exudates reduced the concentration of Ag+ by forming Ag NPs, which were less toxic to T. thermophila compared to Ag+. |
Introduction
The rapid development of nanotechnology over the past few decades has resulted in an increasing presence of engineered nanoparticles (NPs) in consumer commodities. According to the Woodrow Wilson Database in March 2011 there were more than 1300 consumer products on the market incorporating NPs, and 313 of them containing silver (Ag).1 Mainly due to their antimicrobial properties, Ag NPs are used in various biomedical applications, food preservation, water and air purification, household products, cosmetics, clothing, and goods for children.2–4 These antimicrobial properties are shown to rely upon the slow dissolution of NPs,5,6 as Ag ions are considered one of the most toxic forms of heavy metals to microorganisms as well as other forms of life.7Despite the high toxicity of Ag compounds, a number of organisms have been identified to be capable of forming Ag NPs in vivo.8 Specifically, bacteria, fungi, algae and other organisms that are capable of synthesizing Ag NPs either intra- or extracellularly have already been successfully applied for the green synthesis of nanosilver.9
The main driving force for NP biosynthesis by the microorganisms is presumably derived from the need for detoxification of Ag ions into insoluble, less or nontoxic nanoclusters.10,11 Although the current focus on biosynthesis of Ag NPs has shifted from empirical observations towards mechanistic studies, the exact mechanisms explaining the formation of these NPs remain to be elucidated.12 For example, it has been shown that peptides containing arginine, cysteine, lysine and methionine were capable of reducing silver ions and could further promote the growth of Ag nanocrystals.13 Parikh et al.14 reported the formation of Ag NPs by cell-free supernatant of bacteria Morganella sp. and suggested that the NPs were formed as a result of silver-specific proteins secreted by the bacteria. Nitrate reductase has been proposed as one of the key enzymes in reducing Ag ions to metallic silver and the activity of this enzyme was shown to increase in certain bacteria, fungi and plants in the presence of AgNO3.12 Kumar et al.15 provided further evidence on the role of this enzyme in Ag ion reduction, demonstrating that Ag NPs could be synthesized in vitro using purified nitrate reductase from fungi Fusarium oxysporum in the presence of phytochelatin, 4-hydroxyquinoline and NADPH.
Until now the formation of Ag NPs as a possible route to Ag ion detoxification has been studied mainly in fungi and bacteria.11 However, the increasing use of Ag NPs in consumer products is expected to result in elevated Ag levels in the environment16 and thus the capability of other biological species to cope with the toxic amounts of Ag NPs should also be studied. After bacteria, ciliates constitute the second most relevant and abundant community actively participating in the wastewater treatment process.17,18 The unicellular protozoa of genus Tetrahymena are freshwater ciliates that have been used as model organisms for environmental research for decades.19 However, no information on the interactions between protozoa and Ag ions and their possible conversion to NPs by ciliate protozoa in the environment is available. Moreover, according to a recent review by Kahru and Dubourguier,20 toxicity data for Ag NPs towards ciliates are practically non-existent in the literature. Kvitek et al.21 showed that a 1 h LC50 value for Ag NPs towards ciliate Paramecium caudatum was 39 mg L−1 and that the surfactant/polymer modification could increase the toxicity of the Ag NPs against these organisms. According to Shi et al.22 Ag NPs were toxic to T. pyriformis (IC50 = 1.5 mg L−1); however, they demonstrated that light irradiation decreased the toxicity.
In this study, we show that the soluble extracellular fraction (SEF) of Tetrahymena thermophila can affect the toxicity of AgNO3 by reducing Ag ions and promoting the formation of Ag NPs. The formed Ag NPs were characterized by UV-Vis spectroscopy, dynamic light scattering (DLS) and scanning electron microscopy (SEM). Specifically, we show that the protein fraction of SEF facilitates the formation of the Ag NPs.
Experimental
Materials
AgNO3 was purchased from J.T. Baker and silver NPs (nominal particle size < 100 nm; further designated as “Sigma-Ag NPs”) from Sigma-Aldrich. All chemicals used in the study were of analytical grade. The stock suspensions/solutions of Sigma-Ag NPs (54 g L−1) and AgNO3 (54 g Ag L−1) and their further dilutions were prepared in deionized water (MilliQ, Millipore). The stock suspension containing Sigma-Ag NPs was ultrasonicated in Branson Ultrasonic Bath 1510 for 30 min. Both stock suspensions/solutions were stored at room temperature in the dark.Characterisation of commercial silver nanoparticles
The hydrodynamic diameter and zeta potential of Sigma-Ag NPs (100 mg L−1) in MilliQ water were measured using a Zetasizer Nano ZS (Malvern Instruments). Dissolution of Sigma-Ag NPs in MilliQ water was measured at 205 mg L−1 using an Ag ion selective electrode (Van London-pHoenix Company). The fraction of dissolved Ag was calculated based on a calibration curve constructed using aqueous solutions of AgNO3 with different concentrations (10.8 μg–1.08 g Ag+ L−1). SEM images of Sigma-Ag NPs have been published in our previous article by Ivask et al.23Preparation of soluble extracellular fraction of Tetrahymena thermophila
Protozoan culture (T. thermophila strain BIII) was cultivated essentially as described by Mortimer et al.24 The cells were harvested during the exponential growth phase (5 × 105 cells mL−1) by centrifugation at 300g for 5 min at 4 °C and washed twice with MilliQ water. The number of cells was quantified by counting in a haemocytometer (Neubauer Improved, bright line; Germany) after immobilisation in 5% formalin.Harvested and washed T. thermophila was incubated in MilliQ water on an orbital shaker at 100 rpm, 30 °C for 24 h. The cells were pelleted by centrifugation at 300g for 5 min at 4 °C and the supernatant was further purified from the cellular debris and membranous fraction by ultracentrifugation at 390![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 1 h at 20 °C. The obtained supernatant was designated as “soluble extracellular fraction” (SEF).
000g for 1 h at 20 °C. The obtained supernatant was designated as “soluble extracellular fraction” (SEF).
Characterisation of silver nanoparticles formed from silver nitrate in the soluble extracellular fraction of Tetrahymena thermophila
AgNO3 (108 mg Ag L−1) was added to SEF and incubated in 50 mL polypropylene centrifugation tubes (Falcon) on a shaker (150 rpm; Certomat MO II, B. Braun) at 25 °C with illumination from below using Philips TL-D 38 W aquarelle fluorescent tubes. The samples were collected after 0, 2, 24 h and 7 days of incubation. The resulting mixtures were further designated as “SEF-Ag”.Colour changes in SEF-Ag were monitored both visually and using a UV-Vis spectrophotometer. The absorbance spectra induced by the surface plasmon resonance of Ag NPs in SEF were recorded between 300 and 700 nm (Multiskan Spectrum, Thermo Scientific).
The hydrodynamic diameter of formed Ag NPs was measured using a Zetasizer Nano ZS. The particles formed after 2 and 24 h of incubation were visualized using SEM (Zeiss EVO MA 15). Silver ion concentrations in the samples were quantified using an Ag ion selective electrode (see above).
To analyse the protein composition of SEF before and after incubation with AgNO3, SEF and SEF-Ag samples were concentrated 20-fold via freeze-drying. For sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), the samples were denatured with SDS-sample buffer for 5 min at 100 °C. The proteins were separated on 14% acrylamide gel using constant amperage (35 mA). The proteins were visualized with silver staining and a quantitative analysis of proteins was performed using TotalLabQuant software (Totallab, UK).
For the toxicity analysis the SEF-Ag samples collected after 0, 2, 24 h and 7 days were stored at −18 °C. Prior to the assay, the samples were thawed and diluted 2-, 4-, 8-, 16- and 32-fold with MilliQ water.
Exposure of Tetrahymena thermophila to toxicants
For toxicity analysis 100 μL of harvested and washed T. thermophila suspension in MilliQ water was added to 100 μL of the solution/suspension of Ag compounds (AgNO3, SEF-Ag or Sigma-Ag NPs) that were previously diluted in MilliQ water in 96-well polystyrene plates (Falcon). The following nominal concentrations (chosen according to pre-screening results) were used in the toxicity testing: 1.6, 1.9, 3.2, 3.8, 5.1 and 6.4 mg Ag L−1 of AgNO3; 1.7, 3.4, 6.8, 13.5, 27 and 54 mg Ag L−1 of SEF-Ag; and 50, 100, 150, 200 and 500 mg L−1 of Sigma-Ag NPs. Each concentration was tested in three replicates and the final cell density in the test was 5 × 105 cells mL−1. Protozoan suspension in MilliQ water and Ag compounds in MilliQ water were used as non-treated and abiotic controls, respectively. The test plates were incubated on a microplate shaker (300 rpm, Heidolph Titramax 1000) at 25 °C. The pH of T. thermophila suspension in MilliQ water did not change significantly upon the addition of Ag compounds and remained unchanged for 24 h (pH = 6.6 ± 0.3). After 2- and 24 h exposure 100 μL of the cell suspension was sampled from each well, and viability of the cells was determined by measuring the ATP content using the luciferin–luciferase method essentially as described by Mortimer et al.24Cells were visualized using a light microscope (Olympus CX41) equipped with a DP71 camera.
Data analysis
The ATP concentration in the samples was expressed as a percentage of the non-treated control. The EC50 (effective concentration leading to a 50% loss in cell viability) values with 95% confidence interval were calculated from concentration–effect curves using the REGTOX software for Microsoft Excel™.25Results
Toxicity of silver nitrate to Tetrahymena thermophila
The EC50 values of AgNO3 to T. thermophila calculated from the ATP concentration data were not significantly different after 2- and 24 h exposure (1.8 and 1.5 mg Ag L−1, respectively, Table 1). However, visualisation of the AgNO3-exposed T. thermophila cultures under the light microscope indicated a moderate recovery of the protozoan culture after 24 h exposure compared to the 2 h exposed culture, indicated by the increased share of viable cells. Also, during the visual inspection of the assay wells containing T. thermophila culture exposed to the highest concentration of AgNO3 a slight colour change from colourless to maroon was noted. As the toxicity assay was conducted in MilliQ water where no interfering media components were present, it was inferred that the colour change indicated potential changes in the speciation and/or transformation of AgNO3 induced by the protozoan culture. To determine which cellular processes were responsible for the observed changes with AgNO3, we isolated the protozoan exudates and examined their potential role in the transformation of AgNO3.Silver nanoparticle formation in the soluble extracellular fraction of Tetrahymena thermophila
Similar to the incubation of AgNO3 in the protozoan culture, the addition of AgNO3 to cell-free SEF of T. thermophila induced formation of maroon colour (Fig. 1, inset). Remarkably, during the same incubation period (up to 7 days), the colour change was detected only under the illuminated conditions but not in the dark (not shown). The intensity of the colour formed in SEF-Ag under illumination increased over time (Fig. 1, inset). After one week of incubation a black precipitate formed at the bottom of the incubation vessel.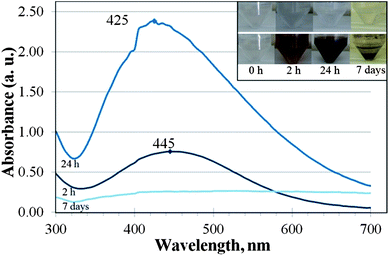 | ||
| Fig. 1 UV-Vis absorption spectra of silver nanoparticles (NPs) formed after 2 h (middle line), 24 h (upper line) and 7 days (lower line) of incubation of AgNO3 (final concentration 108 mg Ag L−1) with the soluble extracellular fraction (SEF) of Tetrahymena thermophila. Inset: photos of SEF of T. thermophila (upper panel; no AgNO3 added) and formed Ag NP suspensions in SEF (lower panel) at different incubation times. No specific absorption peak was detected at the beginning of incubation (t = 0 h; data not shown). | ||
The analysis of the UV-Vis absorbance spectra of the 2- and 24 h incubated SEF-Ag showed a clear surface plasmon resonance peak at 420–450 nm that is characteristic to Ag NPs (Fig. 1). After 7 days of incubation, the plasmon resonance peak of SEF-Ag was significantly broadened and its intensity was low (Fig. 1).
In Fig. 2A the size distribution of the particles formed in SEF-Ag is shown. After 2 h incubation the average hydrodynamic diameter of the formed NPs was 70 nm. In comparison, after 24 h of incubation the mean particle size increased to 105 nm and after 7 days the particle size exceeded the measurement range of the Zetasizer Nano ZS (up to 10 μm). The formation of particles was accompanied by a decrease of silver ion concentration in SEF-Ag (Fig. 2B).
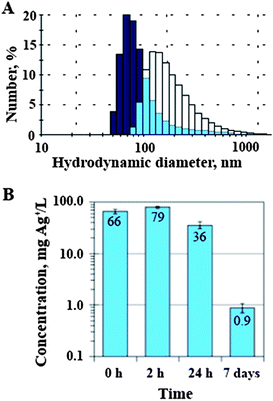 | ||
| Fig. 2 (A) The size distribution of silver nanoparticles (NPs) formed after 2 h (dark blue bars) and 24 h (white bars) of the reaction of AgNO3 (108 mg Ag L−1 added at time zero) with the soluble extracellular fraction (SEF) of Tetrahymena thermophila, light blue bars indicate the overlap of the size distributions. (B) The concentration of silver ions in the mixture of 108 mg Ag L−1 AgNO3 and SEF of T. thermophila after 0, 2 and 24 h and 7 days of reaction; note the logarithmic scale of the y-axis. | ||
Interestingly, although 108 mg Ag L−1 of AgNO3 was added to SEF, only about 70 mg Ag+ L−1 was detectable by the ion selective electrode at the zero time point (Fig. 2B). Thus, the addition of SEF immediately decreased the concentration of Ag ions in AgNO3 solution, apparently due to the immediate binding of silver ions to proteins and/or changed Ag speciation. The concentration of silver ions decreased even more over time and after 7-day incubation the concentration of silver ions in SEF was about 100 times lower (∼0.9 mg Ag L−1) compared to the initial nominal concentration.
The literature suggests that peptides and proteins may be responsible for the biotic formation of Ag NPs from Ag ions. Therefore we studied changes in the protein composition of SEF after its reaction with AgNO3. According to SDS-PAGE, the most prevalent proteins in SEF were within the size range of 22–34 kDa (indicated by a square bracket in Fig. 3). During the reaction of SEF with AgNO3, the amount of those proteins decreased compared to the pure SEF incubated under the same conditions. The intensity of the protein bands of 22–34 kDa size range in the SEF incubated with AgNO3 for 2 h and 24 h decreased by 75% (Fig. 3, lane 4) and 85% (Fig. 3, lane 6), respectively. No proteins within the size range of 22–34 kDa were detected in SEF after 7-day incubation (Fig. 3, lane 8).
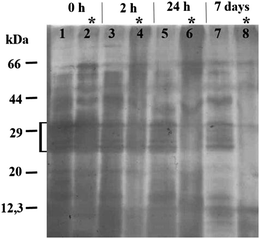 | ||
| Fig. 3 SDS-PAGE analysis of the soluble extracellular fraction (SEF) of Tetrahymena thermophila and SEF incubated with AgNO3 for 2 and 24 h and 7 days. The gel was silver stained; * SEF incubated with AgNO3 (108 mg Ag L−1 added at time zero); ‘[’ proteins within the size range of 22–34 kDa. | ||
Comparison of toxicity of SEF-Ag and Sigma-Ag NPs to Tetrahymena thermophila
The Ag particles formed in SEF-Ag were further analysed by SEM in the backscattered electron imaging mode. After 24 h of incubation of SEF-Ag, silver NPs were formed (Fig. S1†).Given that (i) microscopic evaluation revealed a slight recovery of the T. thermophila culture upon exposure to AgNO3 for 24 h compared to 2 h exposure and (ii) during that time, Ag NPs were formed, we propose that the observed recovery of the protozoan culture with an increasing exposure period could be associated with the decreasing concentrations of Ag ions via Ag NP formation. Indeed, when T. thermophila was exposed to SEF-Ag collected after different incubation times, the longer incubated SEF-Ag was less toxic as indicated by the less steep slope of the dose–effect curve and the higher EC50 values (Fig. 4).
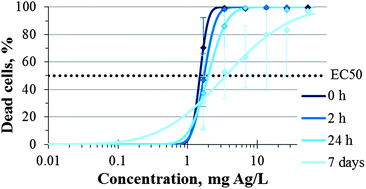 | ||
| Fig. 4 The effect of AgNO3 pre-incubated in the soluble extracellular fraction (SEF) of Tetrahymena thermophila for 0, 2 and 24 h and 7 days on the viability of T. thermophila. On the logarithmic x-axis are the nominal concentrations of AgNO3 added to SEF at t = 0 h. The percentage of dead cells was calculated by measuring the ATP concentration after 2 h of incubation of protozoa with test samples. Data points are the mean values of at least 3 independent experiments, error bars indicate standard deviation. | ||
To confirm that Ag NPs are less toxic to T. thermophila than AgNO3, we chose commercial Ag NPs (herein designated as Sigma-Ag NPs) that were of comparable size with the NPs formed in SEF-Ag. The average hydrodynamic diameter of Sigma-Ag NPs was 147 nm. On the other hand, the polydispersity index of these commercial NPs was 0.48, indicating that the sample was not monodisperse and contained also larger agglomerates. Also, Ag ion concentration in the suspension of Sigma-Ag NPs in MilliQ water was very low: after 24 h only 0.42% (0.86 mg L−1 of 205 mg L−1) of Ag was dissolved (Table 2).
| a The concentration of silver ions, measured in the suspension of Sigma-Ag NPs (nominal concentration 205 mg L−1i.e., 24 h EC50 value for T. thermophila) in MilliQ water. | ||
|---|---|---|
| Hydrodynamic diameter | 147 nm | |
| Zeta potential | −51 mV | |
| Polydispersity index | 0.48 | |
| Solubility in timea | ||
| 0 h | 0.42 ± 0.01 mg L−1 | 0.20% |
| 2 h | 0.56 ± 0.04 mg L−1 | 0.3% |
| 24 h | 0.86 ± 0.06 mg L−1 | 0.42% |
Compared to AgNO3, Sigma-Ag NPs were indeed remarkably less toxic: the EC50 values of the Sigma-Ag NPs were between 205 and 286 mg L−1, while the EC50 values of AgNO3 varied from 1.5 to 1.8 mg Ag L−1 (Table 1). This indicates that the formation of Ag NPs from Ag ions could be one of the defence mechanisms of T. thermophila against the toxic silver ions. Remarkably, we also observed that Ag NPs could be taken up and stored intracellularly in the food vacuoles of T. thermophila (Fig. 5). Visual observations at different time periods revealed that the ingested Ag NPs further agglomerated in the food vacuoles of T. thermophila.
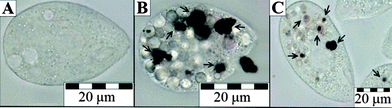 | ||
| Fig. 5 Images of Tetrahymena thermophila under a light microscope. (A) Control cell, (B) after 2 h exposure to Sigma-Ag NPs at 100 mg L−1, and (C) after 24 h exposure to SEF-Ag at 7 mg Ag L−1. Black arrows indicate food vacuoles filled with agglomerates of silver NPs. | ||
Discussion
The increasing use of Ag NPs in industrial and household applications likely leads to the release of Ag NPs to the environment, where the particles may either agglomerate or dissolve to impact the food chain.16,26 Dissolved silver ions are very toxic to most invertebrate, plant and fish species, and the LC50 values of AgNO3 have been documented to be between 0.1 and 1 mg L−1. According to Ratte7 the most sensitive freshwater organisms to AgNO3 are the crustaceans (LC50Daphnia magna,48 h = 0.5–35 μg L−1), and the least sensitive the amphipod Hyalella azteca (LC50 = 1.6–397.7 mg L−1). According to the current study, the freshwater protozoan T. thermophila showed a higher tolerance to silver nitrate (EC50 values 1–2 mg Ag L−1; Table 1) than other freshwater invertebrates. Such results are remarkable considering the fact that the toxicity assays were performed in MilliQ water, where the effects of the medium components on the Ag ion complexation and speciation were eliminated. Nevertheless, our results support the data reported by Shi et al.22 who showed that 1.5 mg Ag L−1 of AgNO3 resulted in ∼60% growth inhibition of Tetrahymena pyriformis. Moreover, T. thermophila has been shown to be less sensitive to most toxic substances compared to other aquatic standard test species27 and it possesses many ABC transporters associated with resistance to multiple drugs and toxins.28 These features are presumably a result of the adaption to high environmental concentrations of pollutants as protozoa are also present in the wastewater purification process.29Even though in the current study the toxicity testing of AgNO3 to T. thermophila was done in MilliQ water, with the aim of minimising any complexation and sedimentation of Ag ions as insoluble salts, the effect of the test organism to the speciation of the toxicant is also an important factor to consider. This was clearly demonstrated as the extensive formation of Ag NPs from Ag ions and further agglomeration of the NPs could occur in the SEF of T. thermophila (Fig. 1, inset). As several microorganisms have been shown to be capable of synthesising Ag NPs from Ag ions both intra- and extracellularly,9 it could be assumed that T. thermophila secreted Ag+-reducing compounds into the surrounding environment. Interestingly, under the illuminated conditions the colour changes in the test environment appeared within minutes, whereas no colour change was observed in the dark even after 7 days of incubation. This evidence is in agreement with the data reported by Nam et al.30 and Mokhtari et al.31 who pointed out that the biosynthesis of Ag NPs from Ag ions is promoted by the visible light. Nam et al.30 showed that silver ions were photoreduced in the presence of the carboxylic acid-containing peptides and ambient light. Peptides and proteins could be most likely involved in the Ag NP biosynthesis also in SEF of T. thermophila as the protozoan is known to release various acid hydrolases into the extracellular medium.32 Madinger et al.33 found that under starving conditions T. thermophila secreted ∼30 different proteins, most of which were proteases. It has been shown that the peptides containing amino acid moieties such as arginine, cysteine, lysine and methionine reduced Ag ions and formed silver NPs of a wide size distribution.13 The Ag NPs formed in the SEF of T. thermophila in the current study were also characterised by high polydispersity in their hydrodynamic size (Fig. 2A) and the broad peaks in the UV-Vis spectra (Fig. 1). It is known that in the biological fluids NPs readily become coated by proteins leading to the formation of protein corona.34 Such corona has been shown to stabilize the NPs resulting in a more stable aqueous suspension35 but may also play a role of creating a chemically reducing environment around the silver clusters and promote crystal growth.12 Our result from SDS-PAGE analysis (Fig. 3) confirmed the involvement of proteins in Ag NP formation as in SEF-Ag the amount of proteins decreased in time. SEF proteins within the size range of 22–34 kDa seemed to show a specifically higher affinity for the Ag NPs at first. Nevertheless, after 7 days of incubation all of the proteins in SEF-Ag appeared to be bound, as indicated by the lack of the protein bands in SDS-PAGE (Fig. 3). This suggests that under the conditions of the current study, where an excess amount of AgNO3 was used, formation of Ag NPs was limited by the amount of proteins secreted by T. thermophila, particularly considering the aggregation and precipitation of Ag particles after 7-day incubation. The role of proteins and peptides in the formation and growth of Ag NPs in SEF was further supported by the UV-Vis analysis of SEF-Ag (Fig. 1). The absorption spectra obtained from the SEF-Ag samples were consistent with the literature, with the absorption maximum of the biosynthesised Ag NPs occurring in the range of 400 to 450 nm.36,37 As the amplitude of the absorbance peak is proportional to the concentration of Ag NPs,38 the increase in absorbance observed after 24 h of incubation compared to the first 2 h of incubation indicated the increased number of Ag NPs over time. As it has been discussed previously in a study on the formation of Ag NP-human serum albumin (HSA) corona, the characteristic peak of the surface plasmon resonance of Ag NPs becomes red-shifted upon the binding of dielectric HSA molecules onto the Ag NP.39 In our study, the characteristic absorbance peak of Ag NPs was identified at 445 nm after 2 h incubation and the peak maximum was shifted towards the shorter wavelength of 425 nm after 24 h incubation of SEF-Ag (Fig. 1). This phenomenon could be explained as follows (schematically illustrated in Fig. 6): during the first hours of the Ag NP formation the number of NPs was low enough to allow sufficient coating of NPs by the proteins excreted by T. thermophila. As the concentration of Ag NPs increased over time, as indicated by the higher absorbance peak in the UV-Vis spectrum after 24 h, the free and weakly bound SEF proteins (soft corona) disassembled from the earlier formed Ag NPs to coat the newly formed Ag crystals. During this process the average thickness of the protein corona surrounding the increasing number of Ag NPs decreased (hard corona was formed), which was reflected by the shift of the absorbance peak towards the lower wavelength. At the final stage, when the proteins were entirely consumed by Ag particles, while continuously reducing the excess Ag ions and promoting the NP formation, the uncoated Ag NPs started to aggregate and precipitate. This process was characterised by the broad and less intense absorbance peak obtained after 7 days of incubation that coincided with the visual observations and the DLS measurements (Fig. 2A). The proposed reaction is also supported by the complete disappearance of the protein bands in the 7-day samples of the SDS-PAGE analysis.
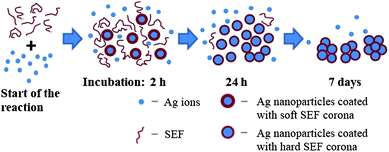 | ||
| Fig. 6 Schematic illustration of the hypothetical mechanism for the formation of silver nanoparticles assisted by the soluble extracellular fraction (SEF) of Tetrahymena thermophila. Soft SEF corona – proteins are weakly bound to nanoparticles; hard SEF corona – proteins are strongly bound to nanoparticles. | ||
The possible toxicity mechanisms of Ag NPs are still under discussion in the scientific community: some have proposed that the toxicity of Ag NPs is solely caused by dissolved Ag ions, others suggest that the particle itself also has a role.40 Conflicting results can also arise as one particle might have different mechanisms in different organisms as has been demonstrated in the case of CuO NPs.24,41 Our results support the contribution of Ag ions to Ag NP toxicity in protozoa as longer incubation of SEF-Ag, which resulted in the formation and growth of Ag NPs accompanied by the decrease in Ag ion concentration, leads to the decreased toxicity of SEF-Ag.
It has been proposed in the literature that the biosynthesis of Ag NPs is one of the detoxification routes for Ag ions.10,11 Although several organisms synthesize Ag NPs intracellularly,9T. thermophila created an environment that reduced Ag ions to Ag NPs extracellularly. Considering the fact that Ag NPs were less toxic to T. thermophila than Ag ions and the toxicity of Ag NPs decreased with the increasing size of the particle, we attribute the formation of Ag NPs catalysed by SEF as one of the primary adaptation mechanisms of T. thermophila to toxic metal ions. Further, the formed Ag NPs were taken up and stored intracellularly in the food vacuoles of T. thermophila. Indeed, Martín-González et al.42 proposed that biocomplexation could be an important mechanism of resistance of ciliated protozoa to toxic metal ions. The latter mechanism may be one of the adaptational tools for protozoa living in the metal-polluted environments.
Conclusions
The current study demonstrated for the first time that Ag ions were rapidly reduced to Ag NPs by the soluble extracellular fraction (SEF) of the ciliated protozoan T. thermophila under illumination at ambient temperature. The proteins of T. thermophila SEF were associated with the NP formation and might have also played a role in promoting NP growth. As incubation of silver in SEF reduced its toxicity to T. thermophila, formation of Ag NPs may be one of the response mechanisms of the organism to toxic metal ions. The results of the study provide insight into the dynamics of nanomaterials in the aquatic environment by broadening the knowledge on NP formation mediated by cellular exudates, toxicity of NPs and released ions on aquatic species and eventually on other species in the food web.Acknowledgements
This work was supported by Estonian targeted funding project SF0690063s08, ETF8561, ETF9001, Estonian Research Council project TERIKVANT and EU FP7 Project NanoValid (grant agreement no. 263147). We would like to thank H. Vija and A. Käkinen for experimental assistance, Prof. U. Kallavus (Tallinn University of Technology) for electron microscopy and Dr M. Samel for helpful consultation on SDS-PAGE.References
- W. Wilson, Consumer products inventory Project on Emerging Nanotechnologies, a project of the Woodrow Wilson International Center for Scholars, 2012, http://www.nanotechproject.org.
- J. S. Kim, E. Kuk, K. N. Yu, J. H. Kim, S. J. Park, H. J. Lee, S. H. Kim, Y. K. Park, Y. H. Park, C. Y. Hwang, Y. K. Kim, Y. S. Lee, D. H. Jeong and M. H. Cho, Nanomedicine, 2007, 3, 95–101 CrossRef CAS.
- C. Marambio-Jones and E. M. V. Hoek, J. Nanopart. Res., 2010, 12, 1531–1551 CrossRef CAS.
- M. K. Rai, S. D. Deshmukh, A. P. Ingle and A. K. Gade, J. Appl. Microbiol., 2012, 112, 841–852 CrossRef CAS.
- G. A. Sotiriou and S. E. Pratsinis, Environ. Sci. Technol., 2010, 44, 5649–5654 CrossRef CAS.
- X. Li and J. J. Lenhart, Environ. Sci. Technol., 2012, 46, 5378–5386 CrossRef CAS.
- H. T. Ratte, Environ. Toxicol. Chem., 1999, 18, 89–108 CrossRef CAS.
- H. Korbekandi, S. Iravani and S. Abbasi, Crit. Rev. Biotechnol., 2009, 29, 279–306 CrossRef CAS.
- R. Vaidyanathan, K. Kalishwaralal, S. Gopalram and S. Gurunathan, Biotechnol. Adv., 2009, 27, 924–937 CrossRef CAS.
- C. K. Carney, S. R. Harry, S. L. Sewell and D. W. Wright, Top. Curr. Chem., 2007, 270, 155–185 CrossRef CAS.
- J. M. Slocik, M. R. Knecht and D. W. Wright, Encycl. Nanosci. Nanotechnol., 2004, 1, 293–308 CAS.
- N. Durán, P. D. Marcato, M. Durán, A. Yadav, A. Gade and M. Rai, Appl. Microbiol. Biotechnol., 2011, 90, 1609–1624 CrossRef.
- R. R. Naik, S. J. Stringer, G. Agarwal, S. E. Jones and M. O. Stone, Nat. Mater., 2002, 1, 169–172 CrossRef CAS.
- R. Y. Parikh, S. Singh, B. L. V. Prasad, M. S. Patole, M. Sastry and Y. S. Shouche, ChemBioChem, 2008, 9, 1415–1422 CrossRef CAS.
- S. A. Kumar, M. K. Abyaneh, S. W. Gosavi, S. K. Kulkarni, R. Pasricha, A. Ahmad and M. I. Khan, Biotechnol. Lett., 2007, 29, 439–445 CrossRef CAS.
- B. Nowack and T. D. Bucheli, Environ. Pollut., 2007, 150, 5–22 CrossRef CAS.
- P. Madoni, Water Res., 1994, 28, 67–75 CrossRef CAS.
- R. J. Seviour and L. L. Blackall, The Microbiology of Activated Sludge, Kluwer Academic Publishers, London, 1999 Search PubMed.
- M. P. Sauvant, D. Pepin and E. Piccinni, Chemosphere, 1999, 38, 1631–1669 CrossRef CAS.
- A. Kahru and H. C. Dubourguier, Toxicology, 2010, 269, 105–119 CrossRef CAS.
- L. Kvitek, M. Vanickova, A. Panacek, J. Soukupova, M. Dittrich, E. Valentova, R. Prucek, M. Bancirova, D. Milde and R. Zboril, J. Phys. Chem. C, 2009, 113, 4296–4300 CAS.
- J.-P. Shi, C.-Y. Ma, B. Xu, H.-W. Zhang and C.-P. Yu, Environ. Toxicol. Chem., 2012, 31, 1630–1638 CrossRef CAS.
- A. Ivask, O. Bondarenko, N. Jepihhina and A. Kahru, Anal. Bioanal. Chem., 2010, 398, 701–716 CrossRef CAS.
- M. Mortimer, K. Kasemets and A. Kahru, Toxicology, 2010, 269, 182–189 CrossRef CAS.
- E. Vindimian, MSExcel macro REGTOX_EV7.0.5.xls, 2011, http://www.normalesup.org/~vindimian/.
- M. Delay and F. H. Frimmel, Anal. Bioanal. Chem., 2012, 402, 583–592 CrossRef CAS.
- A. Gerhardt, A. Ud-Daula and K. W. Schramm, Acta Protozool., 2010, 49, 271–280 Search PubMed.
- J. Xiong, L. Feng, D. Yuan, C. Fu and W. Miao, BMC Evol. Biol., 2010, 10, 330 CrossRef.
- G. Esteban, C. Tellez and L. M. Bautista, Water Res., 1991, 25, 967–972 CrossRef.
- K. T. Nam, Y. L. Lee, E. M. Krauland, S. T. Kottmann and A. M. Belcher, ACS Nano, 2008, 2, 1480–1486 CrossRef CAS.
- N. Mokhtari, S. Daneshpajouh, S. Seyedbagheri, R. Atashdehghan, K. Abdi, S. Sarkar, S. Minaian, H. R. Shahverdi and A. R. Shahverdi, Mater. Res. Bull., 2009, 44, 1415–1421 CrossRef CAS.
- M. Hartmann, A. Guberman, M. Florin-Christensen and A. Tiedke, Appl. Microbiol. Biotechnol., 2000, 54, 390–396 CrossRef CAS.
- C. L. Madinger, K. Collins, L. G. Fields, C. H. Taron and J. S. Benner, Eukaryotic Cell, 2010, 9, 674–681 CrossRef CAS.
- T. Cedervall, I. Lynch, S. Lindman, T. Berggard, E. Thulin, H. Nilsson, K. A. Dawson and S. Linse, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 2050–2055 CrossRef CAS.
- E. Casals, T. Pfaller, A. Duschl, G. J. Oostingh and V. F. Puntes, Small, 2011, 7, 3479–3486 CrossRef CAS.
- J. P. Xie, J. Y. Lee, D. I. C. Wang and Y. P. Ting, ACS Nano, 2007, 1, 429–439 CrossRef CAS.
- J. L. Huang, G. W. Zhan, B. Y. Zheng, D. H. Sun, F. F. Lu, Y. Lin, H. M. Chen, Z. D. Zheng, Y. M. Zheng and Q. B. Li, Ind. Eng. Chem. Res., 2011, 50, 9095–9106 CrossRef CAS.
- J. M. Zook, S. E. Long, D. Cleveland, C. L. A. Geronimo and R. I. MacCuspie, Anal. Bioanal. Chem., 2011, 401, 1993–2002 CrossRef CAS.
- R. Chen, P. Choudhary, R. N. Schurr, P. Bhattacharya, J. M. Brown and P. C. Ke, Appl. Phys. Lett., 2012, 100, 13703–137034 CrossRef.
- J. Fabrega, S. N. Luoma, C. R. Tyler, T. S. Galloway and J. R. Lead, Environ. Int., 2011, 37, 517–531 CrossRef CAS.
- I. Blinova, A. Ivask, M. Heinlaan, M. Mortimer and A. Kahru, Environ. Pollut., 2010, 158, 41–47 CrossRef CAS.
- A. Martín-González, S. Díaz, S. Borniquel, A. Gallego and J. C. Gutiérrez, Res. Microbiol., 2006, 157, 108–118 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: SEM micrograph and the energy-dispersive X-ray spectrometry (EDS) spectrum of SEF-incubated AgNO3 are provided in the supplementary file. See DOI: 10.1039/c2em30731f |
| This journal is © The Royal Society of Chemistry 2013 |
