The influence of ZnO and TiO2 nanoparticles on the toxicity of sewage sludges
Izabela
Jośko
and
Patryk
Oleszczuk
*
Department of Environmental Chemistry, Maria Curie-Skłodowska University, 3 Maria Curie-Skłodowska Square, 20-031 Lublin, Poland. E-mail: patryk.oleszczuk@poczta.umcs.lublin.pl; Fax: +48 81 537 55 65; Tel: +48 81 537 5515
First published on 28th November 2012
Abstract
More and more often sewage sludges become the place of deposition of nanoparticles (NPs), the use of which in consumer products is increasing. In turn, the increasing amount of sewage sludges enforces the need for their utilization (e.g. through the application of sludges to the soil). Therefore, the presence of NPs in sewage sludges may create a new threat to the environment. Thus it becomes important to perform evaluation of the toxicity of sewage sludges in the context of their content of NPs. The objective of the study was to estimate the effect of nanoparticles of ZnO (nano-ZnO) and TiO2 (nano-TiO2) and their bulk counterparts (bZnO and bTiO2) on the toxicity of sewage sludges in relation to selected organisms (plants – Lepidium sativum and Sinapis alba, and microorganisms – Vibrio fischeri and 11 different strains from Microbial Assay for Risk Assessment – MARA). The study also involved the estimation of other factors that may have an effect on the phytotoxicity of NPs in sewage sludge: the size of the particles, the dose of the sewage sludge, the time of NP–sewage sludge contact and light conditions. The effect of both nano-ZnO and nano-TiO2 on the toxicity of the sludges is dependent on the kind of NPs and their concentration. Sludges containing NPs displayed a different level of toxicity from their bulk counterparts. All of the factors estimated (size of particles, dose of sludge, contact time and light conditions) had a significant effect on the phytotoxicity of NPs which was dependent both on the kind of the NPs and on that of the sewage sludge. Estimation of the leachate toxicity indicated a greater sensitivity of plants to the presence of NPs as compared to the sensitivity of microorganisms. Leachates caused a greater reduction of bioluminescence of V. fischeri in the presence of nano-TiO2 than nano-ZnO. Nano-ZnO caused a reduction of the toxicity of the sewage sludge leachates.
Environmental impactThe common application of nanoparticles (NPs) in daily use products may lead to their presence in wastewaters and, consequently, in sewage sludges. In the situation of increasing production of NPs, it is necessary to conduct studies not only on the effect of NPs on the toxicity of sewage sludges but also on their effect on soils amended with such materials. As demonstrated in the present study, the toxicity of sewage sludges containing NPs was affected by various factors (kind and size of the NPs, dose of sludge, ageing, and access to light), hence there is a necessity for further studies, expanding to other environmental conditions, in order to identify the real threat following from the presence of NPs in the environment. |
1 Introduction
The amount of sewage sludges produced (currently averaging at 30 kg of dry matter per inhabitant per year1) is growing. This makes it necessary to undertake actions aimed at the utilization of the sewage sludges produced. One of the desirable solutions is their utilization in agriculture. Due to their high content of organic matter and minerals sewage sludges are a valuable fertilizing material.2 One of the limitations for that direction of sewage sludge utilization is the inorganic (heavy metals) and organic (phenolic compounds, polycyclic aromatic hydrocarbons, polychlorinated biphenyls, polychlorinated naphthalenes, and polybrominated diphenyl ethers) contaminants accumulated in sewage sludges.3 At present we are witnessing the appearance of a new problem, which will probably intensify with time, that is the presence of nanoparticles (NPs) in sewage sludges.4It is estimated that among inorganic nanomaterials (apart from Ag) the highest production is characteristic of nano-ZnO and nano-TiO2.5 These nanoparticles find an application in many consumer products – sunscreen products, textiles, paints, coatings and antibacterial agents.6 The exploitation of materials containing NPs in their composition may, as demonstrated by studies conducted so far,4 lead to their release into various elements of the environment. Sewages as well as sewage sludges can also be burdened with a load of NPs.4 The fact that nano-ZnO and nano-TiO2 find an extensive application in various areas of life is due to their unique properties resulting from their size. However, those new properties achieved, thanks to the nano size, cause an increased hazard to living organisms. This is related, among other things, to easier transportation of NPs inside organisms,7 where they can exert their toxic effect on individual organs, tissues and cells. Therefore, fertilization of soils with sewage sludges containing NPs may involve the risk of their effect on plants and on soil microorganisms.
So far, studies on the toxicity of NPs in relation to plants have been conducted in hydroponic cultures8,9 or, less frequently, in soil.10,11 The studies demonstrated that nano-ZnO displayed notable toxicity8,9 towards plants. In the case of nano-TiO2 no such distinct effect on the growth and development of plants was observed as in the case of nano-ZnO. What is more, in certain studies nTiO2 displayed a stimulating character.12 More extensive studies on the toxicity of NPs have been concerned with microorganisms. However, like in the case of plants, the studies are focused on pure solutions of NPs,8,13 and only a few are concerned with the effect of additional factors.14 The toxicity of ZnO NPs in relation to microorganisms has been demonstrated, whereas TiO2 NPs, even at extremely high concentrations (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1), had no effect on their development.15–17 However, when analyzing the toxicity of NPs one should take into account a number of additional factors on which it may be dependent. Such factors include the kind of NPs and their concentration, the size of nanoparticles, the contact time between NPs and matrix (soil, sediment),17 light conditions18 and the kind of test organism. Among the factors enumerated above, studies concerned with the estimation of the effect of the whole matrix (soil, sediment, and sewage sludge) on the toxicity of NPs are particularly scarce. As demonstrated by our earlier studies on the phytotoxicity of soils containing NPs,11 NPs displayed a considerably stronger toxic effect towards plants in an aquatic environment than in soil.11 It is to be expected that the toxicity of NPs present in sewage sludges will differ notably from their toxicity in other matrices, e.g. either in water or in soil.11 This results from the fact that sewage sludges, due to their qualitative and quantitative “richness” in various components (organic matter and minerals, among others), may alter the fate and the effect of NPs on various organisms to a greater extent than the soil can. Such changes can consist of binding of NPs, their aggregation,19 or other reactions (reduction/oxidation).7 On the other hand, NPs may enter into interactions with pollutants accumulated in sewage sludges, becoming their carrier.20 This may potentially amplify the toxic effect.21
000 mg L−1), had no effect on their development.15–17 However, when analyzing the toxicity of NPs one should take into account a number of additional factors on which it may be dependent. Such factors include the kind of NPs and their concentration, the size of nanoparticles, the contact time between NPs and matrix (soil, sediment),17 light conditions18 and the kind of test organism. Among the factors enumerated above, studies concerned with the estimation of the effect of the whole matrix (soil, sediment, and sewage sludge) on the toxicity of NPs are particularly scarce. As demonstrated by our earlier studies on the phytotoxicity of soils containing NPs,11 NPs displayed a considerably stronger toxic effect towards plants in an aquatic environment than in soil.11 It is to be expected that the toxicity of NPs present in sewage sludges will differ notably from their toxicity in other matrices, e.g. either in water or in soil.11 This results from the fact that sewage sludges, due to their qualitative and quantitative “richness” in various components (organic matter and minerals, among others), may alter the fate and the effect of NPs on various organisms to a greater extent than the soil can. Such changes can consist of binding of NPs, their aggregation,19 or other reactions (reduction/oxidation).7 On the other hand, NPs may enter into interactions with pollutants accumulated in sewage sludges, becoming their carrier.20 This may potentially amplify the toxic effect.21
The objective of the study presented herein was the estimation of the effect of nano-ZnO and nano-TiO2 on the toxicity of sewage sludges. The toxicity of the solid phase was estimated, as well as that of leachates of sewage sludges. The test organisms used in the study were plants: Lepidium sativum and Sinapis alba, and microorganisms: Vibrio fischeri and 11 different strains used in the MARA test. The determination of the effect of NPs on the toxicity of sewage sludges was conducted for various concentrations of NPs and doses of sewage sludge, size of ZnO particles, contact time between NPs and sewage sludges and light conditions.
2 Materials and methods
2.1 Materials
Nanoparticles ZnO, TiO2 (nano-ZnO, nano-TiO2) and their bulk counterparts (bZnO, bTiO2) were purchased from Sigma-Aldrich (USA). The diameter of nanoparticles was as follows: nano-ZnO <50 nm (nano-ZnO50), <100 nm (nano-ZnO100); nano-TiO2 <21 nm (nano-TiO2). Transmission electron microscope (TEM) images of the tested nanoparticles were obtained using a Tecnai™ Spirit TEM (FEI Company, Japan). The surface area of nano-ZnO50, nano-ZnO100, and nano-TiO2 was 10, 8; 15–25; and 35–65 m2 g−1, respectively. Scanning Electron Microscope (SEM) images of sewage sludges with NPs were obtained using a Tescan Vega LMU (USA).2.2 Sewage sludge characteristics
The sewage sludges for the study were taken from two sewage treatment plants situated in the territory of Poland: SL1 (from Przemyśl) and SL2 (from Gdynia). The sewage sludges were air-dried, and then crushed and sieved through the sieves with a mesh size of 1 mm. The chemical properties of sewage sludges studied were determined by standard methods.22 The pH was measured potentiometrically in 1 M KCl after 24 h in the liquid/soil ratio of 2.5. The total organic carbon was determined using a TOC-VCSH (SHIMADZU) with Solid Sample Module SSM-5000. The total nitrogen (Nt) was determined by the Kjeldahl's method without the application of Dewarda's alloy (Cu–Al–Zn alloy-reducer of nitrites and nitrates). The concentrations of P, K and Mg were determined according to “Procedures for Soil Analysis”.22 To determine the heavy metal content in sewage sludge, samples were mineralized in a PROLABO microwave oven (Microdigest 3.6, France) using a wet method. This method uses a mixture of nitric acid and perchloric acid at the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Details of the mineralization are described in Baran et al.23 The total contents of Cd, Cr, Cu, Pb, Ni, Ti and Zn were determined by emission spectrometry using a Varian 810 MS apparatus with Inductively Coupled Plasma Mass Spectrometers (ICP-MS) induction in argon.
1. Details of the mineralization are described in Baran et al.23 The total contents of Cd, Cr, Cu, Pb, Ni, Ti and Zn were determined by emission spectrometry using a Varian 810 MS apparatus with Inductively Coupled Plasma Mass Spectrometers (ICP-MS) induction in argon.
The basic properties of the sewage sludges are presented in Table 1. Both sludges were characterized by acid reaction. The TOC content of sludge SL2 was more than twice as high as that of sludge SL1. Likewise, a lower content of total nitrogen (Nt) was noted in sludge SL1 as compared to sludge SL2. The content of heavy metals in the sludges is presented in Table 1. All the tested sludges met the required standards in terms of the permissible levels of heavy metal content. Noteworthy is the concentration of Ti and Zn in the sludges under study. The sewage sludges (SL1 and SL2) displayed significant differences with respect to the concentration of Ti, amounting to 299 and 914 mg kg−1 for sludges SL1 and SL2, respectively, whereas the content of Zn was on a similar level in both sludges 1686–1696 mg kg−1. Leachates obtained from sewage sludge SL1 contained 0.12 mg L−1 Ti and 0.29 mg L−1 Zn. The addition of bZnO and nano-ZnO100 to the sewage sludge increased the concentrations of these elements in the leachates to 7.96 and 6.18 mg L−1, respectively. Adding TiO2 to the sludge also resulted in a higher content of Ti in the leachates compared to leachates from sewage sludge without these compounds. Leachates contained 2.57 mg L−1 Ti in nano-TiO2 amended sewage sludge and 0.16 mg L−1 in bTiO2 amended sewage sludge.
| Properties | Sewage sludge | Soil | |
|---|---|---|---|
| SL1 | SL2 | OECD | |
| a pH – reactivity in KCl; TOC – total organic carbon content (mg kg−1); Nt – total nitrogen content (mg kg−1); P, K, Mg (g kg−1) – available forms of phosphorous, potassium and magnesium, respectively (mg kg−1); Cd, Zn, Pb, Cr, Cu, Ni, Ti – concentration (mg kg−1). | |||
| pH | 5.7 | 6.1 | 6 |
| TOC | 143.7 | 384.0 | 40 |
| Nt | 35 | 51 | 0.1 |
TOC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nt Nt |
4.1 | 7.6 | 400 |
| P | 5.4 | 5.8 | 0.005 |
| K | 1.4 | 15.9 | 0.027 |
| Mg | 4.6 | 8.2 | 0.063 |
| Cd | 1.57 | 1.18 | — |
| Zn | 1696 | 1686 | 11.2 |
| Pb | 61.7 | 43.7 | — |
| Cr | 26.5 | 70.7 | — |
| Cu | 96.3 | 209 | 2.5 |
| Ni | 22.8 | 56.1 | 6.1 |
| Ti | 229 | 914 | — |
2.3 Experimental
In the experiment regarding the effect of concentration of NPs on the toxicity of different sewage sludges, NPs and their bulk counterparts were spiked with SL1 and SL2 sewage sludges as a powder of ZnO and TiO2 at the concentration of 100, 1000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1. The highest concentration of NPs was used to assess the “worst scenarios”, where extremely high concentrations of NPs in sewage sludge occur. Samples of sewage sludges containing NPs or bulk counterparts were thoroughly mixed with a glass spatula, rolled end over end for 28 days (in the dark). After 28 days aging sewage sludges were mixed with OECD soil and the phytotoxicity was evaluated. Sewage sludges were mixed with OECD soil at the dose of 3%. Each sample was prepared in three replications, and the final results were the arithmetic means.
000 mg kg−1. The highest concentration of NPs was used to assess the “worst scenarios”, where extremely high concentrations of NPs in sewage sludge occur. Samples of sewage sludges containing NPs or bulk counterparts were thoroughly mixed with a glass spatula, rolled end over end for 28 days (in the dark). After 28 days aging sewage sludges were mixed with OECD soil and the phytotoxicity was evaluated. Sewage sludges were mixed with OECD soil at the dose of 3%. Each sample was prepared in three replications, and the final results were the arithmetic means.
The study of the effect of various (i) plant species, (ii) size of NPs, (iii) dose of sewage sludge and (iv) contact time on the toxicity of sewage sludges containing NPs was conducted at the NP concentration level of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1. NPs were spiked with sewage sludges and mixed with OECD soil according to the procedure described above. In the part of the study concerned with the effect of NPs on the toxicity of the sewage sludges in relation to various plants and sludge doses only sewage sludge SL1 was used. For the estimation of the effect of the size of ZnO particles and of the contact time between the NPs and sewage sludge on the toxicity of the sludges, the study was conducted with the use of both the sludges: SL1 and SL2. Samples were incubated in the dark for 28 and 96 days.
000 mg kg−1. NPs were spiked with sewage sludges and mixed with OECD soil according to the procedure described above. In the part of the study concerned with the effect of NPs on the toxicity of the sewage sludges in relation to various plants and sludge doses only sewage sludge SL1 was used. For the estimation of the effect of the size of ZnO particles and of the contact time between the NPs and sewage sludge on the toxicity of the sludges, the study was conducted with the use of both the sludges: SL1 and SL2. Samples were incubated in the dark for 28 and 96 days.
In the experiments regarding the effect of light conditions, TiO2 nanoparticles and their bulk counterparts were spiked with tested sewage sludges (SL1 and SL2) at the dose of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1. After mixing, the samples of sewage sludges were exposed to daylight for 96 days. After 96 days, sewage sludges were mixed with OECD soil at the dose of 3% and their phytotoxicity was measured according to the method described below.
000 mg kg−1. After mixing, the samples of sewage sludges were exposed to daylight for 96 days. After 96 days, sewage sludges were mixed with OECD soil at the dose of 3% and their phytotoxicity was measured according to the method described below.
The toxicity of NPs towards bacteria was determined in relation to the leachates obtained from sewage sludge SL1 contaminated with NPs. Leachates were obtained according to the EN 12457-2 protocol (EC 2002).24 The samples were mixed with de-ionised water in a single-stage batch test performed at a liquid-to-solid (L/S) ratio of 1 L per 100 g. The glass bottles were shaken in a roller-rotating device at 10 rpm. The leachates were filtered using a filter with a porosity of 0.45 μm. The toxicity of leachates was estimated also in relation to plants. The test with plants was based on germination/elongation recommended by OECD (1984).25
2.4 Bioassays
Four bioassays were used for toxicity determination of sewage sludges and sewage sludges containing NPs: one test with the solid phase – Phytotoxkit™ and another three tests with leachates – Microtox™, Microbial Assay for toxic Risk Assessment (MARA) and a germination/elongation test recommended by OECD (1984).25The Phytotoxkit™ measures the decrease (or the absence) of seed germination and of the growth of the young roots after 3 days of exposure of seeds of selected higher plants (cress – Lepidium sativum and mustard – Sinapis alba) to the contaminated matrix in comparison to the controls in a reference soil. Ten seeds of each plant were positioned at equal distances near the middle ridge of the test plate on a filter paper placed on top of the hydrated soil. After closing the test plates were placed vertically in a holder and incubated at 25 °C for 3 days. At the end of the incubation period a digital picture of the test plates with the germinated plants was taken. The analyses and the length measurements were performed using the Image Tool 3.0 for Windows (UTHSCSA, San Antonio, USA). The bioassays were performed in three replicates. The percent inhibition of seed germination (SG) and root growth inhibition (RI) were calculated with the formula:
| SG/RI = (A − B/A)100 |
The Microtox® Toxicity Test was used to evaluate the inhibition of the luminescence in the marine bacteria Vibrio fischeri. Luminescence inhibition of the extract was assessed for 15 min of exposure carrying out the “81.9% Basic test protocol” (screening test) (Azur Environment Ltd, 1998). The light output of the luminescent bacteria from soil's extract was compared with the light output of a blank control sample. The Microtox® test was performed using the Microtox® bacteria reagent (Microtox® Acute Toxicity Testing Reagent) and prepared according to the test protocol (Azur Environment Ltd, 1998).
MARA (Microbial Assay for Risk Assessment) is a multi-species assay which allows measurement of toxic effects of chemicals and environmental samples. The test uses a selection of taxonomically diverse microbial species lyophilised in a microplate. Ten prokaryotic species and a eukaryote (yeast) constitute the biological indicators of toxicity assessment. The growth of the organisms exposed to a dilution series of the test sample is determined with the reduction of tetrazolium red (TZR).26 A scanned image of the microplate obtained using a flatbed scanner is analysed using purpose-built software. The MARA test was performed according to the standard protocol described by Wadhia et al.26 In the present work, the microbial species used consisted of ten bacterial species: Microbacterium sp., Brevundimonas diminuta, Citrobacter freundii, Comamonas testosteroni, Enterococcus casseliflavus, Delftia acidovorans, Kurthia gibsonii, Staphylococcus warnerii, Pseudomonas aurantiaca, Serratia rubidaea, and one yeast species: Pichia anomalia.26
3 Results
3.1 Influence of NP concentration on sewage sludges phytotoxicity
In all the experimental treatments no statistically significant effect of the sewage sludges on the germination of the test plants was observed. Therefore, further on in the paper the term toxicity refers to root growth inhibition.Sewage sludges SL1 and SL2 with no content of NPs had a similar effect on the growth of roots of L. sativum, causing their inhibition at the level of 18.1 and 13.6%, respectively (Fig. 1). The addition of nano-ZnO100 to the sewage sludges caused an increase in their toxicity, the level of which, however, varied in relation to the kind of sewage sludge. In the case of sludge SL1 the three doses applied (100, 1000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1 nZnO100) increased the inhibition of root growth of L. sativum in relation to the sewage sludge without NPs by 9.6, 10.9 and 29.4%, respectively (Fig. 1A). The presence of nano-ZnO100 had a less pronounced effect on the toxicity of sludge SL2 than was observed for sludge SL1. The lowest dose of nano-ZnO100 added to sludge SL2 (100 mg kg−1) had no significant effect on its phytotoxicity (Fig. 1B). The higher doses (1000 and 10
000 mg kg−1 nZnO100) increased the inhibition of root growth of L. sativum in relation to the sewage sludge without NPs by 9.6, 10.9 and 29.4%, respectively (Fig. 1A). The presence of nano-ZnO100 had a less pronounced effect on the toxicity of sludge SL2 than was observed for sludge SL1. The lowest dose of nano-ZnO100 added to sludge SL2 (100 mg kg−1) had no significant effect on its phytotoxicity (Fig. 1B). The higher doses (1000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1) caused an increase in the inhibition of root growth of L. sativum, by 9.6% and 15.1%, respectively, in relation to the sludge with no content of NPs. In this case a relationship was noted between the dose and the effect observed (Fig. 1B). The application of bZnO to the sludges, as opposed to nano-ZnO100, did not result in any case in the appearance of a dose–effect relationship. The lowest dose of bZnO caused the greatest toxic effect both in sludge SL1 and SL2 (higher by 11 and 13.3%, respectively, in relation to the sludges without bZnO). For sewage sludge SL1 the higher doses of bZnO had no significant effect on its toxicity in relation to the sludge with no content of bZnO. The application of bZnO to sludge SL2 in doses of 1000 and 10
000 mg kg−1) caused an increase in the inhibition of root growth of L. sativum, by 9.6% and 15.1%, respectively, in relation to the sludge with no content of NPs. In this case a relationship was noted between the dose and the effect observed (Fig. 1B). The application of bZnO to the sludges, as opposed to nano-ZnO100, did not result in any case in the appearance of a dose–effect relationship. The lowest dose of bZnO caused the greatest toxic effect both in sludge SL1 and SL2 (higher by 11 and 13.3%, respectively, in relation to the sludges without bZnO). For sewage sludge SL1 the higher doses of bZnO had no significant effect on its toxicity in relation to the sludge with no content of bZnO. The application of bZnO to sludge SL2 in doses of 1000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1 resulted in an insignificant (less than 4%) decrease in its toxicity.
000 mg kg−1 resulted in an insignificant (less than 4%) decrease in its toxicity.
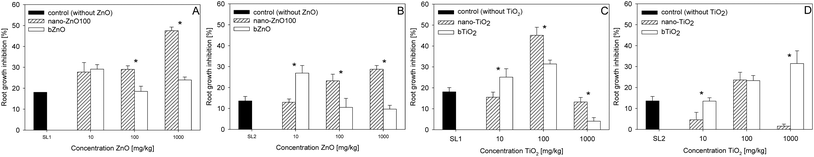 | ||
| Fig. 1 Phytotoxicity of sewage sludges and sewage sludges with nanoparticles and their bulk counterparts. A – SL1 with/without ZnO, B – SL2 with/without ZnO, C – SL1 with/without TiO2, and D – SL2 with/without TiO2. *Values statistically different between bars (P ≤ 0.05). | ||
Depending on the dose applied, the introduction of nano-TiO2 in the sewage sludges caused varied effects (Fig. 1C and D). In the case of both sludges the addition of nano-TiO2 in the dose of 1000 mg kg−1 caused the strongest inhibition of root growth. Again the observed effect was greater in sewage sludge SL1 than in SL2, just as was observed for nano-ZnO100 (Fig. 1). The values obtained were higher by 27% (SL1) and 10% (SL2) in relation to the sewage sludges without nano-TiO2. The lowest dose of nano-TiO2 introduced in sewage sludge SL1 had no significant effect on the toxicity of that sludge, while the dose of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1 reduced its phytotoxicity by 14% (Fig. 1C). In sludge SL2 both the smallest dose (100 mg kg−1) and the largest dose (10
000 mg kg−1 reduced its phytotoxicity by 14% (Fig. 1C). In sludge SL2 both the smallest dose (100 mg kg−1) and the largest dose (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1) of nano-TiO2 caused an almost total reduction of the toxic effect (Fig. 1D). The application of bTiO2 caused different responses of L. sativum as compared to the application of nano-TiO2. In sewage sludge SL1 the level of toxicity was inversely correlated with the dose of bTiO2. The lowest dose of bTiO2 added to sewage sludge SL1 increased its toxicity by 8.8%, while the larger doses caused a decrease in the toxicity by 7.6% (1000 mg kg−1) and 8.4% (10
000 mg kg−1) of nano-TiO2 caused an almost total reduction of the toxic effect (Fig. 1D). The application of bTiO2 caused different responses of L. sativum as compared to the application of nano-TiO2. In sewage sludge SL1 the level of toxicity was inversely correlated with the dose of bTiO2. The lowest dose of bTiO2 added to sewage sludge SL1 increased its toxicity by 8.8%, while the larger doses caused a decrease in the toxicity by 7.6% (1000 mg kg−1) and 8.4% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1) in relation to the sludge with no content of bTiO2. The application of bTiO2 to sewage sludge SL2 caused a gradual intensification of the toxic effect. However, no significant differences between the sludge with the lowest dose and the sludge with no content of bTiO2 were observed. The doses of 1000 and 10
000 mg kg−1) in relation to the sludge with no content of bTiO2. The application of bTiO2 to sewage sludge SL2 caused a gradual intensification of the toxic effect. However, no significant differences between the sludge with the lowest dose and the sludge with no content of bTiO2 were observed. The doses of 1000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1 of bTiO2 increased the toxic effect of the sewage sludge, by 10 and 12%, respectively, but as in the case of sludge SL1 the differences were not statistically significant.
000 mg kg−1 of bTiO2 increased the toxic effect of the sewage sludge, by 10 and 12%, respectively, but as in the case of sludge SL1 the differences were not statistically significant.
3.2 Effect of NPs in sewage sludge on different plants
The toxicity of the sludge as such, as well of the sludge in which ZnO or TiO2 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1) was introduced varied in relation to the test plant species (Fig. 2). The sludge tested without any addition of NPs caused a lower inhibition of root growth in S. alba (12.7%) than in L. sativum (18.1%). L. sativum was also more sensitive than S. alba to the effect of sewage sludge containing nano-ZnO100. Germination inhibition in sewage sludge containing nano-ZnO100 was higher by 18.7% in the case of L. sativum than of S alba. For bZnO also a greater sensitivity of L. sativum than of S. alba was observed. However, the differences were not as significant as those observed for nano-ZnO100.
000 mg kg−1) was introduced varied in relation to the test plant species (Fig. 2). The sludge tested without any addition of NPs caused a lower inhibition of root growth in S. alba (12.7%) than in L. sativum (18.1%). L. sativum was also more sensitive than S. alba to the effect of sewage sludge containing nano-ZnO100. Germination inhibition in sewage sludge containing nano-ZnO100 was higher by 18.7% in the case of L. sativum than of S alba. For bZnO also a greater sensitivity of L. sativum than of S. alba was observed. However, the differences were not as significant as those observed for nano-ZnO100.
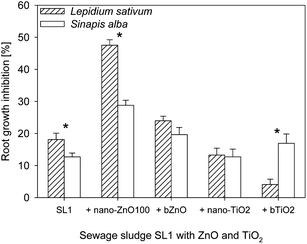 | ||
Fig. 2 Effect of ZnO and TiO2 (nano, bulk) on root growth inhibition of L. sativum and S. alba. The concentration of bulk and nanoparticles in sewage sludge 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1. *Values statistically different between bars (P ≤ 0.05). 000 mg kg−1. *Values statistically different between bars (P ≤ 0.05). | ||
Exposure of L. sativum and S. alba to the sewage sludge to which TiO2 had been added caused different responses than those observed in the case of application of ZnO. The addition of nano-TiO2 to the sewage sludge evoked similar responses in both plants. The inhibition of their root growth was ca. 13%, whereas a significant difference in the tolerance of both plants was noted in the presence of sewage sludge with bTiO2 which resulted in considerably greater toxicity of the sewage sludge towards S. alba (16.9%) than L. sativum (4.1%).
3.3 Influence of ZnO particle size on sewage sludge phytotoxicity
The size of ZnO particles played a significant role in the level of phytotoxicity of the sewage sludges (Fig. 3). In both kinds of sewage sludge a distinct difference was observed between the nanoparticles of ZnO (nano-ZnO50 and nano-ZnO100) and their bulk counterparts (bZnO), whereas no significant differences were noted between nano-ZnO50 and nano-ZnO100. In both sewage sludges, the difference between nano-ZnO50 and nano-ZnO100 did not exceed 3%. The application of nano-ZnO50 and nano-ZnO100 to sludge SL1 caused root growth inhibition at a level of nearly 50%, which was higher by 24% compared to the sludge containing bZnO. The addition of nano-ZnO50 and nano-ZnO100 to sludge SL2 caused a distinctly lower toxic effect than it did in sludge SL1 (30% inhibition of germination) and notably higher than in the sludge containing bZnO – by 19% (nano-ZnO50) and 21.9% (nano-ZnO100).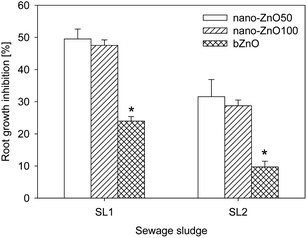 | ||
| Fig. 3 Influence of ZnO size on root growth inhibition of L. sativum. nano-ZnO50 – nanoparticles with diameter <50 nm, nano-ZnO100 – nanoparticles with diameter <100 nm, bZnO – bulk particles. *Values statistically different (P ≤ 0.05). | ||
3.4 Influence of sewage sludge dose on the phytotoxicity
The dose of sewage sludge (SL1) played a significant role in its level of toxicity (Fig. 4). Increase of the dose of sludge with no content of NPs from 3% to 9% caused an almost 2.5-fold increase in its toxicity. No similar relationship was observed in the sewage sludge with nano-ZnO100. Increase of the dose of sewage sludge containing nano-ZnO100 from 3 to 9% did not cause any significant increase in its toxicity compared to the dose of 3%. In this case, however, the level of toxicity was comparable to that observed in sludge with no content of NPs applied at the dose of 9%. The situation was completely different for sewage sludge dose increase in the case of bZnO. Although the sludge containing bZnO differed only slightly in its level of toxicity from the sludge with no ZnO (dose of 3%), increase of the dose of the sludge to 9% caused also an increase in its toxicity (Fig. 4). After the addition of the sludge with bZnO at the dose of 9% the level of toxicity significantly exceeded (by 11.9%) the level observed for the control sludge, with no ZnO, applied at the same dose. An identical tendency was observed in the case of nano-TiO2 which, like bZnO, displayed toxicity higher by 11.4% in relation to the 9% dose of sludge with the addition of NPs. Both at the lower and the higher doses, bTiO2 reduced the toxicity of the sludge by 14.1 (dose of 3%) and 8.4% (dose of 9%), respectively.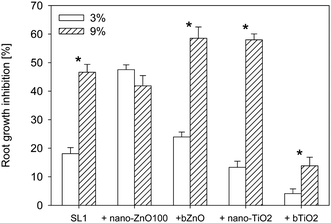 | ||
| Fig. 4 Effect of sewage sludge dose on the toxicity of nanoparticles and their bulk counterparts. *Values statistically different (P ≤ 0.05). | ||
3.5 Effect of the contact time between NPs and sewage sludge on the phytotoxicity
The contact time between the sewage sludge and ZnO or TiO2 also had an effect on the level of phytotoxicity of the sewage sludges (Fig. 5). That effect, however, depended significantly not only on the kind of NPs but also on the sewage sludge. Aging had no effect on the toxicity of sludge SL1 containing nZnO100, the toxicity of which, irrespective of the time of contact, oscillated at a similar level of 47.5 (28 d) and 45.4% (54 d), respectively, whereas a reduction of toxicity (by 6.4%) with the passage of time was observed in the case of sludge SL2 containing nano-ZnO100. Significant lowering of toxicity with extension of the contact time between NPs and sewage sludge was observed also in the case of bZnO and nano-TiO2 in sludge SL1, and bTiO2 in sludge SL2. After 54 days of incubation the inhibition of root growth of L. sativum was lower than after 28 days – by 21.4% in the case of bZnO and by 6.6% in the case of nano-TiO2 in sludge SL1, and by 18.3% in the case of bTiO2 in sludge SL2. Extension of the contact time between NPs and the sewage sludges from 28 to 54 days did, however, cause an increase in their toxicity in the case of bTiO2 (SL1) and nano-TiO2 (SL2). In both cases a several-fold increase of their toxicity was noted as compared to the 28 day period of incubation (Fig. 5). That phenomenon was more pronounced in the case of sludge SL2 than SL1.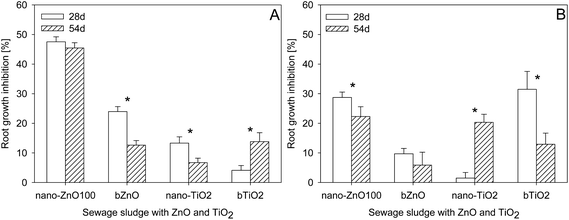 | ||
| Fig. 5 Effect of aging on the toxicity of sewage sludges SL1 (A) or SL2 (B) with nanoparticles and their bulk counterparts. *Values statistically different between bars (P ≤ 0.05). | ||
3.6 Effect of light on TiO2 phytotoxicity in sewage sludges
Light conditions had a significant effect on the phytotoxicity of the sewage sludges in which TiO2 was introduced (Fig. 6). Exposure to light of sludges SL1 and SL2 containing TiO2 (irrespective of its form) caused a significant increase of their toxicity as compared to sludges incubated in darkness. Exposure to light caused an increase in the toxicity of sludge SL1 in which nano-TiO2 and bTiO2 were introduced, by 13.9 and 9%, respectively. Several-fold increase of toxicity under the effect of light was observed also in sludge SL2 containing nano-TiO2 and bTiO2 (Fig. 6).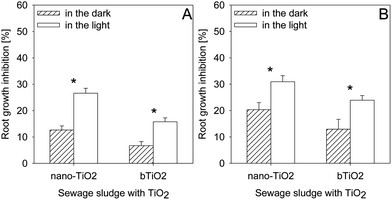 | ||
| Fig. 6 Effect of sunlight on the sewage sludge SL1 (A) and SL2 (B) toxicity depending on the TiO2 forms (nano and bulk). *Values statistically different between bars (P ≤ 0.05). | ||
3.7 Toxicity of extracts from sewage sludges
Table 2 presents the values of ED50 determined for leachates obtained from sewage sludge with and without the content of NPs. The water leachate obtained sludge SL1 had a significant effect on the bacteria tested (Microtox and MARA) and on L. sativum.| Organism | SL1 | SL1 + nano-ZnO100 | SL1 + bZnO | SL1 + nano-TiO2 | SL1 + bTiO2 |
|---|---|---|---|---|---|
| Lepidium sativum | 50.7 ± 4.5 | 22.7 ± 1.9 | 71.2 ± 3.9 | 10.6 ± 0.9 | 58.2 ± 3.5 |
| Vibrio fischeri (Microtox) | 63.3 ± 5.7 | 88.0 ± 9.3 | 88.0 ± 7.9 | 51.3 ± 4.4 | 62.3 ± 5.8 |
The values of ED50 obtained for the leachates from sludge SL1 oscillated around the level of 50.7%. The addition of NPs to the sludge caused an increase of its toxicity, which was exhibited in a decrease of the value of ED50 to 27.0% (in the presence of nano-ZnO) and by 40.1% (in the presence of nano-TiO2) in relation to the sludge with no content of NPs. Leachates obtained from sludge containing bZnO and bTiO2 reduced the negative effect exerted by the leachates on L. sativum. The values of ED50 increased after the addition of bZnO and bTiO2 by 20.5 and 7.5%, respectively, compared to the sludge with no content of the compounds tested.
The ED50 value determined for the sludge tested in the Microtox test oscillated around the level of 63.3%, indicating a slightly lower sensitivity of V. fisheri to the sludge tested compared to L. sativum. The addition of both ZnO and TiO2, as in the case of L. sativum, had a significant effect on ED50. However, the direction of the changes depended on the kind of compound. Both nano-ZnO and bZnO increased the toxicity of the sewage sludges (Table 2). In this case, the determined value ED50 was higher by 24.7% than the value obtained for the sludge with no content of ZnO, whereas TiO2 caused an opposite effect. Extracts obtained from sludges containing nano-TiO2 and bTiO2 reduced the value of ED50 by 12 and 1%, respectively.
The effect of leachates obtained from sludge SL1 on the growth of bacteria in the MARA test depended on the test strain (Fig. 7). Strains M. species and P. anomala displayed the greatest sensitivity to the leachates, manifested in 65% inhibition of growth of those organisms in relation to the test without the leachates. A lower toxicity of the leachates was found in relation to strains S. warneri and S. rubidaea, at 7 and 19%, respectively. In the case of the remaining bacterial strains the leachates had a stimulating effect on their growth.
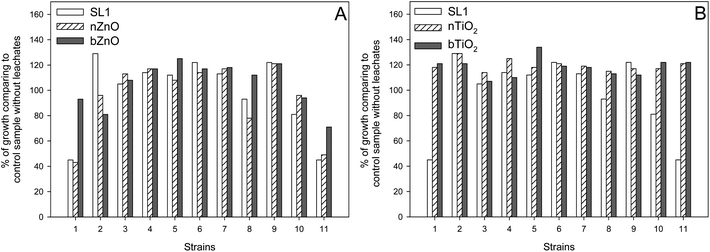 | ||
| Fig. 7 Influence of ZnO (A) TiO2 and (B) on sewage sludge leachate toxicity to bacteria in the MARA test. | ||
The addition of ZnO and TiO2 to sewage sludge SL1 affected the level of toxicity of its leachates in the MARA test only in relation to certain organisms: M. species, B. diminuta (only in the case of ZnO), S. warneri, S. rubidaea, and P. anomala (in the case of all tested elements). For strains M. species, S. warneri and P. anomala the addition of bZnO to the sludges caused a significant reduction of their toxicity. No similar relationship was noted in the case of nano-ZnO, whose toxicity was at a level similar to that of the sludge with no addition of ZnO. The toxicity of leachates obtained from the sewage sludges was reduced after the addition of nano-ZnO and bZnO also in relation to strain S. rubidaea, by 15% and 13%, respectively. An opposite trend was observed in the case of strain B. diminuta, whose growth was inhibited under the effect of leachates from sewage sludge containing both of the tested ZnO, the rate of inhibition being 33% (nano-ZnO) and 48% (bZnO), respectively (Fig. 7A).
The addition of TiO2 to the sludges had no significant effect on most of the organisms tested. The presence of both nano-TiO2 and bTiO2 in sewage sludge SL1 distinctly reduced the toxicity of leachates obtained from sludges with no content of TiO2 only in relation to M. species, S. warneri, S. rubidaea, and P. anomala (Fig. 7B). In this case, the observed decrease of toxicity varied from 20 to 77%. No negative effect of TiO2 (both in the nano and bulk forms) was found in the case of any of the organisms tested. Also, no statistically significant differences were observed between nano-TiO2 and bTiO2 in any of the cases studied.
4 Discussion
Sewage sludges are characterized by a high fertilizer potential due to their high content of organic matter, nitrogen and potassium, which makes them an interesting fertilizer material. Therefore, sewage sludges are frequently used for the amendment of soil properties or for soil reclamation. One of the barriers that limit such utilization of sewage sludges can be the content of organic and inorganic contaminants.3 In recent years, sewage treatment plants have had to face a new challenge in the form of the presence of NPs in sewage and in sewage sludge.4,27,28 In this context, it is necessary to perform an estimation of potential environmental implications related to the introduction of sewage sludge containing NPs into the soil. Special attention should be focused on nano-ZnO and nano-TiO2, as those NPs are used in the production of a variety of consumer products, such as sunscreens, paints, pigments and textiles.27,29 The exploitation of nanoproducts, containing nano-ZnO and nano-TiO2 in their composition, may involve the risk of their release from such materials and then, via sewage treatment plants, they may migrate to sewage sludge. As was demonstrated in model studies conducted by Gottschalk et al.,30 soils amended with sewage sludge can contain 89.2 μg kg−1 per year nTiO2 and 3.25 μg kg−1 per year nano-ZnO. The real scale of the problem can be evaluated by the total concentrations of Zn (1686–1696 mg kg−1) and Ti (299–914 mg kg−1) that occurred in sewage sludges tested (Table 1). It should be emphasized that under environmentally relevant concentration no effect of nano-ZnO or TiO2 on sludges is expected.The presence of NPs in sewage sludge may result in greater consequences on plants and microorganisms in sewage sludge-amended soil than in the case of their bulk counterparts. Due to their nano sizes, NPs can penetrate easily into organisms, which exposes the organisms to the toxic effects of those compounds.31 The results obtained in this study demonstrated that sewage sludges containing nano-ZnO and nano-TiO2 differed in terms of the character and dynamics of effect on the growth of L. sativum from sludges containing their bulk counterparts. Moreover, in certain cases (nZnO100 in sludge SL1), already at the lowest dose the NPs caused an increase of the phytotoxicity of the sludges. The scale of effect of nano-ZnO100 varied among the different sewage sludges. A greater increase of toxicity was noted in the case of sludge SL1, in which the highest concentration of nano-ZnO100 increased the toxicity of the sludge by 29.4%, than in sludge SL2 (increase of 15.1%). The differences observed resulted most probably from different levels of organic carbon content of the sludges (Table 1). Sludge SL1, in which the toxic effect assumed a more pronounced character, had a TOC content of less than half of that in sludge SL2. Natural organic matter (NOM) plays an important role in the bioavailability of various contaminants, including NPs.32 Studies by Yang et al.33 demonstrated the adsorption of humic acids on the surface of nano-ZnO and nano-TiO2, which limited their availability and resulted in the deposition of the NPs. Also in aquatic environment interaction of NOM with NPs brought about positive effects in the form of reduction of the toxicity of NPs towards Daphnia magna34 or Pseudokirchneriella subcapitata.35 Moreover, nano-ZnO can enter into reactions with various components appearing in sewage sludges, e.g. phosphates. The interactions between NPs and the other compounds of sewage sledges should be studied in detail. Lombi et al.36 showed that speciation of ZnO NPs was changed during the wastewater treatment, and it might affect the fate and toxicity of the investigated NPs.
Compared to sludge SL1, sludge SL2 was also characterized by a higher content of available forms of P, K and Mg (Table 1). Research by Lv et al.6 demonstrated that in the presence of phosphates the nanoparticles of ZnO released less Zn2+ ions, which may result in a reduction of their toxicity. The higher NOM content of sludge SL2 could have also had an effect on the process of formation and then deposition of aggregates of NPs7 on the surface of sewage sludge components. Images acquired with the help of SEM show deposition/adsorption of NP aggregates on the surface of the solid phase (Fig. 8). It is commonly known that aggregation of NPs reduces their “nano” properties to a significant degree. As demonstrated by studies of Chowdhury et al.,19 the effect of deposition of NPs may intensify as a result of the formation of a NP–humic acid–bacteria complex.
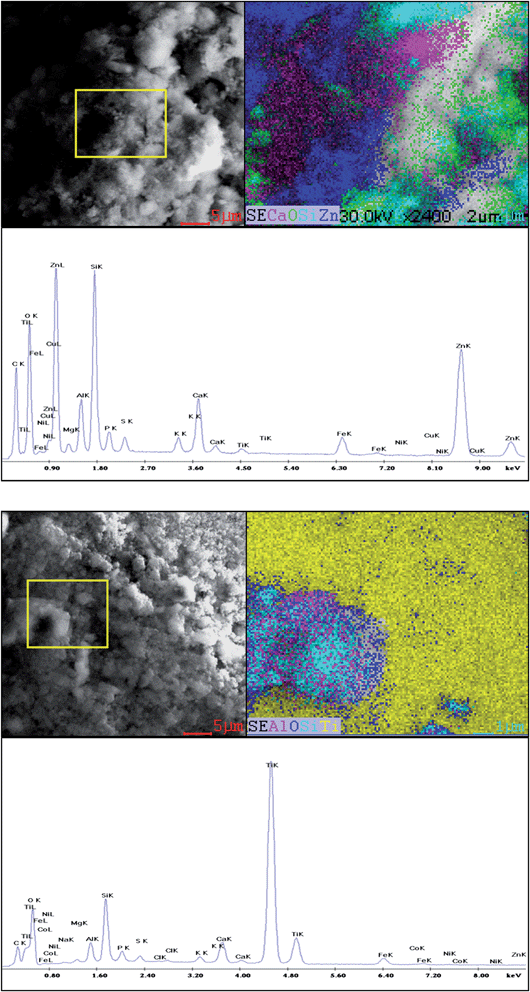 | ||
| Fig. 8 (a) SEM image and map with the EDAX spectrum of sewage sludge SL1 with nZnO. (b) SEM image and map with the EDAX spectrum of sewage sludge SL1 with nTiO2. | ||
As opposed to nano-ZnO, the addition of nano-TiO2 to the sludges did not produce a dose–effect relationship in any case. Both sludges containing nano-TiO2 at a concentration of 1000 mg kg−1 displayed the greatest toxicity towards L. sativum, while the lower as well as the higher dose notably reduced the phytotoxicity of the sewage sludges. The positive aspect of the presence of nano-TiO2 (in a considerable amount – 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1) in sewage sludges may be caused by the adsorption of contaminants on the surface of nano-TiO2 (surface area 35–65 m2 g−1),37 which reduced their bioavailability and – indirectly – also their toxicity. However, it is hard to explain the positive effect of nano-TiO2 at the lowest concentration and then its negative effect at a higher concentration followed by a recurring positive effect at the highest concentration. Our earlier study11 also demonstrated a stimulating effect of nano-TiO2 (in various soils) on L. sativum. In that study also no dose–effect relationship was observed. It is interesting to note that the trends in the effect of nano-TiO2 on toxicity in both sludges were the same, while the sludges differed considerably in their content of Ti. This may indicate a greater importance of the size of the particles than that of their concentration. As in the case of nano-ZnO, a stronger negative effect after the addition of nano-TiO2 was observed in sludge SL1 than in SL2. This confirms again the significant effect of organic matter on the bioavailability, and indirectly also on the toxicity of the NPs under study. Also important in this respect is their aggregation which significantly reduces the toxic effect.38 Noteworthy as well as is the fact that the introduction of the “macro” or bulk counterparts of nano-ZnO and nano-TiO2 to the sewage sludges produced different effects than those of the NPs. The effect of bZnO on the toxicity of the sewage sludges was only slight. NPs, thanks to their size, can penetrate cell walls,39 then the Zn2+ ions can exert their toxic effect on the cell organelles. The nano size of NPs determines their greater specific surface area than that of their bulk counterparts, due to which they can be more effective in their interactions with the components of plants.16
000 mg kg−1) in sewage sludges may be caused by the adsorption of contaminants on the surface of nano-TiO2 (surface area 35–65 m2 g−1),37 which reduced their bioavailability and – indirectly – also their toxicity. However, it is hard to explain the positive effect of nano-TiO2 at the lowest concentration and then its negative effect at a higher concentration followed by a recurring positive effect at the highest concentration. Our earlier study11 also demonstrated a stimulating effect of nano-TiO2 (in various soils) on L. sativum. In that study also no dose–effect relationship was observed. It is interesting to note that the trends in the effect of nano-TiO2 on toxicity in both sludges were the same, while the sludges differed considerably in their content of Ti. This may indicate a greater importance of the size of the particles than that of their concentration. As in the case of nano-ZnO, a stronger negative effect after the addition of nano-TiO2 was observed in sludge SL1 than in SL2. This confirms again the significant effect of organic matter on the bioavailability, and indirectly also on the toxicity of the NPs under study. Also important in this respect is their aggregation which significantly reduces the toxic effect.38 Noteworthy as well as is the fact that the introduction of the “macro” or bulk counterparts of nano-ZnO and nano-TiO2 to the sewage sludges produced different effects than those of the NPs. The effect of bZnO on the toxicity of the sewage sludges was only slight. NPs, thanks to their size, can penetrate cell walls,39 then the Zn2+ ions can exert their toxic effect on the cell organelles. The nano size of NPs determines their greater specific surface area than that of their bulk counterparts, due to which they can be more effective in their interactions with the components of plants.16
It is important to get to know the effect of NPs accumulated in sewage sludge on various plant species. As demonstrated by the study presented here, L. sativum displayed the greatest sensitivity to the sewage sludges and to those with a content of NPs (with the exception of bTiO2 which was more toxic towards S. alba). In an earlier study, concerned with other contaminants, L. sativum was also characterized by greater sensitivity than S. alba.40 The differences in the tolerance of plants to the presence of sewage sludge with a content of the particular NPs may result from their various capacities of absorbing metals.39 A key role can also be played by the size of seeds or the anatomy of root.16 Another potential cause can be the content of the plant mucus covering the surface of the roots, as it absorbs NPs and that may, in turn, interfere with the transport of water and nutrients to the inner tissues of plants.41 Apart from that, plants can produce various exudations which may affect the aggregation of NPs, and thus also their availability.39 The elucidation of differences among plants, however, requires further studies comprising a greater group of plants.
The validity of the study is evidenced by the significant difference between the NPs and their bulk counterparts in their effect on the toxicity of sewage sludges. nano-ZnO50 and nano-ZnO100 caused a notably greater increase (ca. 50%) in the toxicity of the sludges compared to the effect of bZnO. The key role of the size of particles is evidenced by numerous studies on various nanoparticles.42,43 However it is interesting that in this study only a small difference was observed between the effects of NPs with different sizes on the toxicity of the sewage sludges. Perhaps this is related to the small difference in the surface area of the NPs (nano-ZnO50 – 10.8 m2 g−1 and nano-ZnO100 – 15–25 m2 g−1). Moreover, nano-ZnO100 also contains nanoparticles with a diameter of <50 nm (nano-ZnO50) in its composition, which undoubtedly had an effect on the small difference in phytotoxicity between those NPs.
One of the important aspects in the estimation of risk related to sewage sludges and contaminants contained in them, especially in the aspect of their utilization in agriculture, is the determination of the effect of dose on toxicity. As mentioned before, sewage sludges can constitute a rich source of organic matter and minerals. In the case of deficit of those components in soil, application of larger doses of sewage sludge is accepted. Additionally, higher doses of sewage sludge are frequently applied in processes of soil reclamation.2 Therefore, it was necessary to estimate the effect of the NPs as related to the sewage sludge dose. The introduction of nano-TiO2 maintained that tendency, while nano-ZnO100 had a less toxic effect in the larger dose of sludge than in the smaller one. That tendency could have been caused by greater influx of organic matter and components such as P that can immobilize nano-ZnO100, which limited their toxicity. On the other hand, however, NPs can adsorb contaminants, becoming potentially their carrier, which may result in greater toxicity.20,44 That last example may explain the increased toxicity of nano-TiO2 at the higher dose of the sludge.
Another extremely important issue is the effect of the contact time between the NPs and the sludge on the toxic effect observed.15 The problem is of particular importance as depending on the duration of contact between NPs and sewage sludge various results can be observed. This is related to a varied environmental risk. In principle, in the case of most contaminants, extension of the contact time reduces their bioavailability and, indirectly, also their toxicity.45 This rule was confirmed for most of the nanoparticles studied (Fig. 5). Longer time of contact of NPs with sewage sludge causes favorable conditions for interaction of the NPs with sewage sludge components (e.g. organic matter), which may reduce their bioavailability either through adsorption or through aggregation.38 The increase of toxicity of sewage sludges containing TiO2 can be a result of incomplete degradation of other contaminants present in the sludges, that under the effect of TiO2 underwent degradation to forms more toxic than the parent compounds. Although the sewage sludges were incubated in the dark, both the process of preparation of the experiment and subsequent preparation for the Phytotoxkit™ test were conducted with access to light, which could have been conducive to that process.46 This is supported by further studies in which both bTiO2 and nano-TiO2 in sewage sludge SL1 and nano-TiO2 in sewage sludge SL2 exposed to light increase the toxicity of the sewage sludges (Fig. 6). Thanks to its strong photocatalytic properties, TiO2 can degrade organic contaminants.46 However, as mentioned before, those compounds can be degraded to metabolites which give a greater toxic effect than their parent compounds. Another potential cause for the increase of toxicity under the effect of TiO2 can be the production of reactive forms of oxygen (ROS) by nano-TiO2, that are subsequently responsible for producing oxidative stress in living organisms.47,48
Leachates can be useful for evaluating the toxicity of sewage sludge-amended soils to aquatic organisms in hypothetical situations where soil leaching and erosion processes could mobilize contaminants to the surrounding fluvial systems. In relation to the above, it is very important to expand the estimation of the effect of NPs on the toxicity of sewage sludges by also including sewage sludge leachates in the scope of the estimation. As demonstrated by this study, the toxicity of leachates from sludges containing NPs was greater than the toxicity of leachates from sludges with no content of NPs. Despite similar concentrations of Zn, leachates from sewage sludge containing nano-ZnO100 showed higher toxicity to L. sativum in comparison to leachates from sludge containing bZnO (Table 1). This clearly indicates that ZnO NPs have a more negative impact than their bulk counterparts at the same concentration. Similarly higher toxicity in relation to L. sativum was observed for TiO2 NPs than their bulk counterparts. In this case it was associated with significantly higher contents of Ti in the leachates from sewage sludge containing NPs than bulk forms. Tested bacteria showed to be a relatively poor indicator of the toxicity of NPs in the sewage sludge leachates. Apart from a few exceptions, in most cases both in the Microtox and the MARA tests, bacteria were fairly tolerant to the negative effect of the sludges.
5 Conclusion
The common application of NPs, ZnO and TiO2 in particular, in daily use products may lead to their presence in wastewaters and, consequently, in sewage sludges. As demonstrated in the present study, NPs contained in sewage sludges may have a significant effect on their toxicity, and thus may constitute an additional factor limiting the application of sewage sludges in soil reclamation. In the situation of increasing production of NPs it is necessary to conduct studies not only on the effect of NPs on the toxicity of sewage sludges but also on their effect on soils amended with such materials. Application of sewage sludges containing NPs to soil entails the risk of direct exposure of plants and soil microorganisms to the effect of those “new contaminants”. Hence there is a need for research on the effect of NPs on the toxicity of sewage sludges in relation to various species of organisms, and plants in particular. As demonstrated in the present study, the toxicity of sewage sludges containing NPs was affected by various factors (kind and size of the NPs, dose of sludge, ageing, and access to light), hence there is a necessity of further studies, expanding to other environmental conditions, in order to identify the real threat following from the presence of NPs in the soil environment.Acknowledgements
The work was funded in the frame of Grant no. NN523 616639 financed in 2010–2012 from the budget of Ministry of Science and Higher Education (Poland).References
- A. Hospido, M. Carballa, M. Moreira, F. Omil, J. M. Lema and G. Feijoo, Water Res., 2010, 44, 3225–3233 CrossRef CAS.
- E. Epstein, Land Application of Sewage Sludge and Biosolids, Lewis, Boca Raton, 2003 Search PubMed.
- N. Roig, J. Sierra, M. Nadal, E. Marti, P. Navalon-Madrigal, M. Schuhmacher and J. L. Domingo, Sci. Total Environ., 2012, 425, 99–109 CrossRef CAS.
- S. K. Brar, M. Verma, R. D. Tyagi and R. Y. Surampalli, Waste Manage., 2010, 30, 504–520 CrossRef CAS.
- B. Kiss, T. Biro, G. Czifra, B. I. Toth, Z. Kertesz, Z. Szikszai, A. Z. Kiss, I. Juhasz, C. C. Zouboulis and J. Hunyadi, Exp. Dermatol., 2008, 17, 659–667 CrossRef CAS.
- J. Lv, S. Zhang, L. Iuo, W. Han, J. Zhang, K. Yang and P. Christie, Environ. Sci. Technol., 2012, 46, 7215–7221 CrossRef CAS.
- G. V. Lowry, K. B. Gregory, S. C. Apte and J. R. Lead, Environ. Sci. Technol., 2012, 46, 6891–6892 CrossRef CAS.
- D. Lin and B. Xing, Environ. Pollut., 2007, 150, 243–250 CrossRef CAS.
- D. Lin and B. Xing, Environ. Sci. Technol., 2009, 42, 5580–5585 CrossRef.
- W. Lee, J. Kwak and Y. An, Chemosphere, 2012, 86, 491–499 CrossRef CAS.
- I. Jośko and P. Oleszczuk, Influence of soil type and environmental conditions on ZnO, TiO2 and Ni nanoparticles phytotoxicity, submitted.
- L. Zheng, F. Hong, S. Lu and C. Liu, Biol. Trace Elem. Res., 2005, 104, 83–91 CrossRef CAS.
- J. Geisler-Lee, Q. Wang, Y. Yao, W. Zhang, M. Geisler, K. Li, Y. Huang, Y. Chen, A. Kolmakov and X. Ma, Nanotoxicology, DOI:10.3109/17435390.2012.658094, in press.
- W. Lee, Y. An, H. Yoon and H. Kweon, Environ. Toxicol. Chem., 2008, 27, 1915–1921 CrossRef CAS.
- P. Oleszczuk, I. Jośko and B. Xing, J. Hazard. Mater., 2011, 186, 436–442 CrossRef CAS.
- C. M. Rico, S. Majumdar, M. Duarte-Gardea, J. R. Peralta-Videa and J. L. Gardea-Torresdey, J. Agric. Food Chem., 2011, 59, 3485–3498 CrossRef CAS.
- Y. Beak and Y. An, Sci. Total Environ., 2011, 409, 1603–1608 CrossRef.
- Y. Ma, E. Lombi, A. L. Nolan and M. J. Mclaughlin, Environ. Toxicol. Chem., 2006, 25, 652–658 CrossRef CAS.
- I. Chowdhury, D. M. Cwiertny and S. L. Walker, Environ. Sci. Technol., 2012, 46, 6968–6976 CrossRef CAS.
- N. B. Hartmann, S. Legros, F. von der Kammer, T. Hofmann and A. Baun, Aquat. Toxicol., 2012, 118–119, 1–8 CrossRef CAS.
- D. Wang, J. Hu, D. R. Irons and J. Wang, Sci. Total Environ., 2011, 409, 1351–1356 CrossRef CAS.
- L. P. van Reeuwijk, Procedures for Soil Analysis, ISRIC, Wageningen, The Netherlands, 2002 Search PubMed.
- S. Baran, P. Oleszczuk, A. Lesiuk and E. Baranowska, Pol. J. Environ. Stud., 2000, 11, 299–305 Search PubMed.
- EC, European Commission, Council decision of 19 December 2002 establishing criteria and procedures for the acceptance of waste at landfills pursuant to Article 16 of and Annex II to Directive1999/31/EC. 2003/33/EC, OJ L11, 27, 2002.
- OECD, Organization for Economic Co-operation and Development, Guideline for Testing of Chemicals 208. Terrestrial Plants, Growth Test, 1984 Search PubMed.
- K. Wadhia, T. Dando and K. C. Thompson, J. Environ. Monit., 2007, 9, 953–958 RSC.
- F. Gottschalk and B. Nowack, J. Environ. Monit., 2011, 13, 1145–1155 RSC.
- L. Windler, C. Lorenz, N. von Gotz, K. Hungerbuehler, M. Amberg, M. P. Heuberger and B. Nowack, Environ. Sci. Technol., 2012, 46, 8181–8188 CrossRef CAS.
- B. Nowack, J. F. Ranville, S. Diamond, J. A. Gallego-Urea, C. Metcalfe, J. Rose, N. Horne, A. A. Koelmans and S. J. Klaine, Environ. Toxicol. Chem., 2012, 31, 50–59 CrossRef CAS.
- F. Gottschalk, T. Sonderer, R. Scholz and B. Nowack, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS.
- B. Nowack and T. D. Bucheli, Environ. Pollut., 2007, 150, 5–22 CrossRef CAS.
- A. Kabata-Pendias and H. Pendias, Biogeochemistry of Trace Elements, PWN, Warszawa, 1993 Search PubMed.
- K. Yang, D. Lin and B. Xing, Langmuir, 2009, 25, 3571–3576 CrossRef CAS.
- S. Lee, K. Kim, H. K. Shon, S. D. Kim and J. Cho, J. Nanopart. Res., 2011, 13, 3051–3061 CrossRef CAS.
- K. Van Hoecke, K. A. C. De Schamphelaere, P. Van der meeren, G. Smagghe and C. R. Janssen, Environ. Pollut., 2011, 159, 970–976 CrossRef CAS.
- E. Lombi, E. Donner, E. Tavakkoli, T. W. Turney, R. Naidu, B. W. Miller and K. G. Scheckel, Environ. Sci. Technol., 2012, 46, 9089–9096 CrossRef CAS.
- B. Kim, M. Murayama, B. P. Colman and M. F. Hochella, J. Environ. Monit., 2012, 14, 1129–1137 RSC.
- J. Chen, Z. Xiu, G. V. Lowry and P. J. J. Alvarez, Water Res., 2011, 45, 1995–2001 CrossRef CAS.
- J. D. Judy, J. M. Unrine, W. Rao, S. Wirck and P. M. Bertsh, Environ. Sci. Technol., 2012, 46, 8467–8474 CrossRef CAS.
- P. Oleszczuk, Ecotoxicol. Environ. Saf., 2008, 69, 496–505 CrossRef CAS.
- S. Asli and M. Neumann, Plant, Cell Environ., 2009, 32, 577–584 CrossRef CAS.
- Z. Wang, X. Xie, J. Zhao, X. Liu, W. Feng, J. C. White and B. Xing, Environ. Sci. Technol., 2012, 46, 4434–4441 CrossRef CAS.
- A. Praetorius, M. Scheringer and K. Hungerbuhler, Environ. Sci. Technol., 2012, 46, 6705–6713 CrossRef CAS.
- J. Farkas, L. Nizzetto and K. V. Thomas, Sci. Total Environ., 2012, 425, 283–288 CrossRef CAS.
- M. Alexander, Environ. Sci. Technol., 2000, 34, 4259–4265 CrossRef CAS.
- G. Shan, S. Yang, R. D. Tyagi, R. Y. Surampalli and T. C. Zhang, Pract. Period. Hazard., Toxic, Radioact. Waste Manage., 2009, 13, 110–119 CrossRef CAS.
- Y. Ma, A. Brennan and S. A. Diamond, Environ. Toxicol. Chem., 2012, 31, 2099–2107 CrossRef.
- H. Ma, A. Brennan and S. A. Diamond, Environ. Toxicol. Chem., 2012, 31, 1621–1629 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
