Sorption of phenanthrene on single-walled carbon nanotubes modified by DOM: effects of DOM molecular weight and contact time
Hongwen
Sun
*,
Qi
Song
,
Pei
Luo
,
Wenling
Wu
and
Jizhou
Wu
MOE Key Laboratory of Pollution Processes and Environmental Criteria, College of Environmental Science and Engineering, Nankai University, Tianjin, China. E-mail: sunhongwen@nankai.edu.cn; Fax: +86 22 23508807; Tel: +86 22 23509241
First published on 6th December 2012
Abstract
The impacts on the sorption of phenanthrene (PHE) onto single-walled carbon nanotubes (SCNT) by loading of dissolved organic matter (DOM) fractions with different molecular weights (MWs) were studied. Moreover, the influence of contact time on the sorption capacity of DOM-modified SCNT was evaluated for the first time. Preloading of DOM on SCNT for 2 days led to a reduction in PHE sorption and the nonlinearity of the sorption isotherm; however further increasing the contact time between the DOM and SCNT from 2 days to 20 days enhanced the sorption capacity, and the sorption nonlinearity increased also. This is due to the transferring of DOM molecules from the external surface to interstitial channels trapped in SCNT particles, restoring the sorption sites. The loading of DOM fraction with MWs larger than 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da (DOM>14
000 Da (DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) brought a stronger suppression of PHE sorption by SCNT compared to smaller ones (DOM<1000 and DOM1000–14
000) brought a stronger suppression of PHE sorption by SCNT compared to smaller ones (DOM<1000 and DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), with the Freundlich nonlinear index n increasing from 0.236 to 1.093 and the Freundlich affinity coefficient log KF decreasing from to 7.34 to 4.57. These are similar to the sorption characteristics of DOM>14
000), with the Freundlich nonlinear index n increasing from 0.236 to 1.093 and the Freundlich affinity coefficient log KF decreasing from to 7.34 to 4.57. These are similar to the sorption characteristics of DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, suggesting a complete coverage of the SCNT by DOM>14
000, suggesting a complete coverage of the SCNT by DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 molecules. Though the loading of DOM fractions with smaller MWs made SCNT more polar, the reduction in PHE sorption on DOM-preloaded SCNT was limited due to their lesser sorbed amount and smaller molecular size.
000 molecules. Though the loading of DOM fractions with smaller MWs made SCNT more polar, the reduction in PHE sorption on DOM-preloaded SCNT was limited due to their lesser sorbed amount and smaller molecular size.
Environmental impactOnce CNT are released into the environment, they will inevitably interact with DOM, which would in turn affect the environmental behavior of the CNT and their interaction with organic pollutants. Both the chemical composition and steric conformation of DOM were found to influence its interaction with SCNT. DOM fractions with larger MWs (DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) showed stronger interactions with SCNT and a greater binding capacity for PHE than smaller ones (DOM<1000 and DOM1000–14 000) showed stronger interactions with SCNT and a greater binding capacity for PHE than smaller ones (DOM<1000 and DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), leading to the strongest suppression of sorption of PHE. Increasing the contact time between the DOM and SCNT from 2 days to 20 days enhanced the sorption capacity and increased the sorption nonlinearity of PHE on DOM-preloaded SCNT. 000), leading to the strongest suppression of sorption of PHE. Increasing the contact time between the DOM and SCNT from 2 days to 20 days enhanced the sorption capacity and increased the sorption nonlinearity of PHE on DOM-preloaded SCNT.
|
Introduction
Carbon nanotubes (CNT) have attracted great attention due to their unique properties, such as electrical conductivity, optical activity, and mechanical strength, since their discovery by Iijima in 1991.1 This fascinating new class of materials has shown promising applications in many areas, including battery and fuel electrodes, catalysts, super capacitors, conductive coating, sensors, and adsorbents in water treatment.2,3Serious environmental concerns have been aroused regarding the fate and impacts of CNT since they might be released into the environment with increasing manufacture and applications. CNT exhibit great sorption capacity for organic contaminants, especially hydrophobic organic contaminants (HOCs) because of their great surface areas and highly hydrophobic nature, and the sorption coefficients on CNT for most HOCs are usually hundredfold that of those on natural geosorbents.4,5 Hence, one major concern is that the existence of CNT would alter the fate, transport and bioavailability of HOCs in the environment. Both inhibition and enhancement of the bioavailability of organic contaminants due to the sorption onto CNT have been reported. For example, addition of 3.0 mg g−1 CNT in soil substantially decreased the bioaccumulation of pyrene by earthworms.6 This is in agreement with the common recognition that sorbed organic chemicals on solids are less bioavailable. However, opposite results were also reported. The existence of CNT increased accumulation of sorbed pyrene in Lumbriculus variegatus and the authors ascribed this to the easier passage of nanosized CNT into and through organisms.7 Similarly, the mobility of HOCs may change greatly which is controlled by both the sorption capacity and the stability of CNT in the water phase. Hence the sorption of organic chemicals on CNT has drawn much attention, and considerable literature has come forth.6–9 Multiple mechanisms have been proposed for the sorption by CNT, such as hydrophobic interactions, π–π electron donor–acceptor interactions, hydrogen bonds and electrostatic interactions.8
Dissolved organic matter (DOM) is ubiquitous in the aquatic environment,10,11 and it is composed of a complex mixture of organic compounds with varying molecular weights (MWs), from small hydrophilic acids, amino acids, proteins to large humic and fulvic acids. DOM affects the distribution of organic compounds between the interface of solution and solids through several pathways. Kilduff and Wigton found that the presence of DOM could reduce the sorption of trichloroethylene (TCE) on activated carbon due to pore blockage of the activated carbon, which can reduce the effective surface area available to TCE.12 Pignatello et al. reported that humic substances in adsorbed and coflocculated states inhibited adsorption of organic chemicals onto black carbon because of the similar mechanisms.13 An opposite result has also been reported by Murphy et al. that DOM coating would enhance the adsorption of HOCs on natural mineral particles.14 The effects of DOM depend on the nature of the solids as well as the physicochemical properties of DOM, such as polarity, molecular size and configuration, aromaticity and alkyl moieties. It has been reported that different DOM fractions show different properties. Kang et al. found that the macromolecule fraction of DOM contained higher amounts of aromatic moieties and carbohydrate-like substances and more hydrophobic groups.15 This will lead to different effects on the fate of organic chemicals. Chin et al. observed a strong correlation between the pyrene binding coefficient, Kdoc, MWs and aromaticity of the aquatic humic substances.16 Chen et al. reported that DOM fractions with high MW had more effect on the mobility of prometryne in soils than those with low MW.17
Once CNT are released into the environment, they will inevitably interact with DOM, which would in turn affect the environmental behavior of CNT and their interaction with organic pollutants. Hyung et al. reported that CNT can interact with DOM in aquatic systems which could enhance their stability and transport in aquatic systems.18 It was also observed that CNT had higher sorption capacity for humic acids of higher MW and greater hydrophobicity than for fulvic acids.19 Zhang et al. reported that the presence of natural organic matter (NOM) greatly suppressed the adsorption of phenanthrene (PHE), biphenyl, and 2-phenylphenol by CNT and the effects of NOM were determined by the planarity and hydrophobicity of these three organic chemicals.20 Wang et al. reported that peptone coating made the strongest suppression of the sorption of PHE, naphthalene, and 1-naphthol by CNT among three DOMs (humic acid, peptone, and α-phenylalanine).21 However, so far no study has examined the influence of DOM fractions with different MWs on the adsorptive behavior of HOCs by CNT.
Besides, it is well known that many interactions in the environment are long-term processes, which are quite different from laboratory experiments with a short time usually of one or two days.22,23 It has been well documented that both the sorption amount and combination state of organic chemicals on geosorbents change with increasing contact time (aging effect); and the change in the combination state of sorbed chemicals leads to decreased desorption and bioavailability.24,25 In our earlier study, we found that extending contact time between soil and black carbon led to a reduced sorption capacity of the mixture in the presence of water, but no significant change was found under dry conditions.26 The reduced sorption capacity of the mixture was ascribed to the interaction of soil organic matter with black carbon, which was facilitated by water molecules. The interaction of DOM and CNT may also change with extended time, which may influence the environmental behavior of their complex in the environment. However, sorption on DOM–CNT complexes under different contact times has never been studied.
Therefore, the objectives of this study were to investigate the influence of contact time and DOM chemical properties on the sorption capacity of DOM-loaded CNT for organic chemicals and the mechanisms therein. To this end, we studied the sorption of PHE on original commercial single-walled carbon nanotubes (SCNT), SCNT preloaded with bulk DOM (extracted from a peat) and its fractions with different MWs, and SCNT preloaded with DOM experiencing different contact times.
Materials and methods
Materials
The SCNT (2–10 nm diameter × 1–5 μm length) were purchased from Sigma-Aldrich Chemical Company (MO, USA) and were used as received. PHE (purity >98%) was purchased from Acros Corporation (NJ, USA).The DOM was extracted from a peat material collected from Mount Wuyi, China with double-distilled water using a solid to water ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 (w/v, dry weight basis) on a magnetic stirrer for 24 h at room temperature. After the suspension was centrifuged at 3000 rpm for 30 min, the supernatant was filtered through a 0.45 μm membrane, and the filtrate was stored at 4 °C. Cellulose dialysis membrane bags (Spectrum Inc., Texas, USA) were used to separate DOM fractions at MWs of 1000 and 14
2.5 (w/v, dry weight basis) on a magnetic stirrer for 24 h at room temperature. After the suspension was centrifuged at 3000 rpm for 30 min, the supernatant was filtered through a 0.45 μm membrane, and the filtrate was stored at 4 °C. Cellulose dialysis membrane bags (Spectrum Inc., Texas, USA) were used to separate DOM fractions at MWs of 1000 and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da as described by Cheng and Wong.27 The bags were cleaned by overnight soaking in deionized water and thorough rinsing before use. Fifty milliliters of the DOM extract was added to a dialysis bag with a MW cutoff of 1000 Da, and dialysis was conducted in 100 mL of deionized water in the dark at 10 °C with magnetic stirring for 40 h. Then, the solution that remained in the bag was transferred to another dialysis bag with a MW cutoff of 14
000 Da as described by Cheng and Wong.27 The bags were cleaned by overnight soaking in deionized water and thorough rinsing before use. Fifty milliliters of the DOM extract was added to a dialysis bag with a MW cutoff of 1000 Da, and dialysis was conducted in 100 mL of deionized water in the dark at 10 °C with magnetic stirring for 40 h. Then, the solution that remained in the bag was transferred to another dialysis bag with a MW cutoff of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, and the dialysis was conducted under the same conditions as described above. The three fractions are designated as DOM<1000, DOM1000–14
000 Da, and the dialysis was conducted under the same conditions as described above. The three fractions are designated as DOM<1000, DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and DOM>14
000, and DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, respectively. The NaN3 used in the aqueous stock solutions as the biocide to inhibit microorganisms was of analysis grade.
000, respectively. The NaN3 used in the aqueous stock solutions as the biocide to inhibit microorganisms was of analysis grade.
Characterization of SCNT
The SCNT were measured for Brunauer–Emmett–Teller (BET) surface areas (SBET) and pore size distribution based on nitrogen sorption–desorption isotherms at 77 K using a high-resolution gas adsorption analyzer (AutoSorb-1-MP, Quantachrome, USA). DFT (density functional theory) was used to analyze the pore size distribution using a Quantachrome NOVA automated gas sorption system, NOVA for Windows Version 1.12.28 All samples were outgassed at 115 °C for 10 h before N2 sorption. Bulk elemental contents (C, H, and N) of the samples were measured using a CHN elemental analyzer (Elementar Vario EL CUBE, Germany) and are reported as w/w percentages. The amount of ash was determined by mass loss after heating the samples at 900 °C for 10 h. Oxygen content was calculated by mass difference. To get information on surface functionalities and elemental composition of the samples, X-ray photoelectron spectra (XPS) were obtained using a Kratos Axis Ultra DLD multi-technique X-ray photoelectron spectrometer (Shimadzu Corporation, Japan). Binding energies were calibrated using the C1s hydrocarbon peak at 284.8 eV with deconvolution processing of the C1s spectra by the Xpspeak software package, and the C1s binding energy levels were assigned as follows: 284.6 eV to C–C, 286.2 eV to C–H, 287.6 eV to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and 289.1 eV to COO–.29
O, and 289.1 eV to COO–.29
Sorption experiments
Sorption experiments were carried out in 40 mL US EPA standard sample vials with Teflon-lined septa and screw caps. Five milligrams of SCNT powder was weighed into the vials containing 40 mL of 200 mg L−1 NaN3 solution, and specific amounts of PHE stock solution were added to the vials as experimental design to initiate the sorption. The vials were shaken for 8 days in a shaker operated at 150 rpm and 20 ± 0.5 °C in the dark. The data of the sorption kinetic experiments showed that it took 5 days for PHE to obtain apparent sorption equilibrium on SCNT and DOM-loaded SCNT. When the impacts of the contact time of DOM with SCNT were studied, the extracted bulk DOM was allowed to react with the SCNT for 2 and 20 days to represent fresh and aged DOMbulk–SCNT. For the influence of DOM fractions with different MWs, the solutions of DOM MW fractions were allowed to react with SCNT for 2 days, which are denoted as DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT, DOM1000–14
000–SCNT, DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT, and DOM<1000–SCNT, respectively. The levels of DOM before and after interaction with SCNT were recorded. Phenanthrene was spiked directly in vials without separation of DOM residue in the solution phase to initiate the sorption. This design is thought more realistic since DOM exists both in the solution phase and on the solid phase in the natural environment. After sorption experiments, the solution and the SCNT were separated by centrifugation at 2000 rpm for 20 min. An appropriate aliquot of the supernatant was removed, and PHE concentration was analyzed. All sorption experiments were conducted in duplicates and all the data in figures are the average of two replicates.
000–SCNT, and DOM<1000–SCNT, respectively. The levels of DOM before and after interaction with SCNT were recorded. Phenanthrene was spiked directly in vials without separation of DOM residue in the solution phase to initiate the sorption. This design is thought more realistic since DOM exists both in the solution phase and on the solid phase in the natural environment. After sorption experiments, the solution and the SCNT were separated by centrifugation at 2000 rpm for 20 min. An appropriate aliquot of the supernatant was removed, and PHE concentration was analyzed. All sorption experiments were conducted in duplicates and all the data in figures are the average of two replicates.
PHE binding to bulk DOM and its MW fractions
To elucidate the binding capacity and possible impact of DOM on PHE, PHE binding to DOMbulk and its MW fractions in solution were measured. Concentrations of DOMbulk and its MW fractions were adjusted by dilution in deionized water, and a series of DOM solutions with concentrations ranging from 0 to 40 mg C L−1 were used in the experiments. The initial concentration of PHE was set at 1 mg L−1. The sorption experiments were conducted in 22 mL US EPA standard sample vials, and after hand-shaking, the vials were allowed to interact for 10 min at 25 °C before analysis. Experiments were conducted in duplicates. PHE blanks without DOM and DOM blanks without PHE were prepared.Analysis
Aqueous PHE was quantified by HPLC (Agilent 1200, USA) equipped with a fluorescence detector (G1321A) and a reverse-phase column (Agela, Venusil XBP C-18, 150 mm × 4.6 mm × 5 μm). The flow rate of the mobile phase composed of 90% methanol and 10% HPLC pure water was 1.0 mL min−1, and the injection volume was 10 μL. The excitation and emission wavelengths for the fluorescence detector were 250 nm and 364 nm, respectively.Concentration of DOM was measured by a TOC analyzer (TOC-5000A, Shimadzu, Japan). The equilibrium DOM concentration after interaction with SCNT for 2 or 20 days was analyzed after removing the SCNT with a 0.45 μm Acrodisc nylon membrane syringe filter. The sorbed amount of DOM onto the SCNT (Q) was calculated based on the difference in DOM concentrations before and after the interaction with SCNT. To characterize the aromaticity of DOM, the absorbances of DOM solutions were measured at 254 nm using a Varian UV-005 spectrophotometer (Varian, USA).
To quantify the interaction of PHE with DOM in solution, the fluorescence intensities of PHE–DOM mixtures and blank samples without DOM were measured at 25 °C using a fluorescence spectrometer (Perkin-Elmer LS 55, UK) with 294 and 365 nm as excitation and emission wavelengths, respectively. The amount of bound PHE could be calculated from the reduction in fluorescence in the PHE–DOM mixtures as compared to the blank, and the KDOM [L (kg C)−1] of PHE were calculated using the Stern–Volmer plot (eqn (1)) described by Lakowicz:30
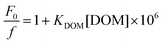 | (1) |
Isotherm modeling
The Freundlich model was employed to analyze sorption isotherm data:| Q = KFCen | (2) |
Results and discussion
Properties characterization of SCNT and DOM-preloaded SCNT
The structural parameters for the original SCNT, and SCNT preloaded with bulk DOM of different contact times, and SCNT preloaded with DOM fractions of different MWs are shown in Table 1. The bulk of the original SCNT was rich in carbon and ash but low in oxygen and hydrogen contents; while the surface of the original SCNT was rich in both carbon and oxygen (Table 1), which was different from the bulk element composition, indicating that the residue O-containing moieties were mainly located on the outer surface of the SCNT. The preloading of extracted bulk DOM on SCNT for 2 days increased the oxygen content from 0.62% to 3.53% and nitrogen content from 0 to 1.21%, respectively, indicating that substantial O-containing and N-containing moieties were introduced to the SCNT by DOM, leading to an increase in polarity, as indicated by the increased polar index [(O + N)/C]. The original SCNT had a BET surface area and micropore volume of 243 m2 g−1 and 0.026 cm3 g−1, respectively; while the fresh DOMbulk–SCNT had a substantially reduced surface area (110 m2 g−1) and micropore volume (0 cm3 g−1). The average pore diameter of aged DOMbulk–SCNT with a contact time of 20 days increased and the BET surface area and total pore volume decreased compared to those of fresh DOMbulk–SCNT.| Sorbents | S BET (m2 g−1) | V total (cm3 g−1) | V micro (cm3 g−1) | D average (Å) | A cal (m2 g−1) | V cal (cm3 g−1) | Bulk element composition (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | Ash | H/C | (O + N)/C | |||||||
| a S BET, BET surface area. b V total, total pore volume. c V micro, micropore volume. d D average, average pore diameter. e A cal and Vcal, the calculated surface area and micropore volume from the surface area of SCNT (243 m2 g−1) and micropore volume of SCNT (0.026 cm3 g−1) and the adsorbed DOM content by the following equation: Acal = 243 × (1 − fss) and Vcal = 0.026 × (1 − fss), fss = QDOM/100.9 | |||||||||||||
| Original SCNT | 243 | 0.413 | 0.026 | 67.9 | 243 | 0.026 | 67.8 | 2.29 | 0 | 0.62 | 29.3 | 0.405 | 0.007 |
| Fresh DOMbulk–SCNT | 110 | 0.467 | 0 | 170 | 160 | 0.017 | 65.4 | 1.81 | 1.21 | 3.53 | 28.0 | 0.332 | 0.056 |
| Aged DOMbulk–SCNT | 93.1 | 0.410 | 0 | 176 | 129 | 0.014 | 64.7 | 1.84 | 1.55 | 3.67 | 28.2 | 0.341 | 0.063 |
| DOM<1000–SCNT | 125 | 0.494 | 0 | 158 | 214 | 0.023 | 67.2 | 1.47 | 1.53 | 5.04 | 24.7 | 0.262 | 0.076 |
DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT 000–SCNT |
133 | 0.477 | 0 | 144 | 189 | 0.020 | 64.4 | 1.49 | 1.11 | 2.29 | 30.7 | 0.278 | 0.041 |
DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT 000–SCNT |
136 | 0.456 | 0 | 134 | 159 | 0.017 | 67.2 | 1.55 | 1.65 | 0.96 | 28.6 | 0.277 | 0.032 |
| Surface functionalities and element composition (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| C–C | C–O | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
C | N | O | O/C | (O + N)/C | |
| Original SCNT | 59.4 | 29.4 | 5.41 | 5.80 | 83.2 | 0 | 16.8 | 0.15 | 0.15 |
| Fresh DOMbulk–SCNT | 35.0 | 24.6 | 34.6 | 5.70 | 80.2 | 0.62 | 19.2 | 0.18 | 0.18 |
| Aged DOMbulk–SCNT | 54.3 | 5.99 | 28.5 | 11.3 | 81.3 | 1.46 | 17.3 | 0.16 | 0.17 |
| DOM<1000–SCNT | 47.3 | 41.0 | 0.65 | 11.0 | 84.7 | 0.54 | 14.7 | 0.13 | 0.14 |
DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT 000–SCNT |
53.8 | 25.4 | 17.2 | 3.56 | 83.9 | 0.87 | 15.2 | 0.14 | 0.14 |
DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT 000–SCNT |
58.7 | 4.27 | 20.7 | 16.3 | 83.0 | 1.37 | 15.6 | 0.14 | 0.15 |
The micropore volume was zero for all the DOM-preloaded SCNT. This is because of the diffusion limitation of N2 molecules to pass through loaded DOM layer into micropores at 77 K, and hence the micropore volume and surface area of the sorbents may be underestimated by this method.31 The calculated surface area Acal and micropore volume Vcal of fresh DOMbulk–SCNT based on the unit loading mass of DOM were 160.0 m2 g−1 and 0.017 cm3 g−1 (Table 1), suggesting that DOM sorption occurred through both surface-adsorption and micropore-blocking processes, which is consistent with previous reports on DOM adsorption by CNT.20,21 This can also be proved by DFT differential pore volume distribution of the original SCNT, fresh DOMbulk–SCNT and aged DOMbulk–SCNT (Fig. 1), showing that after reacting with DOM, the pore volume of SCNT with pore width within 14 Å sharply decreased. The total pore volume of fresh DOMbulk–SCNT slightly increased from 0.413 to 0.467 cm3 g−1, and average pore diameter substantially increased from 67.9 to 170 Å (Table 1), indicating that DOM-preloading had changed the structural features of the SCNT greatly. After aging, these parameters changed further, indicating the location and conformation of the loaded DOM changed during the increased contact time.
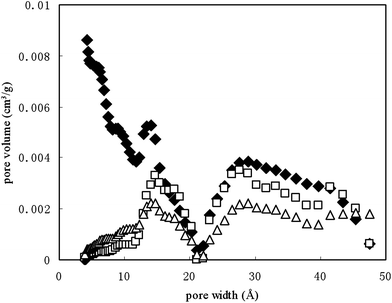 | ||
| Fig. 1 DFT differential pore volume distribution of original SCNT (◆), fresh DOMbulk–SCNT (□) and aged DOMbulk–SCNT (△). | ||
Binding of PHE with DOM fractions with different MWs in solution
The DOM extracted from peat consisted of significantly more DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (46.9%) and relatively less of DOM<1000 (35.2%) and DOM1000–14
000 (46.9%) and relatively less of DOM<1000 (35.2%) and DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (18.0%), as calculated based on the TOC values of each respective fraction. Notable differences in the PHE binding capacity existed for different DOM MW fractions; however, KDOM (eqn (1)) values of PHE were still in the same magnitude order, being 3.16 × 104, 2.79 × 104, and 2.19 × 104 L (kg C)−1 for DOM>14
000 (18.0%), as calculated based on the TOC values of each respective fraction. Notable differences in the PHE binding capacity existed for different DOM MW fractions; however, KDOM (eqn (1)) values of PHE were still in the same magnitude order, being 3.16 × 104, 2.79 × 104, and 2.19 × 104 L (kg C)−1 for DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, DOM1000–14
000, DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and DOM<1000 (Table 2), which descended with decreasing MW of the DOM. The sum of the KDOMs of the different DOM MW fractions based on their percentage weights was 2.76 × 104 L (kg C)−1, which agrees closely with the measured KDOM for DOMbulk [2.45 × 104 L (kg C)−1]. Several interactions may control the binding of PHE to DOM, such as van der Waals forces, solubilization (partition) effect, specific forces like π–π and n–π electron donor–acceptor (EDA) interactions, as well as physical engagement in the steric structure of DOM aggregates.32,33 Hence, both chemical composition and steric conformation of DOM influence the binding of organic chemicals. The UV light absorbance of DOM depends on the electronic structure of its macromolecules. The UV absorbance in the range of 254–280 nm is in the region of π–π* electronic transitions in conjugated systems of bulky DOM and can be used as an indicator of aromaticity.34,35 The UV absorbance at 254 nm followed the order: DOM>14
000, and DOM<1000 (Table 2), which descended with decreasing MW of the DOM. The sum of the KDOMs of the different DOM MW fractions based on their percentage weights was 2.76 × 104 L (kg C)−1, which agrees closely with the measured KDOM for DOMbulk [2.45 × 104 L (kg C)−1]. Several interactions may control the binding of PHE to DOM, such as van der Waals forces, solubilization (partition) effect, specific forces like π–π and n–π electron donor–acceptor (EDA) interactions, as well as physical engagement in the steric structure of DOM aggregates.32,33 Hence, both chemical composition and steric conformation of DOM influence the binding of organic chemicals. The UV light absorbance of DOM depends on the electronic structure of its macromolecules. The UV absorbance in the range of 254–280 nm is in the region of π–π* electronic transitions in conjugated systems of bulky DOM and can be used as an indicator of aromaticity.34,35 The UV absorbance at 254 nm followed the order: DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 > DOM1000–14000 > DOMbulk > DOM<1000 (Table 2), and significant positive correlation between the molar absorption of different DOM MW fractions at 254 nm and their KDOMs for PHE occurred (r2 = 0.94), which agrees with the view that the aromaticity of DOM is a primary factor in controlling its binding capacity to hydrophobic aromatic hydrocarbons. This finding is consistent with former reports,36–38 but contrary to a study on pyrethroid, which indicated that aromaticity was not the main property influencing pyrethroid binding.39 The different results in the latter study may be because of the specific reactions between DOM and polar groups in pyrethroid.
000 > DOM1000–14000 > DOMbulk > DOM<1000 (Table 2), and significant positive correlation between the molar absorption of different DOM MW fractions at 254 nm and their KDOMs for PHE occurred (r2 = 0.94), which agrees with the view that the aromaticity of DOM is a primary factor in controlling its binding capacity to hydrophobic aromatic hydrocarbons. This finding is consistent with former reports,36–38 but contrary to a study on pyrethroid, which indicated that aromaticity was not the main property influencing pyrethroid binding.39 The different results in the latter study may be because of the specific reactions between DOM and polar groups in pyrethroid.
| DOM fraction | UV254nm (L (m mg)−1) | Stern–Volmer plot | R 2 | K DOM [L (kg C)−1] | Log KDOM |
|---|---|---|---|---|---|
| a x is the concentration of DOM (mg C L−1) and Y is F0/f (eqn (1)). | |||||
| DOMbulk | 345 | Y = 0.0245x + 0.99 | 0.995 | 2.45 × 104 | 4.39 |
| DOM<1000 | 281 | Y = 0.0219x + 0.99 | 0.989 | 2.19 × 104 | 4.34 |
DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
358 | Y = 0.0279x + 0.98 | 0.987 | 2.79 × 104 | 4.44 |
DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
392 | Y = 0.0316x + 0.99 | 0.994 | 3.16 × 104 | 4.50 |
Sorption of PHE on original SCNT and fresh DOMbulk–SCNT
Sorption isotherms of PHE on original SCNT and fresh DOMbulk–SCNT are shown in Fig. 2. Parameters of the sorption of DOM on original SCNT and PHE on unmodified and modified SCNT are listed in Table 3.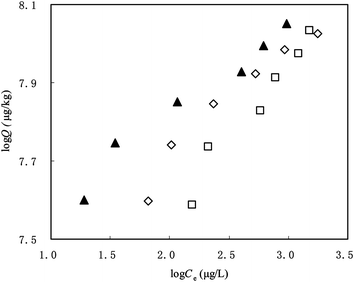 | ||
| Fig. 2 Sorption of PHE on original SCNT (▲), fresh DOMbulk–SCNT (□) and aged DOMbulk–SCNT (◇). | ||
| Sorbents | DOM | PHE (the Freundlich equation (eqn (2)) was employed) | ||||||
|---|---|---|---|---|---|---|---|---|
| C 0 (mg C L−1) | C e (mg C L−1) | Q (mg C g−1) | Log KF | n | R 2 | K d (L kg−1) | ||
| C e = 100 μg L−1 | C e = 1000 μg L−1 | |||||||
| a C 0 (mg C L−1) is the initial concentration of DOM before reacting with SCNT. b C e (mg C L−1) is the equilibrium concentration of DOM in the aqueous solution. c Q (mg C g−1) is the equilibrium concentration of DOM on SCNT. d K d (L kg−1) values were derived from the Freundlich model: Kd = Q/Ce = KF(Ce)n−1. | ||||||||
| Original SCNT | — | — | — | 7.34 | 0.236 | 0.966 | 6.49 × 105 | 1.12 × 105 |
| Fresh DOMbulk–SCNT | 18.4 | 14.2 | 34.2 | 6.75 | 0.402 | 0.959 | 3.54 × 105 | 8.92 × 104 |
| Aged DOMbulk–SCNT | 18.4 | 12.5 | 46.9 | 7.14 | 0.283 | 0.946 | 5.06 × 105 | 9.72 × 104 |
| DOM<1000–SCNT | 12.0 | 10.5 | 11.8 | 7.22 | 0.273 | 0.952 | 5.87 × 105 | 1.10 × 105 |
DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT 000–SCNT |
12.1 | 9.29 | 22.2 | 7.05 | 0.343 | 0.924 | 5.41 × 105 | 1.17 × 105 |
DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT 000–SCNT |
12.1 | 7.82 | 34.6 | 4.57 | 1.093 | 0.963 | 5.69 × 104 | 7.04 × 104 |
The original SCNT showed great sorption capacity for PHE, with log KF being 7.34 (Table 3). The sorption was highly nonlinear, with the Freundlich nonlinear index n being 0.23, suggesting heterogeneous sorption sites existing on the SCNT. The heterogeneous sites of SCNT for PHE may come from the external surfaces and interstitial channels trapped in the SCNT. Besides, aggregation of SCNT may create heterogeneous sites with different sorption energies for PHE sorption.40 From the thermodynamic view, at low PHE concentration, high-energy sites played a dominant role in adsorption, hence, Kd at Ce = 100 μg L−1 was much higher than that at Ce = 1000 μg L−1.
After loading of DOM, the Freundlich nonlinear index n of PHE adsorption on fresh DOMbulk–SCNT increased, suggesting that DOM molecules were preferably sorbed to high-energy adsorption sites of SCNT making sorption sites of SCNT less heterogeneous for PHE. Yang et al. also showed that cetylpyridinium chloride precoating on SCNT increased the linearity of the naphthalene adsorption isotherm due to the occupancy of high-energy adsorption sites.9
When preloaded with DOM, PHE sorption on SCNT declined significantly, and log KF decreased from 7.34 to 6.75 for the fresh DOM-preloaded SCNT (Table 3). Several mechanisms may be involved for the reduction. First, DOM preloading introduced polar moieties to SCNT, as indicated by the increased O content (Table 1). A slight increasing in O-containing moieties would greatly facilitate the formation of water clusters on the surface of SCNT. The water clusters and polar functionalities on the SCNT surface would affect the surface hydrophobicity of SCNT, and thus inhibit the hydrophobic interactions between SCNT and PHE. Zhang et al. found that surface functionalized SCNT showed reduction in adsorption of PHE but enhancement in adsorption of biphenyl and 2-phenylphenol due to the increased O-containing functional group content on the surface of SCNT.39 The latter is due to the strengthened polar interactions. Secondly, as DOM sorption occurred through both surface-adsorption and micropore-blocking processes, DOM molecules would occupy sorption sites of SCNT and block the micropores, which suppressed the sorption of PHE by “competition”. This could be confirmed by the reduced micropore volume, especially the portion with micro-size (<14 Å) and surface area (Table 1 and Fig. 1). Besides the change in individual SCNT particles, DOM-preloading might reduce the aggregation of CNT particles and enhance their repulsion. Enhanced dispersion of SCNT by DOM was visually observed in our DOM-preloading experiments. This may have two effects. First, as mentioned above, the interstitial spacing between neighboring nanotubes may sequester PHE and contribute partially to PHE sorption. Reduction in SCNT aggregation led to a loss of this sorption mechanism. On the other hand, reduction in aggregation resulted in more surface sites being exposed,18,41 which should have favored the sorption of PHE. Hence, the apparent impact on PHE sorption on SCNT by DOM preloading is an integrated result of the above multiple processes. In the present study, the increase in sorption of PHE due to the newly exposed sites was much weaker than the decrease in sorption. Our observation is consistent with the previous findings that coating DOM on black char strongly reduced its affinity for hydrophobic organic molecules.42 Kilduff and Wigton also reported that sorption of trichloroethylene by humic-preloaded activated carbon was inhibited.12
Sorption of PHE on aged DOMbulk–SCNT
Although sorption kinetics have shown that apparent sorption equilibrium occurred in less than 2 days, increasing the contact time made SCNT sorb more DOM molecules, as indicated by the increased Q values of aged DOMbulk–SCNT compared to that for fresh DOMbulk–SCNT (Table 3). According to this, we have expected that the sorption capacity and nonlinearity of PHE on aged DOM–SCNT should have decreased. However, PHE sorption on the aged DOM–SCNT increased significantly as compared to that on the fresh DOM–SCNT (Fig. 2), with the log KF value increasing from 6.75 to 7.14. The nonlinearity of the sorption isotherm increased simultaneously, with n decreasing from 0.402 to 0.283 (Table 3). To explain this, the properties were compared between the aged and fresh DOMbulk–SCNT. Micropore volume (Fig. 1, pore width within 12 Å) in aged DOMbulk–SCNT, which is thought to contribute primarily to the sorption of organic chemicals like PHE (169.5 Å3), increased slightly compared to that of the fresh DOMbulk–SCNT (Fig. 1). Bulk O content of fresh and aged DOMbulk–SCNT was 3.53 and 3.67, respectively; whereas the corresponding surface O content was 19.2 and 17.3 (Table 1). These different variation tendencies of O content in bulk and surface of DOM-preloaded SCNT suggested that DOM molecules that contained O atoms had transferred from the external surface to interstitial channels in the SCNT aggregate, making the sorption sites restore their heterogeneity and become more available for PHE molecules.Sorption of PHE on SCNT preloaded with DOM fractions with different MWs
The interaction of SCNT and DOM fractions of different MWs differed a lot (Table 3), with the sorption amount following the order: DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (Q = 34.6 mg C g−1) > DOM1000–14
000 (Q = 34.6 mg C g−1) > DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (Q = 22.2 mg C g−1) > DOM<1000 (Q = 11.8 mg C g−1). Though the most was loaded onto SCNT for DOM>14
000 (Q = 22.2 mg C g−1) > DOM<1000 (Q = 11.8 mg C g−1). Though the most was loaded onto SCNT for DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, the O content of DOM>14
000, the O content of DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT was the least (0.96%) compared to those of DOM1000–14
000–SCNT was the least (0.96%) compared to those of DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT (2.29%) and DOM<1000–SCNT (5.04%) (Table 1). This is due to the differences in the chemical composition of the DOM fractions with different MWs. Our parallel study found that C content increased and O content decreased with increasing DOM MW, indicating that DOM>14
000–SCNT (2.29%) and DOM<1000–SCNT (5.04%) (Table 1). This is due to the differences in the chemical composition of the DOM fractions with different MWs. Our parallel study found that C content increased and O content decreased with increasing DOM MW, indicating that DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was less hydrophilic followed by DOM1000–14
000 was less hydrophilic followed by DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and DOM<1000.43 The difference in the polarity of DOM fractions could explain the difference in their affinity to SCNT. Thus, the preloading of DOM>14
000 and DOM<1000.43 The difference in the polarity of DOM fractions could explain the difference in their affinity to SCNT. Thus, the preloading of DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 made SCNT more hydrophobic than the other DOM fractions did, as indicated by the increase in polar index [(O + N)/C] from 0.032 for DOM>14
000 made SCNT more hydrophobic than the other DOM fractions did, as indicated by the increase in polar index [(O + N)/C] from 0.032 for DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT to 0.076 for DOM<1000–SCNT. As has been discussed above, the absorbance of the different DOM fractions at 254 nm followed the order: DOM>14
000–SCNT to 0.076 for DOM<1000–SCNT. As has been discussed above, the absorbance of the different DOM fractions at 254 nm followed the order: DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 > DOM1000–14
000 > DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 > DOM<1000 (Table 2), suggesting that DOM>14
000 > DOM<1000 (Table 2), suggesting that DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was more aromatic than DOM1000–14
000 was more aromatic than DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and DOM<1000. As π–π interactions exist between SCNT and DOM, and this could also explain why SCNT had a stronger interaction with DOM>14
000 and DOM<1000. As π–π interactions exist between SCNT and DOM, and this could also explain why SCNT had a stronger interaction with DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 than with DOM1000–14
000 than with DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and DOM<1000. Hence, we attribute the strongest sorption of DOM>14
000 and DOM<1000. Hence, we attribute the strongest sorption of DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 on SCNT to hydrophobic and π–π interactions. This result is consistent with the previous reports showing that hydrophobic and π–π interactions were responsible for DOM sorption by activated carbon and multi-walled carbon nanotubes.44,45 We also observed that the loading of DOM>14
000 on SCNT to hydrophobic and π–π interactions. This result is consistent with the previous reports showing that hydrophobic and π–π interactions were responsible for DOM sorption by activated carbon and multi-walled carbon nanotubes.44,45 We also observed that the loading of DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 more significantly reduced the aggregation degree of SCNT particles than DOM<1000 and DOM1000–14
000 more significantly reduced the aggregation degree of SCNT particles than DOM<1000 and DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 did, as indicated by the color of the SCNT suspensions after standing. This may lead to an enhancement of repulsion and increasing the number of sorption sites on the SCNT. Therefore, the sorption of PHE on SCNT preloaded with different DOM MW fractions was the result of the balance of these two opposite effects.
000 did, as indicated by the color of the SCNT suspensions after standing. This may lead to an enhancement of repulsion and increasing the number of sorption sites on the SCNT. Therefore, the sorption of PHE on SCNT preloaded with different DOM MW fractions was the result of the balance of these two opposite effects.
The loading of all the three DOM fractions with different MWs inhibited PHE sorption on SCNT; however, there were notable differences in the sorption of PHE on SCNT loaded with DOM fractions with different MWs (Fig. 3, Table 3). DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT showed the weakest sorption capacity, with log KF decreasing from 7.34 for the original SCNT to 4.57; whereas the log KF for DOM1000–14
000–SCNT showed the weakest sorption capacity, with log KF decreasing from 7.34 for the original SCNT to 4.57; whereas the log KF for DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT and DOM<1000–SCNT was 7.05 and 7.22, respectively, which did not differ a lot from the value for the original SCNT. Combining the changes in SCNT properties, the most negative effect of DOM>14
000–SCNT and DOM<1000–SCNT was 7.05 and 7.22, respectively, which did not differ a lot from the value for the original SCNT. Combining the changes in SCNT properties, the most negative effect of DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 on PHE sorption was ascribed to the occupying of sorption sites and blockage of micropores of SCNT by DOM>14
000 on PHE sorption was ascribed to the occupying of sorption sites and blockage of micropores of SCNT by DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 due to its greater sorbed amount and larger MW. The log KF of DOM>14
000 due to its greater sorbed amount and larger MW. The log KF of DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT (4.57 in Table 3) is similar to the log KDOM of DOM>14
000–SCNT (4.57 in Table 3) is similar to the log KDOM of DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (4.50 in Table 2), suggesting that the SCNT was completely occupied by DOM>14
000 (4.50 in Table 2), suggesting that the SCNT was completely occupied by DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and the sorption sites of SCNT were inaccessible to PHE. The Freundlich linearity sorption index n of PHE on DOM>14
000, and the sorption sites of SCNT were inaccessible to PHE. The Freundlich linearity sorption index n of PHE on DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT is 1.093, indicating a linear sorption. This confirms that the highly heterogeneous sorption sites of SCNT with high energy were almost completely covered by the DOM>14
000–SCNT is 1.093, indicating a linear sorption. This confirms that the highly heterogeneous sorption sites of SCNT with high energy were almost completely covered by the DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 molecules. Though the loading of DOM1000–14
000 molecules. Though the loading of DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and DOM<1000 makes SCNT more polar, which can partially explain the reduction in PHE sorption: most of the sorption sites of SCNT are still accessible to PHE due to the lesser sorbed amount and smaller MWs of these two fractions. Hence, the reduction in PHE sorption is very limited.
000 and DOM<1000 makes SCNT more polar, which can partially explain the reduction in PHE sorption: most of the sorption sites of SCNT are still accessible to PHE due to the lesser sorbed amount and smaller MWs of these two fractions. Hence, the reduction in PHE sorption is very limited.
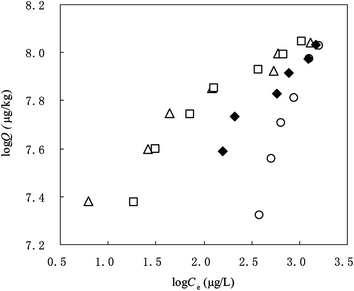 | ||
Fig. 3 Effect of DOM fractions of different molecular weights on the sorption of PHE as compared to bulk DOM. Fresh DOMbulk–SCNT (◆); DOM<1000–SCNT (△); DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT (□); DOM>14 000–SCNT (□); DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–SCNT (○). 000–SCNT (○). | ||
There was no significant correlation between the sorption capacity of PHE on different SCNT and their structural parameters, such as surface area, micropore volume, surface and bulk polarity among DOM-preloaded SCNT (data not shown). This confirms that PHE sorption by SCNT was determined by multiple factors and no single SCNT characteristic controls PHE sorption. In addition, the surface area and micropore volume measured from nitrogen sorption–desorption isotherms at 77 K may be underestimated. Yang et al. reported that the adsorbed volume capacity of PHE on SCNT was greater than both the monolayer adsorption volume capacity of N2 and micropore volume of SCNT.4 Pignatello et al. showed that N2 molecules were unable to access micropores after condensation of humic substances on black carbon surface.13
Conclusions
DOM sorption on SCNT occurred through both surface adsorption and micropore blocking, which reduced the sorption of PHE on DOM-preloaded SCNT by occupying sorption sites and blockage of micropores. Both the chemical composition and steric conformation of DOM were found to influence its interaction with SCNT. The DOM fraction with larger MW (DOM>14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) showed stronger interaction with SCNT and a greater binding capacity of PHE than smaller ones (DOM<1000 and DOM1000–14
000) showed stronger interaction with SCNT and a greater binding capacity of PHE than smaller ones (DOM<1000 and DOM1000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), leading to the strongest suppression of sorption of PHE on DOM-preloaded SCNT. Increasing the contact time between DOM and SCNT from 2 days to 20 days enhanced the sorption capacity and increased the sorption nonlinearity of PHE on DOM-preloaded SCNT due to the transferring of DOM molecules from the external surface to interstitial channels trapped in SCNT, making the sorption sites restore their heterogeneity and be more available for PHE molecules. In addition, sorption of PHE by SCNT was determined by multiple factors and no single SCNT characteristic controls PHE sorption.
000), leading to the strongest suppression of sorption of PHE on DOM-preloaded SCNT. Increasing the contact time between DOM and SCNT from 2 days to 20 days enhanced the sorption capacity and increased the sorption nonlinearity of PHE on DOM-preloaded SCNT due to the transferring of DOM molecules from the external surface to interstitial channels trapped in SCNT, making the sorption sites restore their heterogeneity and be more available for PHE molecules. In addition, sorption of PHE by SCNT was determined by multiple factors and no single SCNT characteristic controls PHE sorption.
Acknowledgements
This study was supported by Natural Science Foundation of China (no. 41073087 and 41225014) and by the Fundamental Research Funds for the Central Universities in China.Notes and references
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- R. H. Baughman, A. A. Zakhidov and W. A. de Heer, Science, 2002, 297, 787–792 CrossRef CAS.
- T. Masciangioli and W. X. Zhang, Environ. Sci. Technol., 2003, 37, 102–108 CrossRef.
- K. Yang, L. Z. Zhu and B. S. Xing, Environ. Sci. Technol., 2006, 40, 1855–1861 CrossRef CAS.
- S. Gotovac, J. Honda, Y. Hattori, K. Takahashi, H. Kanoh and K. Kaneko, Nano Lett., 2007, 7, 583–587 CrossRef CAS.
- E. J. Petersen, R. A. Pinto and P. F. Landrum, Environ. Sci. Technol., 2009, 43, 4181–4187 CrossRef CAS.
- E. J. Petersen, Q. G. Huang and W. J. Weber, Environ. Health Perspect., 2008, 116, 496–500 CAS.
- B. Pan and B. S. Xing, Environ. Sci. Technol., 2008, 42, 9005–9013 CrossRef CAS.
- K. Yang, Q. Jing and W. Wu, Environ. Sci. Technol., 2010, 44, 681–687 CrossRef CAS.
- E. A. Ghabbour and G. Davies, Humic Substances: Versatile Components of Plants, Soil and Water, Royal Society of Chemistry, Cambridge, UK, 2000 Search PubMed.
- K. Kalbitz, S. Solinger, J. H. Park, B. Michalzik and E. Matzner, Soil Sci., 2000, 165, 277–304 CrossRef CAS.
- J. E. Kilduff and A. Wigton, Environ. Sci. Technol., 1999, 33, 250–256 CrossRef CAS.
- J. J. Pignatello, S. Kwon and Y. F. Lu, Environ. Sci. Technol., 2006, 40, 7757–7763 CrossRef CAS.
- E. M. Murphy, J. M. Zachara, S. C. Smith and J. L. Philips, Sci. Total Environ., 1992, 117–118, 413–423 CrossRef CAS.
- K. H. Kang, H. S. Shin and H. Park, Water Res., 2002, 36, 4023–4032 CrossRef CAS.
- Y. P. Chin, G. R. Aiken and K. M. Danielsen, Environ. Sci. Technol., 1997, 31, 1630–1635 CrossRef CAS.
- G. Chen, C. Lin and L. Chen, Chemosphere, 2010, 79, 1046–1055 CrossRef CAS.
- H. Hyung, J. D. Fortner, J. B. Hughes and J. H. Kim, Environ. Sci. Technol., 2007, 41, 179–184 CrossRef CAS.
- H. Hyung and J. H. Kim, Environ. Sci. Technol., 2008, 42, 4416–4421 CrossRef CAS.
- S. J. Zhang, T. Shao, S. Sule and B. Kaplan, Water Res., 2010, 44, 2067–2074 CrossRef CAS.
- X. L. Wang, J. L. Lu and B. S. Xing, Environ. Sci. Technol., 2008, 42, 3207–3212 CrossRef CAS.
- M. Alexander, Environ. Sci. Technol., 2000, 34, 4259–4264 CrossRef CAS.
- H. W. Sun, T. Masafumi, I. Michihiko and F. Masanori, Water Res., 2003, 37, 2960–2968 CrossRef CAS.
- J. G. Li, H. W. Sun and Y. Zhang, Soil Sediment Contam., 2007, 16, 1–9 CrossRef.
- H. W. Sun and J. G. Li, Water, Air, Soil Pollut., 2005, 166, 353–365 CrossRef CAS.
- W. Zhang and H. W. Sun, Soil Sediment Contam., 2012, 21, 407–418 CrossRef CAS.
- K. Y. Cheng and J. W. C. Wong, Chemosphere, 2006, 62, 1907–1916 CrossRef CAS.
- P. I. Ravikovitch, G. L. Haller and A. V. Neimark, Adv. Colloid Interface Sci., 1998, 76–77, 203–226 CrossRef CAS.
- C. H. Cheng, J. Lehmann, J. E. Thies, S. D. Burton and M. H. Engelhard, Org. Geochem., 2006, 37, 1477–1488 CrossRef CAS.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Stern–Volmer, Plenum Press, New York, 2006 Search PubMed.
- W. J. Braida, J. J. Pignatello, Y. Lu, P. I. Ravikovitch, A. V. Neimark and B. S. Xing, Environ. Sci. Technol., 2003, 37, 409–417 CrossRef CAS.
- T. D. Gauthier, W. R. Seitz and C. L. Grant, Environ. Sci. Technol., 1987, 21, 243–248 CrossRef CAS.
- D. Zhu, S. Hyun and J. J. Pignatello, Environ. Sci. Technol., 2004, 38, 4361–4368 CrossRef CAS.
- D. J. Dryer, G. V. Korshin and M. Fabbricino, Environ. Sci. Technol., 2008, 42, 6644–6649 CrossRef CAS.
- J. L. Weishaar, G. R. Aiken and B. A. Bergamaschi, Environ. Sci. Technol., 2003, 37, 4702–4708 CrossRef CAS.
- T. Polubesova, N. M. Sherman and B. Chefetz, Environ. Sci. Technol., 2007, 41, 5389–5394 CrossRef CAS.
- S. Kang and B. S. Xing, Environ. Sci. Technol., 2005, 39, 134–140 CrossRef CAS.
- G. Chen, C. Lin and L. Chen, Chemosphere, 2010, 79, 1046–1055 CrossRef CAS.
- M. L. Delgado, L. Wu and J. Gan, Environ. Sci. Technol., 2010, 44, 8473–8478 CrossRef.
- S. J. Zhang, T. Shao, S. S. K. Bekarogle and T. Karanfil, Environ. Sci. Technol., 2009, 43, 5719–5725 CrossRef CAS.
- B. Pan and B. S. Xing, Environ. Sci. Technol., 2008, 42, 9005–9013 CrossRef CAS.
- S. Kwon and J. J. Pignatello, Environ. Sci. Technol., 2005, 39, 7932–7939 CrossRef CAS.
- J. Z. Wu, H. W. Sun, C. P. Wang and Y. H. Li, Chem. Res. Chin. Univ., 2012, 28, 624–630 CAS.
- J. C. Crittenden, S. Sanongraj, J. L. Bulloch, D. W. Hand, T. N. Rogers, T. F. Speth and M. Ulmer, Environ. Sci. Technol., 1999, 33, 2926–2933 CrossRef CAS.
- X. L. Wang, S. Tao and B. S. Xing, Environ. Sci. Technol., 2009, 43, 6214–6219 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
