Potential environmental implications of nano-enabled medical applications: critical review
Indrani Mahapatraa, J. Clarka, Peter J. Dobsonb, Richard Owenc and Jamie R. Lead*ad
aSchool of Geography, Earth and Environmental Sciences, University of Birmingham, Birmingham, B15 2TT, UK. E-mail: j.r.lead@bham.ac.uk
bBegbroke Science Park, University of Oxford, Begbroke, OX5 1PF, UK
cBusiness School, University of Exeter, Exeter, EX4 4PU, UK
dDepartment of Environmental Health Sciences, Arnold School of Public Health, University of South Carolina, Columbia, South Carolina 29208, USA
First published on 11th December 2012
Abstract
The application of nanotechnology and nanoscience for medical purposes is anticipated to make significant contributions to enhance human health in the coming decades. However, the possible future mass production and use of these medical innovations exhibiting novel and multifunctional properties will very likely lead to discharges into the environment giving rise to potentially new environmental hazards and risks. To date, the sources, the release form and environmental fate and exposure of nano-enabled medical products have not been investigated and little or no data exists, although there are a small number of currently approved medical applications and a number in clinical trials. This paper discusses the current technological and regulatory landscape and potential hazards and risks to the environment of nano-enabled medical products, data gaps and gives tentative suggestions relating to possible environmental hotspots.
 Indrani Mahapatra | Indrani Mahapatra has worked in the areas of health risk assessment due to industrial and indoor air pollution, corporate sustainability reporting and assurance, corporate sustainable sourcing strategies, hazardous waste issues and management, and implementation of renewable energy projects at community level. She has a Master's in Environment Management and is currently pursuing Ph.D. at University of Birmingham, U.K. |
 J. Clark | J. Clark is Senior Lecturer in Geography at University of Birmingham. His research focuses on political geographies of Europe, the political governance of natural resources, and the interrelations between political geography and political science. He has published widely on political geography and environmental governance and has research monographs published by Oxford University Press and Routledge-Cavendish. |
 Peter J. Dobson | Peter J. Dobson currently holds the position of Academic Director at Begbroke Science Park, University of Oxford and is the Chief Strategic Advisor on Nanotechnology to the Research Councils in the UK. His research interests primarily lies in the areas of most aspects of nanotechnology with focus on applications to medicine. He founded two spin-off two companies, Oxonica plc and Oxford Biosensors Ltd., in the decade of 1990–2000. He was named the Innovator of the Year in 2005 by the Small Times Magazine. He advises several corporate and government organisations on nanotechnology and knowledge transfer. |
 Richard Owen | Richard Owen holds the Chair in Responsible Innovation at the University of Exeter Business School, where he is Director of Postgraduate Research. Richard's research involves understanding the responsible emergence of innovation and new technologies in democratic society, with a particular emphasis on governance. Richard is a member of EPSRC's Strategic Advisory Network where he advises on societal and ethical issues. |
 Jamie R. Lead | Jamie Lead is the Chair in Environmental Nanoscience and Risk and Director of the SmartState Center for Environmental Nanoscience and Risk for at University of South Carolina, U.S.A and adjunct Professor of Environmental Nanoscience and Founder and Co-Director of the Facility for Environmental Nanoscience and Characterisation at the University of Birmingham, UK. His research interests cover nanoscale phenomena in environmental systems, including natural and manufactured materials, their interactions, chemistry, fate and effects, risk and regulation. |
Environmental impactThe application of nanotechnology in healthcare is emerging as an important strategic area of research and it is likely to make significant contributions to improving healthcare in coming decades. However, the likely emergence of large scale nanomedicine production is likely to lead to significant environmental exposure of bioactive and potentially toxic nanomaterials. The bioactive nature of these materials might lead to enhanced risk e.g. in enhanced ‘Trojan Horse’ type mechanisms in drug delivery vehicles. By controlling exposure and/or by designing drugs effectively with environmental concerns in mind will maximize benefit and minimize risk. In order to prompt a discussion of this emerging area, this review presents the current status of nanomedicine developments, brings together the knowledge from the field of environment impact research of pharmaceuticals and nanomaterials and the existing regulatory guidelines to identify knowledge gaps and uncertainties which need to be addressed to ensure a safe, sustainable and effective technology is produced and used. |
Introduction
Nanotechnology is a large, convergent, multidisciplinary enabling field that is rapidly growing.1,2 Size dependent properties due to spatial confinement along with their high specific surface area and surface energies, give nanomaterials (NMs) unique, size dependent and tuneable electronic and optical behaviour. Such properties can be exploited in a range of applications including in nanomedicine,† which also utilise other nanoscale properties such as their ability to interact with the cellular machinery and potential for targeted cellular and sub-cellular compartmentalization.3,4 Applications of nanotechnology in healthcare can be as platforms for drug delivery, for enhancing outcomes of various treatment types such as photothermal therapy, thermal ablation and hyperthermia treatments, in vitro detection of biomarkers, imaging, and as combined drug and diagnostics devices – “theranostics” (for more details, see ref. 5–8).Applications of nanotechnology can increase the efficacy of therapeutics by providing solutions to the traditional problems associated with pharmaceutical solubility,9 limiting systemic toxicities,‡10 bioavailability,12 immunocompatibility13 and cellular uptake.14 Nanotechnology based therapeutics can be produced either through top down processes such as milling, high pressurised homogenisation, etching, lithography and other methods, or through bottom up processes, such as chemical synthesis both for inorganic NMs or complex polymeric designs to act as therapeutic agents or carriers of therapeutic agents.§ These nanocarriers¶ can be used to encapsulate and/or conjugate therapeutic agents like pDNA, siRNA and traditional chemotherapeutics (e.g., paclitaxel, doxorubicin, irinotecan)9,15–17 which have had limited applications in clinical settings due to biological degradation, high systemic toxicities or other factors. Additionally, functionalization of the surface of these NMs using aptamers, antibodies, and cell receptor recognising proteins helps to achieve targeted drug delivery.
Nano-enabled in vitro diagnostic devices can help in the detection and early diagnosis of diseases like cancer (e.g., see a recent review, ref. 18) with increased sensitivity19 and in a non-invasive manner.20 In addition to in vitro diagnostics, nanotechnology is being used to develop new MRI contrast agents for in vivo diagnostics for contrast enhancement. For instance, a ten-fold increase in MRI contrast in comparison to clinical Gd(III) contrast agents e.g. Gd–DTPA and Gd–DOTA was observed by using nanodiamond conjugated to Gadolinium(III) [Gd(III)–ND].21 Due to these advantages, research and development investment of nanotechnology and nanomedicine has increased rapidly over the last 10 years, and it is likely that production and use of nano-enabled medical applications will rise in the coming years.
A recent, detailed study has estimated that there are currently 247 nanomedical products that have been approved and are in the market or are in early or late stages of clinical trials, with many more in development.22 A market research firm, BCC research, has estimated that the nanomedical global market value increased from USD 53 billion in 2009 to USD 72.8 billion in 2011. Additionally, the firm has projected a compound annual growth rate in the global market for nanomedicines to be 12.5%, between the years 2011 and 2016, with largest growth in the therapeutic area of oncology and disorders of the central nervous system (CNS).23,24 The applications currently in clinical development belong to the categories of liposomal formulations, polymer–protein and polymer–drug conjugates, micelles, antibody–drug conjugates, dendrimers, metal and metal oxide NMs. Please see Table 1 exemplifying each of these categories.
| Details | Nanocomponent (size) | Drug or device | Reference |
|---|---|---|---|
| a Though the exact sizes are not known here, superparamagnetic and ultrasuperparamagnetic iron oxide nanoparticles are mainly made of γ Fe2O3 and Fe3O4 and have core diameter <25 nm (see ref. 89) and 5–12 nm (cited in ref. 90) respectively. These magnetic particles are coated with silica, dextran, etc. for specific applications. | |||
| Diagnosis of lung cancer, breast, colon cancer, gastric lesions, etc. and multiple sclerosis from biomarkers in exhaled breath | Gold NPs (5 nm), SWCNT | Device | 20 and 52–56 |
| Alkylated polyethylenimine (PEI) nanoparticles (NPs) incorporated in composite resin dental restorative materials to reduce bacterial infections and dental caries | PEI NPs | Device | 57 |
| Obturators lined with silicon incorporated with quaternary ammonium polyethylenimine (PEI) NPs for managing post surgery infection in head and neck cancer | PEI NPs | Device | 58 |
| Liposomal formulation of two anti-leishmaniasis drugs for treatment of leishmaniasis | Liposome | Drug | 59 |
| Liposome formulation of combination anticancer drugs: | Liposome | Drug | 60 |
| • cytarabine and daunorubicin for acute myeloid leukemia | |||
| • irinotecan and floxuridine for colorectal cancer | |||
| Chemotherapeutic drug (paclitaxel) associated albumin nanoparticles used as last line treatment for metastatic breast cancer | Albumin NPs (130 nm) | Drug | 61 |
| Chemotherapeutic drug doxorubicin associated with polyethylene glycol (PEG) liposomes | PEGylated liposomes | Drug | 62 |
| • PEGylated interferons + drug for treatment of hepatitis C | PEGylated proteins | Drug | Summarised in ref. 5, see also ref. 63 |
| • methoxy PEGylated erythropoietin receptor activators for anaemic patients with chronic kidney disease or myeloma | |||
| • monoclonal antibody directed against TNF-α with a PEG tail for treatment of rheumatoid arthritis and Crohn's disease | |||
| Thermosensitive liposome in combination with doxorubicin and radiofrequency ablation or hyperthermia or high intensity focussed ultrasound for treatment of breast cancer, colorectal liver and bone metastases | Thermosensitive liposome | Drug | 64–66 |
| Chemotherapeutic drug paclitaxel in polymeric micelles for treatment of breast cancer, lung cancer, advanced ovarian cancer | PEG and poly(D,L-lactic acid) polymeric micelle | Drug | 41 and 67 |
| Anticancer drugs paclitaxel, cisplatin and oxaliplatin in polymeric micelles | Polymeric micelle | Drug | 68 |
| Radioactive Yttrium-90 conjugated with monoclonal antibody directed against CD40 antigen of B cells for treatment of relapsed or refractory, low-grade, follicular or B-cell non Hodgkin's lymphoma (nuclear medicine or biotech medicine) | — | Drug | Summarised in ref. 5 |
| Circulating tumour cells test/assay for diagnosis of metastatic breast, colorectal or prostate cancer: ferro-magnetic NPs labelled with monoclonal antibodies corresponding to specific antigen expressed in cancer cells | Magnetic nanoparticles | Device | 69 |
| Infectious disease tests, cardiac tests, etc. using gold NPs functionalised with specific biomolecules like oligonucleotides, antibodies | Gold NPs (13–20 nm) | Device | 70 |
| Fifth generation PAMAM dendrimers used for immunoassays as confirmation test for the occurrence of myocardial ischemia | Dendrimers | Device | 71 and 72 |
| Mannosylated polyethylenimine (PEI) polymers containing plasmid DNA as therapeutic vaccine for HIV/AIDS | PEI polymers | Device (the delivery patch) | 17 and 73 |
| A fourth generation L-lysine dendrimer with naphthalene disulfonic acid surface groups in a gel base for treatment of bacterial vaginosis, coating of condoms for sexually transmitted diseases, etc. | Lysine dendrimer | Drug | 74 and 75 |
| PEGylated colloidal gold bound TNF for treatment of advanced solid tumors | Gold NPs (∼27 nm) | Drug | 76 |
| Gold coated silica nanoparticles for thermal ablation of refractory head and neck cancer | Au–Si NP (150 nm) | Device | 77 |
| Bio bar code assay: it has a magnetic micro particle plate functionalised with target specific recognition agents (in this case monoclonal anti-PSA antibodies) and the second component is AuNP probes, average 30 nm size, carrying hundreds of DNA strands and PSA strands leading to amplification of the signal. Detection of prostate cancer relapse post-prostatectomy | AuNP probes (average 30 nm) | Device | 19 |
| Superparamagnetic iron oxide nanoparticles as contrast agents for delineating the bowel, glioblastoma multiforme, lymph nodes in prostate cancer | Iron oxide NPsa | Drug | 78–80 |
| Iron oxide nanoparticles coated with polyglucose sorbitol carboxymethyl ether for treatment of iron deficiency anaemia in chronic kidney disease | Iron oxide NPs (30 nm with coating) | Drug | 81 and 82 |
| SPIONs (superparamagnetic iron oxide NPs) for tracking of inflammatory (mononuclear) cells | Iron oxide NPsa | Drug | 83 |
| SPIONS with aminosilane coating for treatment of multiforme glioblastoma (an aggressive brain cancer) in combination with application of external magnetic field | ∼15 nm iron oxide NPs | Device | 84 |
| Silver nanoparticles associated with wound dressings | Nanocrystalline silver | Device | 85 |
| Nano silver hand gel | Nanosilver | Drug | 86 |
| Nano silver impregnated activated carbon wound dressing | Nanosilver | Drug and device combination | 87 |
| Nano silver coated latex central venous catheters to reduce chances of infections | Nanosilver | Device | 88 |
Only a few earlier publications to date have discussed potential issues related to nanotoxicity of nanomedicine and the majority of these have drawn extensively upon from the literature relating specifically to health impacts of fine particulates.25–28 Occupational exposure from nanomedical applications and ways of monitoring was discussed by Murashov.29 To the best of our knowledge, Baun and Hansen30 were the first to publish their perspective specifically regarding nanomedicine and the environment. These early statements of problem formulation strongly suggest the need for research on ecotoxicology, environmental exposures and risks. Subsequently, a handful of empirical studies have been published in this area (see Table 3). Additionally, the European Medicines Agency in 2010 conducted a workshop on nanomedicines and had a discussion on specific methodological issues for environmental risk assessment of nanomedicines.31 However, concrete steps are yet to be taken in this area. The nascent stage of the nanomedicine field, the assumption that more targeted medicines will reduce dosages, and that safety issues are covered in the stringent and well controlled development pathway of therapeutics are all reasons for this lack of action in this area. However, assumptions for pharmaceuticals have previously been made, e.g., that their small amount compared to other chemicals would not pose any risk and that pharmaceutical industries follow good manufacturing practices, but the current environmental research focusing on pharmaceuticals indicates that problems are arising.32 In addition, a number of other issues are pertinent: (1) it is appropriate to consider these issues at an early stage before development is ‘locked’ in by economic and other factors, (2) targeting will increase solubility and bioavailability in the environment even though medical dosages used might be low and (3) stringent regulatory controls are evident in the human area but less so for environmental exposure and behaviour. Therefore, it is appropriate to critically review the available information in this area, understand the knowledge gaps and address this potential issue at an early stage.
In this review, we discuss (i) the existing status of definitions of nanomedicine and a few relevant examples of nanomedicines under development, (ii) the current status of knowledge regarding environmental hazards and risks of conventional pharmaceuticals and nanomaterials with a view to understanding the environmental risks of nanomedicines, (iii) existing regulatory framework for pharmaceuticals for human use and medical devices and its coverage in the context of nanomedical applications and (iv) uncertainties and knowledge gaps.
This paper focuses on nanomedical applications which are marketed or are in advanced stages of clinical development. The nanomedical applications that find mention in the text belong to the category of therapeutics and diagnostics. Developments and clinical applications in regenerative nanomedicine are excluded in this review. There are a large number of proof-of-concept works related to nanocarriers based on fullerenes, carbon nanotubes, nanodiamonds, graphene, etc. which have immense potential for a myriad of applications in drug delivery. However, to the best of the knowledge of the authors, disease treatment strategies which have shown potential either in vitro or in proof-of-concept experiments using these materials are yet to move beyond pre-clinical stage, therefore, this paper doesn't focus on these materials. A lot of ecotoxicity research has been conducted on quantum dots (CdTe/CdSe as core), but these ecotoxicity studies are not being reviewed here because the use of quantum dots is not directly related to clinical applications. Of course, quantum dots are used in life sciences for cell imaging, tracking and elucidating biochemical mechanisms, hence they are important for medical research and innovation, but they are beyond the scope of this review. There is only one in vitro diagnostic device in the clinical trial phase which possibly uses SWCNT (single walled carbon nanotube), therefore, this paper also doesn't focus on the ecotoxicity research conducted on carbon nanotubes. The governance and regulatory framework explained in the text is EU centric and the paper refrains from touching upon other regulatory guidelines existing beyond the EU, unless exceptionally necessary to explain a particular section.
Nanotechnology in healthcare applications
The aim of nanomedicine is the comprehensive monitoring, repair and improvement of all human biological systems, working from the molecular level using engineered devices and nanostructures to achieve medical benefit. The concept includes nanoscale active components or objects ranging in size from 1 nm to 100s of nm.33 The European Commission's recommendation on the definition of nanomaterial creates a special case for pharmaceuticals and medical devices. The recommendation mentions that the proposed size cut-off value of 1 to 100 nm, with more than 50% of particles by number in this range, should not prejudice pharmaceutical and the medical devices sectors.34 Thus, though mainstream nanotechnology explores particles between 1 and 100 nm in diameter, for nanomedicine, the size of the nanomaterials might in totality exceed 100 nm. The US FDA is yet to define nanomaterial or nanomedicine. Therefore, a widely accepted and clear definition is essential for appropriate regulation of nanomedicine but is not in place as yet due to novelty and multidisciplinarity of the field and broad range of applications that can be developed in the context of other convergent technologies. Nevertheless, rapid progress towards a definition is essential for regulatory purposes.Nanomaterials are broadly classified into two categories based on the type of interactions exploited for designing nanomedicine. These two categories are ‘hard’ and ‘soft’. Hard nanomaterials are metal and metal oxide nanoparticles, fullerenes, etc. formed via ionic or covalent bonds, whereas ‘soft’ nanocarriers use weak interactions.35 The key types of hard nanomaterials currently being investigated for clinical applications and in the market are AgNPs, AuNPs and FexOy NPs. AgNPs has an antimicrobial effect and finds it uses in bandages for burn injuries, catheters and others. The potential for AuNPs in biomedical applications is due to its surface chemistry which makes it amenable to coating and functionalising with various targeting ligands, relative biocompatibility and photo-optical properties.36 FexOy NPs are used because at nanoscale iron oxide exhibits super-paramagnetism and can be used as contrast agents as well as for hyperthermia treatments for cancer.37,38 Formulating metal and metal oxide NMs of different sizes and surface coating can help develop various biomedical applications.
The key types of ‘soft’ nanocarriers in the market or in advanced stages of development are:
• Liposomes are spherical vesicles composed of amphiphilic phospholipids and cholesterol, which self-assemble into bilayers to encapsulate an aqueous interior. They are one of the oldest and widely recognised nanocarriers and they can serve as a platform for delivery of both hydrophilic and hydrophobic therapeutic agents (see ref. 39 and references therein). Liposomes vary greatly in size from 25 nm to 5000 nm (ref. 40) and can be classified in terms of composition and mechanism of intracellular delivery into five types: conventional liposomes, pH-sensitive liposomes, cationic liposomes, immunoliposomes, and long-circulating or PEGylated liposomes.41
• Dendrimers are synthetic polymers (e.g. polyamidoamine, polypropylene imine) in which the atoms are arranged in many branches and sub-branches radiating out from a central core and sizes can be in the nm to μm range. Dendrimers can be categorized based on the number of the branches they possess which are called generations (G1, G2, G3, etc.). Dendrimers are being identified as ideal nanoscale drug delivery systems due their capacity to carry multiple modalities (therapeutic, imaging, and targeting). A plethora of different dendrimer compositions and chemical surface modifications can be synthesized and dendrimers can themselves act as therapeutic agents (for further details, see ref. 42–44).
• Micelles are nanosized, spherical colloidal particles with a hydrophobic interior (core) and a hydrophilic exterior (shell). Drugs or contrast agents may be entrapped within the hydrophobic core or linked covalently to the surface of micelles (for examples, see ref. 45).
Nanocarrier design requirements: The key principles governing rational design considerations of nanocarriers are:46,47
• physiologically stable nanocarriers capable of evading the reticuloendothelial system/mononuclear phagocytic system
• amenability to surface functionalisation with targeting moieties such as antibodies and cell penetrating peptides
• ability to cross the biological barriers of the body
• availability of a clearance mechanism that does not harm other organs
• ability to release the drug payload at the required site (delivery can also be designed so that it is modulated by pH or redox changes, enzymatic cleavage of bonds or activated by external stimulus such as electro-magnetic fields)
• biodegradability and biocompatibility, i.e., low or no immunotoxic, genotoxic, mutagenic, reproductive and developmental toxic effect for human beings.
These design features of nanocarriers will have the potential to increase therapeutic efficacy by protecting the therapeutic agents from being physically, chemically, biologically ‘degraded’ before they reach the disease target site and being released at this site. For instance, siRNA has been encapsulated in a 70 nm cyclodextrin based polymer, conjugated with a protein as a targeting ligand and polyethylene glycol (PEG) polymer to promote stability.15 Design strategies can also help in escaping the biological barriers, have better control over drug release profile, increase absorption in tumours tissues, prevent therapeutic agents from interacting with normal cells and hence less systemic toxicities.48–50 If these carriers are designed with the aim to treat CNS disorders, these features can also help the small drug molecules to cross the blood brain barrier and hence improve the therapeutic outcomes.
However, the design features necessary to make efficacious therapeutics might prove to have deleterious impacts on environmental biota and ecological health, when these medicines find their way into the environment. Furthermore, not all design features listed above can be met for development of a particular therapeutic. For example, PEG coated therapeutics, so-called ‘stealth’ nanoparticles are in advanced stages of clinical trials, however it has been found that PEG is not easily biodegradable in the human body (mentioned in ref. 5 and 51). At the same time, more targeted and in some cases reduced dosages might decrease the future environmental burden of conventional pharmaceuticals.
The context of existing pharmaceuticals and environmental risks
The primary focus of drug delivery research in nanomedicine has been to design delivery agents that would have the ability to cross the various biological barriers in the body and deliver therapeutic agents to the target site with the aim to increase therapeutic efficacy. The therapeutic agent might be conventional small molecule drugs which have found limited use in the clinic due to systemic toxic effects or poor solubility. This necessitates the review of the existing scientific literature on conventional pharmaceuticals in the environment. Concerns due to effects of pharmaceutical products (PPs)|| in the environment have been expressed91,92 and this is now an active area of research. PPs from various therapeutic classes have been detected in the range of ng L−1 (and ng g−1) to low μg L−1 (mg kg−1) in different environment compartments, – sewage effluents,93 surface waters,94,95 receiving coastal waters,96 estuaries,97 sediments and soils,98 landfill leachates,99 and to a lower concentration and frequency in groundwater and drinking water sources.100,101 In many cases (see Table 2) concentrations of PPs has shown to exceed the current PECsw** threshold limit value of 0.01 μg L−1 suggested by the EMA.102 PPs have also been found in aquatic biota.32,103,104 The widely detected pharmaceutical products in the environment belong to the therapeutic class of antibiotics, non-steroidal anti-inflammatory drugs, blood lipid lowering agents, sex hormones, central nervous system (CNS) disorder drugs and β-blockers (reviewed and summarized in ref. 105). A few examples of the occurrence of pharmaceuticals and their metabolites in different environmental compartments are presented in Table 2. Spatial, temporal and geographic variations (e.g., ref. 93, 106 and 107) have been shown to occur in the concentrations and type of pharmaceutical products. Fluctuations in the concentrations of pharmaceutical products have also been shown in effluents and receiving water bodies during special episodes, e.g., of disease outbreaks.108,109 In addition to monitoring campaigns, models such as SimpleTreat, LowFlow 2000-WQX, PhATE have been used to predict environmental concentrations in various compartments.110,111| Category | Pharmaceutical product | Concentration | Environmental compartment | Region | Reference |
|---|---|---|---|---|---|
| a Max. Conc.: Maximum Concentration; LOQ: Limit of Quantification; STP: Sewage Treatment Plant; WWTP: Waste Water Treatment Plant. | |||||
| Analgesics/NSAIDs | Acetaminophen | 1.89 μg L−1 (max. conc.) | Groundwater | U.S.A. (California) | 100 |
| 0.08–13.8 μg L−1 | Treated effluents (5 STPs) | Spain | 93 | ||
| 1.4–5.9 μg L−1 | Untreated effluent from 2 hospitals | Italy | 113 | ||
| 0.012–0.058 μg L−1 | STP effluent | Italy | 113 | ||
| Diclofenac | 0.052–1.76 μg L−1 | Effluents (12 municipal WWTPs) | South Korea | 142 | |
| 230 ng L−1 (median values) | WWTP effluents | Spain (Galicia) | 143 | ||
| LOD = 2.95 μg L−1 | Effluents (3 WWTPs) | Ireland (Dublin) | 144 | ||
| 100–131 ng L−1 | STP effluent | Taiwan | 96 | ||
| 53.6 ng L−1 (max. conc.) | Coastal receiving area(6.6 km offshore) | Taiwan | 96 | ||
| Mefenamic acid | LOD = 1.73 μg L−1 | Effluents (3 WWTPs) | Ireland (Dublin) | 144 | |
| 44–392 ng L−1 | Effluents (5 STPs) | South Korea | 145 | ||
| Ibuprofen | 5 μg L−1 (max. mean conc.) | Effluents (5 STPs) | Spain | 93 | |
| 552–1600 ng L−1 | STP effluent | Taiwan | 96 | ||
| 57.1 ng L−1 (max. conc.) | Coastal receiving area (6.6 km offshore) | Taiwan | 96 | ||
| <MDL – 26.6 ng L−1 | Treated drinking water | U.S.A | 101 | ||
96.9–166![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 624 ng L−1 624 ng L−1 | Landfill leachate and inlet of leachate treatment | Norway | 99 | ||
| 120 ng L−1 (max. conc.) | River water | U.S.A.(Washington, D.C.) | 146 | ||
| Beta-blockers | Propranolol | 1–24 ng L−1 | Estuary, harbour | Belgium | 147 |
| 3.18 ng L−1 (max. conc.) | Estuary | Portugal | 107 | ||
| 1.51–2.60 ng g−1 | Sediments of marshy areas | Valencia, Spain | 98 | ||
| 107.4 ± 36 μg kg−1 | Achieved biosolids (collected in year 2001) from 94 WWTPS | U.S.A. | 148 | ||
| Atenolol | 80–293 ng L−1 | Estuary, harbour | Belgium | 147 | |
| 511 ng L−1 (median value) | WWTP effluents | Galicia, Spain | 143 | ||
| 1.1–15 μg L−1 | Effluents (5 STPs) | Spain | 93 | ||
| 261–5911 ng L−1 | Effluents (5 STPs) | South Korea | 145 | ||
| Blood lipid lowering agents | Fenofibrate | 13.20–17.23 ng g−1 | Sediments of marshy areas | Valencia | 98 |
| LOQ = 2.5 ng g−1 | Sludge (3 WWTPs) | Spain | 122 | ||
| Gemfibrozil | 0.15–1.24 μg L−1 | Effluent water | Spain (Valencia) | 149 | |
| 0.08–19.4 μg L−1 | Effluent water | U.S.A. (Texas) | 150 | ||
| 0.11–6.86 μg L−1 | Groundwater below Land application site | U.S.A. (Texas) | 150 | ||
| Estrogens | EE2 (17α-ethinylestradiol) | 2 ng L−1 | Effluents (11 STPs) | UK | 151 |
| Levenogesterol | 11 ng L−1 | Groundwater | France | 152 | |
| Antibiotics | Ciprofloxacin | 1.9 μg L−1 (max. conc.) | Effluents (5 STPs) | Spain | 93 |
| Norfloxacin | 256 ± 64 ng L−1 | Secondary effluent (1 STP) | China (Beijing) | 153 | |
| 7.23 ± 0.22 mg kg−1 | Dewatered sludge (1 STP) | China (Beijing) | 153 | ||
| Ofloxacin | 8.95–12.03 ng g−1 | Sediments of marshy areas | Spain | 98 | |
| 528 ± 89 ng L−1 | Secondary effluent (1 STP) | China (Beijing) | 153 | ||
| 2.8 μg L−1 (max. conc.) | Effluents (5 STPs) | Spain | 93 | ||
| 7.79 ± 0.55 mg kg−1 | Dewatered sludge (1 STP) | China (Beijing) | 153 | ||
| Sulfamethoxazole | 13–96 ng L−1 | Estuary, harbour | Belgium | 147 | |
| 9.14–53.3 ng L−1 | Estuary | Portugal | 107 | ||
| 0.17 μg L−1 (max. conc.) | Groundwater | USA (California) | 100 | ||
| 0.047–0.397 μg L−1 | Effluents (12 municipal WWTP) | South Korea | 142 | ||
| 4.2–485 ng L−1 | Yangtze Estuary | China | 97 | ||
| Lincomycin | 1.06–45.7 μg L−1 | Effluents (12 municipal WWTP) | South Korea | 142 | |
1437–21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 278 ng L−1 278 ng L−1 | Effluents (5 STPs) | South Korea | 145 | ||
| Antineoplastics | Ifosfamide | 4–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 647 ng L−1 (median, 151 ng L−1) 647 ng L−1 (median, 151 ng L−1) | Hospital effluent (21 hospitals) | China | 154 |
| Cyclophosphamide | 6–2000 ng L−1 (median, 100 ng L−1) | Hospital effluent (21 hospitals) | China | 154 | |
| 5-Fluorouracil | 27 ng L−1 (max. conc.) | Hospital effluent | Switzerland | 155 | |
| 0.09–4 μg L−1 (max. mean concentration = 0.8 μg L−1) | Monitoring point where hospital effluent was discharged to the sewage network | France | 156 | ||
| Tamoxifen | 102 ng L−1 (max. conc.) | STP effluent | France | 157 | |
| 26.5 ng L−1 (median conc.) | |||||
| 11 ng L−1 (median conc.) | River | France | 157 | ||
| 120–127 ng L−1 | Yangtze Estuary | China | 97 | ||
| Psychiatric drugs | Fluoxetine | 8–44 ng L−1 | 5 main rivers (Madrid) | Spain | 94 |
| Norfluoxetine (metabolite of fluoxetine) | 41.6 ± 25.1 μg kg−1 | Achieved biosolids (collected in year 2001) from 94 WWTPS | U.S.A | 148 | |
| Diazepam | Upto 80 ng L−1 | Effluents (5 STPs) | Spain | 93 | |
| Pipamperone | 1.4–17.3 ng L−1 | Surface water | Belgium | 158 | |
| Sertraline | 458 ± 168.3 μg kg−1 | Achieved biosolids (collected in year 2001) from 94 WWTPS | U.S.A | 148 | |
| Carbamazepine | 4–321 ng L−1 | Estuary, harbour, sea | Belgium | 147 | |
| 0.37–178 ng L−1 | Estuary | Portugal | 107 | ||
| 1.43–5.77 ng g−1 | Soils of marshy areas | Valencia, Spain | 98 | ||
| 1.81–6.85 ng g−1 | Sediments of marshy areas | Valencia, Spain | 98 | ||
| 2–272 ng L−1 | River (Lopan) | Ukraine | 159 | ||
| 0.208–21 μg L−1 | Effluents (12 municipal WWTPs) | South Korea | 142 | ||
| 3.6 μg L−1 (max. conc.) | Groundwater | U.K. | Cited in ref. 160 | ||
| 0.42 μg L−1 (max. conc.) | Groundwater | U.S.A (California) | 100 | ||
| Upto 6.8 ng L−1 | Treated drinking water | U.S.A | 101 | ||
| 161 ng L−1 (max. conc.) | Stormwater collection system (discharge outfalls) | Canada | 117 | ||
Pharmaceuticals are metabolised and excreted out of the body either unchanged or in conjugated form (e.g. glucuronide, sulphates, glycinate conjugates) and hence the main sources for human pharmaceuticals and their metabolites in the environment have been identified as effluents of waste water treatment plants (WWTPs) from communities112 hospitals113 and pharmaceutical manufacturing facilities.32 Approximately, 28% of the world's population in 2008 was not connected to sewage systems114 and ca. 9% of the wastewater in EU countries is not treated or the waste water treatment systems do not have secondary treatment steps,115 sewage systems are leaky116 or contamination of storm water from waste water exists117 thereby giving rise to the possibility of further environmental contamination. It has been established that the fate, removal and partitioning of these compounds are dependent on the design of the WWTP,118e.g., ibuprofen's removal efficiency was below 25% for a WWTP having primary treatment process compared with a removal efficiency of 90% in a WWTP having secondary treatment.96 Furthermore, a few drugs have been shown to have negative removal percentages in the WWTPs, and it has been suggested that these negative removal percentages might be due to analytical instrumental errors, sampling variations, etc., but, it also gives credence to the hypothesis that the conjugated metabolites may undergo bio-transformation in the environment to form the parent drug (removal efficiencies in WWTPs has been reviewed in ref. 119). PPs can also undergo abiotic transformations in the environmental matrices including photo-transformation and hydrolysis and can be deactivated to ecologically benign molecules or form harmful transformation products. For instance, the photo-transformation of diazepam and its metabolites was recently studied120 and the investigators concluded that diazepam (a widely prescribed antidepressant) would be transformed under conditions present in the environment. However, the photoproducts that were identified had chemical structures similar to identified endocrine disruptors (photolytic and oxidative transformation of drugs has been reviewed in ref. 121).
PPs like ibuprofen, acetaminophen, ciprofloxacin, ketoprofen, etc. have high removal efficiencies in WWTPs which have secondary treatment steps. However, studies have also established that certain PPs (e.g. fenofibrate and anthracyclines from the blood lipid regulator and anticancer therapeutic class respectively) sometimes get removed from the aqueous phase and get adsorbed to the sludge/solids.122,123 This may be due to hydrophobicity, binding interactions with particles in soils, etc. and thereby contributing to a new exposure pathway when this nutrient rich sludge is used for agricultural purposes.
Pharmaceuticals are designed to affect biological receptors and hence it should not come as a surprise that they have stimulatory or inhibitory or dual effects on non-target organisms upon exposure to different concentrations especially when the targets and biochemical pathways are similar. A well-known example of this is the ER-receptor agonist and antagonist behaviour associated with naturally occurring and synthetic hormones (e.g. ethinylestradiol—the active ingredient in the oral contraceptives) which can result in endocrine disruption.124 Sometimes non-target organisms, e.g. algae, cyanobacteria, which have non-related biochemical and metabolic pathways can also be effected upon exposure to pharmaceuticals.125 Organisms might show limited toxicity in acute toxicity tests whereas higher toxicity in chronic tests for particular chemicals.126 Mixture toxicity of PPs is of on-going concern as is their potential effects over many generations.127 It has been observed that exposure to pharmaceutical products affects growth and behaviour of organisms, resulting in physical malformations, feminisation of males, changes in photosynthetic activity and metabolic processes. To date, very few ecotoxicity studies on pharmaceuticals have been conducted at environmentally relevant concentrations and hence there is inconclusive evidence to understand the true implications of their presence in the environment. See ref. 105 for a review of ecotoxicological studies of key pharmaceuticals. A key concern for nanomedicines is that their dual carrier and targeting functions may make existing PPs more bioavailable in the environment.
Early ecotoxicity studies reported toxicity effects at higher exposure concentrations and focused on growth inhibitory and reproductive effects. Recently, the shift in emphasis has been towards assessing impacts at low concentration and assessing the increased number of physiological biomarkers such as studying ROS production, transcription of genes, etc. For example, a decrease in nitrate reduction potential for groundwater bacterial communities was observed at 5 nM concentration of exposure to sulfamethoxazole128 and changes in behaviour of marine amphipods by exposure to fluoxetine (antidepressant, a selective serotonin reuptake inhibitor) at concentrations of 10 ng L−1 was reported.129 A long term study in an experimental lake to assess the population level sustainability of the fathead minnow upon exposure to low levels (5–6 ng L−1) of synthetic estrogens130 has shown that there can be collapse of a population due to feminization of the males. Various publications have suggested the need for assessing mixture toxicity131–133 because of the additive, cooperative and antagonistic effects of different class and compounds of pharmaceuticals.
Many knowledge gaps have been identified which makes the task of conducting a plausible environment risk assessment for pharmaceuticals challenging. These gaps in knowledge create large uncertainties and hence inconclusive results. Furthermore, analytical challenges, e.g., non-extractable residues, interference by other contaminates in complex mixtures of sewage and hospital wastewater, and trace level of these compounds complicates the matter. We summarise here the few repeatedly mentioned knowledge gaps in the literature. Limited knowledge exists on:
• occurrence, fate and activity of metabolites and their transformation products in the environment, mode of action of pharmaceuticals, metabolites and excretion rates
• long-term exposure to low levels of pharmaceuticals, ecosystem level impact, mixture toxicity
• exposure and effects data on soil organisms and marine species, effects data on ionic and polar compounds, and
• bioconcentration factors and bioaccumulation.
Intensive research in the field of environmental occurrence, fate and consequences of drugs and transformation products took off in the 2000s since the first findings of occurrence of pharmaceuticals and their metabolites in the late 1970s in sewage effluents in the US; however, their impacts on ecosystems are yet to be established with certainty. Only population level impact attributed with certainty to pharmaceuticals is the >95% decline in the population of the vultures in the Indian subcontinent due to extensive diclofenac use in veterinary medicines.134 This case study is also widely cited as an example of unexpected routes of exposure and bioaccumulation in the published literature on the environmental impacts of pharmaceuticals. Many national and international collective and cross-sectoral efforts over the past few years (e.g., ERApharm 2007,135 KNAPPE 2008 (ref. 136) in the EU, MistraPharma of Sweden) have been able to bring the discussion to the forefront. Publicly accessible websites such as Pharmaceuticals in the environment, Information for Assessing Risks (http://www.chbr.noaa.gov/peiar/) and the Swedish medicine information portal (http://www.fass.se/) provides information on medicines in the environment. More recently, key gaps in knowledge to help streamline research and efforts in this field have been identified.137 Additionally, different approaches for prioritisation schemes for environment risk assessment for pharmaceuticals have been suggested, e.g. fish plasma model, LogP ranking, hazard based rankings, QSAR approach, and these ranking schemes have been conducted for a substantial number of pharmaceuticals.138–140 In January this year, the European Commission put forward the proposal to amend the Water Framework Directives to include three pharmaceuticals in the list of priority substances in Annex X of the Directive.141 The positive outcome of the above-mentioned initiatives are that regulatory steps are being taken, however, it also exemplifies the time lag between knowing, proving environmental impacts and development or amendment of regulatory guidelines.
This table gives a very small number of the different types of pharmaceuticals monitored in the environment. It is meant to provide to the reader an overview of measured concentrations from recently published studies (2009–2012), the different environmental compartments where PPs have been found, and in different regions of the world. For detailed reviews on occurrence, fate and ecotoxicity of pharmaceuticals and their metabolites the reader is suggested reviews elsewhere, e.g., ref. 105, 121 and 161–163.
Possible sources, fate and effects of nanomaterials (NMs)†† in the environment
The continued thrust on nanotechnology as an innovation and economic driver‡‡164 will result in development of a larger number of new and complex materials and will inevitably result in release of these materials in the workplace165 and subsequently in the environment.166 The possible entry routes of NMs in the environment includes intentional (e.g., for remediation purposes) or accidental releases, emissions from manufacturing facilities, abrasion and weathering of NMs containing products. The specific entry route into a particular environmental compartment will depend on the life cycle of the product and disposal method used, for example, washing of nanofunctionalised textiles will result in release of the NMs in the sewerage system and finally transportation to natural waters.167 To the best of the knowledge of the authors, no actual field level environmental monitoring of NMs have been reported in the literature, though model environmental concentration estimates have been calculated and reported for selected NMs.168As is the case of pharmaceuticals, NMs also give rise to transformation products in various environmental conditions. The type of transformation products that will form will depend on the nanoscale properties of the NMs and on the conditions of the environmental matrices, e.g., magnetic iron nanoparticles aggregate at near neutral pH due to their near neutral zeta potential but at higher pH they are more dispersed.169 The key transformation mechanisms can be aggregation (homo- and hetero-aggregation), dissolution, oxidation/reduction and adsorption.170
Under simulated situations it has been found that natural organic matter (NOM) like humic substances171 and extracellular polymeric substances172,173 influence fate and behaviour of NMs. Studies have shown that NOMs in the environment influence stability of the NM174,175 and hence bioavailability and toxicity (reduction in toxicity shown in ref. 176, 177 and 178) although less effect of NOM on polymer coated NPs in some cases.179 It has also been demonstrated that silver NPs in organic matter rich soil become more bioavailable after aging.180 In addition to NOM, physico-chemical properties of the aqueous environmental compartment like pH, ionic strength, salinity, mineral content/hardness, etc. have also been shown to influence fate, behaviour and toxicity. For instance, it was shown that E. coli survived at pH 10 even at concentrations of iron oxide NPs (βeq of 109 mg L−1 of FeOx), however, S. cerevisiae (an eukaryote) had survival rates of less than 10%.181 Hardness of water, at near neutral pH, can result in formation of aggregates and biouptake by filter feeders182 or settling down and available to pelagic organisms or earthworms. High total organic carbon (TOC) and low ionic strength, conditions in freshwaters, can stabilise NMs and make it persistent and be available for filter feeders, fish and algae and low TOC and high ionic strength such as in sea water can aid in their rapid aggregation and settling.183 Please see ref. 184–187 for further studies on possible fate, behaviour and effects of engineered nanomaterials in the environment.
The key factors influencing toxicity are composition, size, surface properties (both for the NM and the transformed products), sensitivity of the species and presence of other contaminants. For example, cationic branched polymer [polyethyleneimine (PEI)] coated AgNPs (10 nm) was shown to have increased toxicity to a Gram +ve bacterium, bacillus sp. when compared to citrate and PVP coated AgNPs.188 Similarly, positively charged AuNPs of 2 nm size was reported to lyse Bacillus subtilis, but observed to have no effect on E. coli, a Gram −ve bacterium.189 Bioaccumulation and trophic transfer of NMs can also happen.190,191 Many investigators have shown biofilms to be an effective sink for NMs190,192,193 and aquatic organisms like filter feeders to uptake and biotransform suspended and dispersed NMs present in the aquatic matrix.194,195 Furthermore, exposure of organisms to NMs would be dependent on the presence of other environmental contaminants, mode of action of the chemical and the differences in physiology of species. Phenanthrene adsorbed on n-C60 was shown to be more bioavailable to algae and daphnids, but was found to be more toxic to algae196 and the investigators suggested the difference in physiology to be the reason for this observation. Whereas, n-C60 fullerenes was shown to sequester the synthetic hormone, 17-α ethinylestradiol (EE2) hence reducing its bioavailability.197 Similarly, it was demonstrated that mixed iron oxide NMs in organic matter rich environment (humic acid >20 mg L−1), low salinity (tap water and deionised water), and in a pH range of 5–7 could adsorb the antibiotic chlorotetracycline.198 Interactions between hydrophobic NMs and pharmaceutical products can sequester PPs from the environment hence reduce or increase their bioavailability in a given time frame.
Possible sources, fate and effects in the environment of nanomedicines
There is insufficient knowledge regarding possible amounts and entry routes of nanomedicine products in the environment, however it should not deter one from making estimates of likely routes of entry, based on knowledge of environmental release and transport of pharmaceuticals. Fig. 1 is a conceptual model of likely release and exposure pathways of nanomedicine and a key research issue is to quantify the concentrations and fluxes within this conceptual model. There are different entry routes for various medical NMs in the environment. In case of therapeutic applications, the obvious route is the renal and the hepatobiliary§§ routes of excretion to domestic sewage and then subsequently to waste water treatment plants and to receiving water bodies and land (with the caveat mentioned earlier that a large percentage of waste water even in the EU goes untreated). Other possible routes are likely to be NM release into the air from inhalers, disposal of unused medicine at hospitals, R&D labs, and clinical research facilities by casual and ill trained staff, NM release during manufacturing, transport and accidental spills, and from the incinerators. Due to high specific surface areas NMs might get adsorbed on to waste solids of the combustion chamber in an incinerator or be present in the off-gas.199 However, complete removal of NPs below 100 nm from the off gas of the incinerator might not happen, even after state-of-the art effluent gas treatment processes.200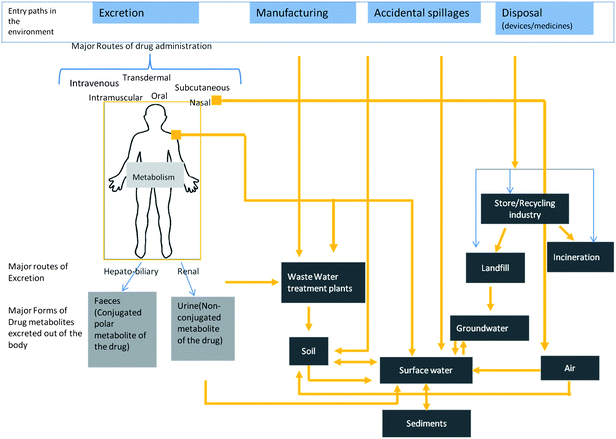 | ||
| Fig. 1 A conceptual schematic showing likely sources and transport of nanomedicine into the environment. Nanomedicines and transformed products may be released into the water through the excretory routes, washing off from skin, from manufacturing facilities, spillages and disposal of product. They can be released in the atmosphere from nasal inhalers. Incineration of composite medical products and abrasion and weathering in landfills may lead to release into the atmosphere. | ||
In case of medical devices, improper disposal of the devices at end of their life, especially disposable of in vitro diagnostic products is likely to release NMs into the environment. Although we know quantitatively very little at the moment, a priori it might be expected that certain pathways are likely to be of major importance, including: (1) waste water and storm water to waste water and sludge and then to freshwater and soil; (2) treated flue gas from incineration to atmosphere and then to water and soil and (3) landfill to groundwater and soil and then to surface water.
Biotransformation and excretion
Ingested and injected PPs are amenable to biotransformation in the body due to the action of various enzymes and are excreted primarily with urine and/or faeces.201 Studies have been performed to find out the clearance mechanisms of NMs with prospective use in medicine. Biodistribution studies with gold nanoparticles have been conducted by different investigators and it has been found that the clearance pathway is dependent on size, surface coating and charge of the particle. Lipka et al.202 showed that 10 kDa PEG coated 5 nm AuNPs followed the hepato-biliary clearance route, whereas another study203 showed uncoated 20 nm AuNPs to be primarily recovered in the urine. A recent study showed that hepato-biliary clearance was inversely related to negatively charged AuNPs of different sizes (1.4, 2.8, 5, 18, 80, 200 nm). The investigators concluded that the small sized negatively charged AuNPs were excreted out via the hepato-biliary excretion pathway because of “dynamic protein binding and exchange which are major mechanisms determining the AuNP accumulation in the various organs and tissues…”.204 By extension, there might be differences in biodistribution profile of encapsulated and free drug. A study showed that urinary excretion of the unaltered drug when encapsulated in poly-epsilon-caprolactone (a widely researched polymer for medical applications) nanocarriers increased by ∼15% in comparison to the administration of non-encapsulated drug,205 hence would result in more discharge to the environment unless dosages are altered. Biological fate of a model PEG–protein was studied and it was found that 46.5% of PEG of the administered dose was excreted out of the urine over a period of few days. The same study also reported that intact PEGylated model protein was excreted out in the first few days post administration of the model protein.206In many cases, nanoformulations help to reduce systemic toxicities especially for anticancer drugs. For example, nab-paclitaxel, a nanomedicine in the market, is a formulation of paclitaxel (empirical formula: C47H51NO14, a plant alkaloid) bound to albumin nanoparticles with mean particle size 130 nm has been shown to have better therapeutic efficacy than conventional paclitaxel (Taxol®) for breast cancer. The dosage of this new formulation is 260 mg m−2 of body surface area every 3 weeks (Taxol's prescribed dosage ranges from 135 mg m−2 to 175 mg m−2 of body surface area every 3 weeks),207,208 showing the possibility of higher doses that can be achieved through nanoformulation. Traditional chemotherapeutics are highly hydrophobic drugs, and hence are generally assumed to be adsorbed on to the sludge of STP123 and then mainly incinerated or spread on agricultural soils (Switzerland and UK, respectively). The possibility of administering increased dosages and changed excretion profiles due to the new nanoformulations will likely increase the environmental concentration of these highly cytotoxic pharmaceutical products. The above examples indicate the likely problem areas; however, the authors acknowledge the fact that more targeted medicines, customised for small populations (personalised medicines) and possibility of reduction in premedication amounts might result in a more favourable benefit-risk balance when all aspects are taken into consideration.
Fate and behaviour
Polymer coating on NMs will affect the fate and behaviour of NMs in waste water treatment plants. Tween 20 coated silica oxide nanoparticles (∼56 nm) were shown to remain in the sludge, whereas uncoated silica oxide NP did not flocculate and remained in the effluent.209 Biofilms can act as potential ‘sinks’ for NMs either in the secondary treatment stage of a sewage treatment plants or in fresh water ecosystems. Sometimes, the polyethylene glycol (PEG) coating on NM can integrate itself with the protein component of the biofilm and change the roughness co-efficient of the biofilm but shielding the toxic effects of the core particle.192 The size can also have an influence of changing the morphological properties of a biofilm. Stojak et al. reported an increase in roughness coefficient and significant decrease in plankton biomass in a L. pneumophila mature biofilm after 2 days of exposure to citrate capped of Au nanoparticles of sizes 4 and 18 nm.193 However, no change was observed in the biofilm exposed to 50 nm gold NPs. Furthermore, it was observed that the 4 nm and 18 nm AuNPs got adsorbed onto the exopolysaccharides of bacterial cell wall and also got entrapped in the bacterial cell.193 Polymers may be utilised as carbon/energy sources. It was recently demonstrated that PEG coated NM could be degraded by bacteria from an urban stream. However, the degradation rate and aggregation depended on the available chain end groups of the two different types of polymer coated NMs studied.210 Polymer (poly ethylene glycol-b-ε-caprolactone) coated nanoparticles were found to irreversibly adsorb onto cellulosic surface and the adsorption mechanism was found to be size dependant. The investigators hypothesised that adsorption could be due to interdigitation and entanglement of the nanoparticles with the D-glucose chains of cellulose.211Biouptake and effects
Monodispersed, stable and targeted nanomaterials are important for medical applications, e.g., monodispersed iron oxide nanoparticles7,212 to fully exploit their novel properties, increase shelf life and to influence physiological responses with increased effectiveness. These same design requirements may dictate the fate and risk of the nanomaterial in the environment by not only arresting growth and reproduction, but also interfering with metabolic processes and hence in turn impacting key ecosystem services. However, to a certain extent organisms might be able to tolerate the exposure of nanomedicines although this is poorly quantified.Nanoparticles are coated with organic polymer coatings to escape the mononuclear phagocytic system in the body (‘stealth’ properties) or to especially target cells. There are limited numbers of ecotoxicity studies which have been conducted with polymers used in medical applications, of the few that we are aware of, most of the studies have been done on dendrimers.
Dendrimer toxicity has been shown to increase with increase in the generation of cationic dendrimers for various model organisms (D. magna, V. fischeri, P. subcapitata, T. platyurus). Table 3 gives a summary of selected ecotoxicity studies which can be linked with NMs used in medical applications. The environmental sources, fate and effects of AgNPs have been widely reported in the scientific literature and therefore this table does not cover ecotoxicity studies of AgNPs. Readers are referred elsewhere for a review on fate and effects of AgNPs in the environment.184
| The ‘nano’ component in nanomedicine | Short description of select ecological studies of toxicity effects, uptake and bioaccumulation for NMs and nanocarriers with likely use in nanomedicine | References |
|---|---|---|
| Polyethyleneimine (PEI) polymer | For tadpole larva, Xenopus laevis, both PEI and PEI:DNA (polyplex) showed teratogenic effects at concentrations 0.1 μg L−1. PEI also showed higher toxicity for the algae Pseudokirchneriella subcapitata; EC10 = 40.8 μg L−1. The polyplex was found to be less toxic to the algae than the free polymer | 213 |
| Dendrimers | Commercially available amine terminated G4 dendrimer showed sublethal toxicity in zebrafish embryos at 0.2 μM concentrations, whereas COOH terminated G3.5 dendrimers did not exhibit toxicity even at concentrations of 200 μM. Dose and time dependant mortality was observed for amine terminated G4 dendrimer, 100% mortality at dose 20 μM in 24 h post fertilisation | 214 |
| Green algae Chlamydomonas reinhardtii exposed to commercially available amine terminated G2 (2.6 nm), G4 (4.4 nm), G5 (5.7 nm) poly(amidoamine) PAMAM dendrimers showed decreased cell viability at 2.5 mg L−1 (median IC 50 as per dendrimers generation: G2, G4, G5, – 2 mg L−1, 3 mg L−1, 5 mg L−1). O2 evolution from photosynthesis significantly increased at concentrations 1 mg L−1 and 2.5 mg L−1 for G2 and G4 dendrimer (increase in PSII reaction centres and e− transport). Exposure concentrations: 0.3, 1, 2.5 and 10 mg L−1. Exposure duration: 72 h | 215 | |
| Commercially available G4 cationic PAMAM dendrimers with diaminobutane core at 15 nM, 25 nM and 35 nM concentrations resulted in a linear increase in ROS level and photosynthetic oxygen level in the algae, C. reinhardtii. Also, most of the transcripts encoding proteins involved in photosynthesis and antioxidant genes were down regulated except for the gene which encodes light-harvesting polypeptide for PSII (this was upregulated). Measured size range: ∼90 nm in MQ water. Exposure duration: 6, 24 h | 216 | |
| A dose-effect study of amine terminated G4 and G1 PAMAM dendrimers with ethylenediamine core was conducted for P. subcapitata. The amine-terminated G4 dendrimer was found to be comparatively more toxic than the amine terminated G1 dendrimer. The negatively charged hydroxyl terminated G4 dendrimer had least toxicity | 217 | |
| SPIONS and USPIOs (<25 nm core) | Pumpkin plants (Cucurbita maxima), when grown hydroponically could translocate and accumulate coated magnetite (Fe3O4) nanoparticles (20 nm size). Strong magnetisation signals were observed in the leaves. Exposure concentration: 0.5 g L−1; exposure period: ∼20 days. Also, when pumpkin plants were grown in soil, no magnetisation signal was noticed in the plants but when grown in sand, the pumpkin plants accumulated the iron nanoparticles. Lima bean plants didn't accumulate Fe3O4 nanoparticles | 218 |
| Higher activity of the oxidative stress enzyme catalase was reported for PVP coated magnetite (Fe3O4) nanoparticles (25 nm) and bulk iron oxide NPs in the shoots of both rye grass and pumpkin plants and roots of rye grass plants. Exposure concentrations: 30 mg L−1, 100 mg L−1 of nano-(Fe3O4) and 30 mg L−1 and 100 mg L−1 of Fe3O4 bulk. Exposure duration was 18 days. Lipid peroxidation was also reported. No magnetism was observed in the shoots of both the plants, indicating, that the iron NPs were not translocated. The oxidative stress enzyme superoxide dismutase activity was found to be higher in the roots of both the plants for iron bulk and nanoparticles at 30 mg L−1 concentration | 219 | |
| In the presence of sublethal concentrations of As(V) (to rule out the probability that environmental arsenic is causing the toxicity), commercial nano-Fe2O3 (20–40 nm) was shown to have increased toxic effect on C. Dubia. It was established nano-Fe2O3 and As(V) caused the toxic effect in a synergistic mode (nano-Fe2O3 alone didn’t exhibit mortality under the concentrations used in the study). It was found that 48 hour mortality was dose dependant but 24 h mortality was not. At 20 mg L−1 of nano-Fe2O3, the 48 hour mortality increased from 30% to 70% and then the mortality rate remained nearly constant for higher exposure doses. Depuridation (upto 75%) occurred after an hour for solutions having algal feed. It was observed that maximum bioaccumulation occurred at neutral pH. Exposure concentration: 1, 5, 10, 20, 50 mg L−1 | 220 | |
| Gold nanoparticles (different sizes for different medical applications; general size range 5–30 nm) | Decrease in colony forming units of soil microbial community after 15 days of exposure to commercial available AuNPs. Higher shoot/root ratio of lettuce exposed to AuNPs observed indicating that AuNPs acted as a growth promoter. Concentration: 0.013% w/w in soil. Exposure time in soil: 15 days | 221 |
| Size selective uptake of citrate capped AuNP by tobacco plants. 3.5 nm AuNPs were found in the leaves of the plants and the 18 nm AuNPs remained agglomerated/aggregated at the root surface. Necrotic lesions in leaves and death of plants occurred after 30 days of exposure. Concentration: 3.5 nm – 48 ppm; 18 nm – 76 ppm. Exposure duration: 3 to 30 days | 222 | |
| Mean ‘Au’ concentrations in tobacco plants were found to be between 2.2 mg kg−1 to 53.5 mg kg−1 when hydroponically exposed to tannate and citrate coated 10, 30, 50 nm of AuNPs. In contrast, wheat plants when exposed to the same exposure concentrations didn't show any uptake. However, it was found that aggregation of AuNPs occurred more in the wheat than the tobacco plant suspensions. Exposure concentration: 30 mg L−1. Exposure time: 7 days for tobacco and 3 days for wheat | 223 | |
| No obvious toxic effects of cucumber and lettuce seeds could be seen when exposed to AuNPs of mean size 10 nm at 2.4 × 1012 NP mL−1 concentration | 224 | |
| Polyvinyl alcohol (PVA) capped Au NPs [size range: 15–35 nm (spherical shape)]. Exposure concentrations: 10, 25, 50, 75, and 100 μg mL−1 were used to study their impact on embryos of zebra fish. The embryos developed normally (similar to the controls) – eyes, tail, brain and otoliths. No change in blood flow or cardiovascular development was observed. Detectable AuNP accumulation was observed in the body of treated embryos (25 and 50 μg mL−1) | 225 | |
| Zebrafish exposed to citrate stabilised gold nanoparticles (12 and 50) nm through feed. Daily dose 90 ng and 106 ng for 12 and 50 nm Au NPs. Exposure duration 36 days. Daily dose: 36 and 42 ng for 12 and 50 nm AuNPs respectively. Exposure duration: 60 days. AuNPs were found in brain, liver and skeletal muscles with highest concentration of 12 nm AuNPs in the brain. Up-regulation of DNA repair genes, increase in mutation and mitochondrial impairment was observed and was more for the 60 days exposure duration | 226 | |
| (1) Feed containing citrate capped 15 nm AuNPs fed to D. melanogaster. Max. dose: 12 μg per g per day. Life span and fertility was negatively affected. Reproductive performance decreased with exposure to increasing AuNP doses (from 1.9 pmol L−1 to 380 pmol L−1) during embryonic and larval development. Overexpression of heat shock protein occurred reflective of ER stress and DNA fragmentation in the gastrointestinal tissue was observed | 227 | |
| (2) Feed containing citrate capped 15 nm AuNPs fed to D. melanogaster. Max. dose: 3 μg per g per day. Mutagenic effects were observed and aberrant phenotypes were observed in subsequent (F1 and F2) generations | 228 | |
| Oxidative stress observed in Mytilus edulis, a marine bivalve mollusc, when exposed to 750 ppb AuNPs (5.3 nm size), for 24 h. The study also showed that larger size Au-NPs resulted in lower oxidative stress in the animal. It was found that the AuNPs accumulated mainly in the digestive gland | 229 | |
| Filter-feeding bivalve Corbicula fluminea (1–2 years of age) exposed to Bovine Serum Albumin (BSA) coated AuNPs of sizes 7.8 nm, 15 and 46 nm. Exposure concentration: 2 mg L−1. Total exposure time: 180 h | 194 | |
| • Efficiency of removal of AuNPs from solution due to filtration by the bivalves was size dependant and removal efficiency increased with particle size | ||
| • When the bivalves were exposed to varying concentrations (2 mg L−1, 4 g L−1 and 8 mg L−1) of 46 nm size BSA–Au NPs, it showed that removal efficiencies from solution to be positively related with concentration of BSA–AuNPs solution | ||
| • The AuNPs were evidenced in the digestive gland and regions of the digestive tract and mass concentration inside the body of the clam was more for the 15 nm of BSA–AuNP exposed bivalves | ||
| • The bivalves exposed to 7.8 nm AuNPs particles, didn't efficiently excrete it during the experimental period and it was found that AuNPs concentrations remained elevated in these bivalves. 15 and 46 nm BSA–AuNP exposed bivalves excreted out the AuNPs more efficiently | ||
| Evaluation of toxigenomic response of Caenorhabditis elegans (soil nematode) exposed to 4 nm citrate capped AuNPs (LC10 = 5.9 mg L−1 at 24 h and same concentration used for the genetic response analysis to AuNPs). Observations reported are as follows | 230 | |
| • Upregulation of genes related to clathrin-mediated endocytotic pathway | ||
| • Upregulation of Ca+ signalling and amyloid processing pathway (protein unfolding and denaturation) | ||
| • Upregulation of heat shock protein genes (reflective of ER stress) | ||
| Silicon oxide NPs | Commercially available silica NPs (50% were below size 100 nm, 40% were between 100 and 200 nm) at concentrations of 0.825 mg mL−1 was shown to affect the endoplasmic reticulum function leading to ER stress response, in a fish fibroblasts cell line | 231 |
| Commercially available silica NPs of size 10–20 nm diameter were found to decrease growth rate of S. obliquus (a fresh water green algae) as a function of concentration (50, 100, 150 mg L−1) and time (48, 72, 96 h). | 232 | |
| Contents of chlorophyll-a and b decreased by 86.4% and 94.8% as compared to the control group, but the amount of carotenoids didn't decrease. Exposure duration: 96 h of exposure at 50 mg mL−1 | ||
| SiO2 NPs (commercially available) of 12.5 and 27 nm shown to be toxic to P. subcapitata (green algae) EC20 was found to be 20 ± 5 mg L−1 and 28.8 ± 3.2 mg L−1 for 12.5 nm and 27 nm. Adsorption to the cell wall was seen but no cellular uptake was observed by the investigators. Exposure duration: 72 h | 233 |
Regulatory framework for medicines and medical devices in the EU
Regulations for pharmaceuticals for human use
Extensive studies for assessing toxicity are conducted after identification of a promising new entity that has a therapeutic potential. A battery of tests and assays are performed to understand whether there are risks for human carcinogenicity, genotoxicity, reproductive and development toxicity, immunotoxicity, etc.). Pharmacodynamics and pharmacokinetics studies are conducted in small animal models to assess the distribution of the drug, the mode of action and physiological effects, metabolism and excretion. Data from these studies is required to be submitted to the relevant medical regulatory agency before enrolling human subjects to establish the safety and efficacy (Phase I to Phase III clinical trial) of the new drug. The preclinical and the clinical trial data form the basis of the marketing authorisation application (MAA) in the EU and member states. Applications for therapeutics for cancer, neurodegenerative diseases, HIV/AIDS and immune dysfunctions, and viral diseases are submitted to the centralised medical regulatory agency in the EU, the European Medicines Agency. A few other therapeutics which go through the centralised procedure include officially designated ‘orphan’¶¶ medicines, biotechnology based therapeutics, tissue engineering products. Marketing surveillance (‘pharmacovigilance’) of the medicine post authorisation are other regulatory steps which should help to monitor the therapeutic agent's safety. Fig. 2 is a simplified depiction of the medicine innovation pathway, key checkpoints with regard to regulatory agency involvement and the underlying guidelines for ethics and safety during innovation. The decision for approving a medicine is based on the careful evaluation of the benefit-risk assessment of a particular therapeutic for the target group of patients.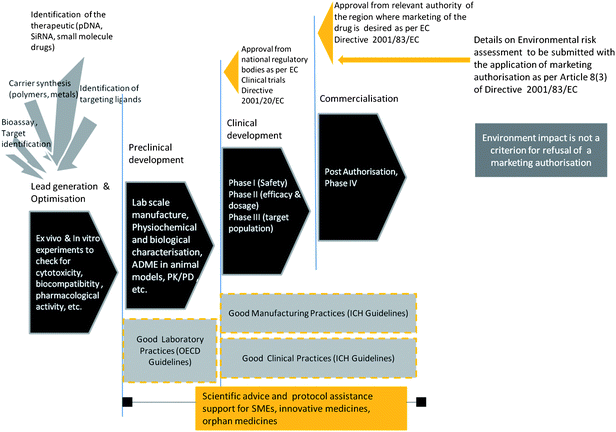 | ||
| Fig. 2 General stages of nanomedicine development and the key points of interaction between regulatory agencies and nanomedicine developers. Good Laboratory Practices, Good Manufacturing Practices, Good Clinical Practices are the quality and ethical guidelines followed by pharmaceutical companies and researchers and monitored by regulators. Rapid advancement in technology and science and need for innovation to be an economic driver, regulatory agencies are present in earlier stages of product development. | ||
Medical devices legislation
The regulatory context with regard to medical devices in the EU is substantially different to that of medicines for human use. Medical device and medicine regulatory pathway have distinct and clearly demarcated regulatory process. The medical device directives are implemented at EU member state level with no overarching body at the EU level. There are three different regulations to capture all the different types of devices used in the medical industry – the Medical Devices Directive, Active Implantable Medical Device Directive, and In Vitro Diagnostic Medical Device Directive. Combination products i.e. integration of a medical device and medicine, spurred by nanotechnology, has blurred the distinction between the two distinct regulatory pathways and need for revisions in the current legal framework has been voiced in the public consultation process, a part of the undergoing process of revision (2008-onwards) of the medical devices directives. Also, few member state regulators (e.g. UK, Sweden) have taken required steps towards addressing the issue.Medical devices are classified according to their perceived level of risk – Class I representing the lowest level of expected/perceived risk and Class III representing the highest level of risk. The degree of risk assigned then determines the level and type of evidence required for award of CE (Conformité Européenne) mark. Clinical data is required for awarding a CE mark for Class III medical devices but are not mandatory; literature analysis showing clinical investigations and experience related to similar devices and appropriate justification can be used for submission of application for approval.235 Tests to identify human toxicological risks from materials used in medical device components, (e.g. polymers) need to be performed. However, no environmental risk assessment of medical devices is required. Unfortunately, the medical devices directives do not cover the entire life cycle of the product and their disposal in the EU was according to the WEEE Directive (Waste Electronic and Electrical Equipment Directive 2002/96/EC) which did not mandate recycling and recovery percentages for medical devices giving rise to the concern that similar environmental and occupational health problems as electronic waste 236 may occur for the use of cost-effective mass produced nano-enabled in vitro diagnostic tools. However, the recently revised WEEE Directive has mandated recovery and recycling targets for certain medical devices.237
Current regulatory context (human medicines) related to environmental safeguard
The European Medicines Agency's Guideline on Environmental Risk Assessment (ERA) of pharmaceuticals102 follows a tiered assessment approach: Phase I and Phase II (Tier A and Tier B). Fig. 3 gives a concise schematic explanation of the approach. An ERA needs to be provided with every new marketing authorisation application (MAA) for a pharmaceutical; however, granting of market authorisation is independent of environment impact. | ||
| Fig. 3 Key requirements in the tiered environment risk assessment process for medicines for human use. For anti-microbials, if the PNEC:PECsw is less than 0.1, need for extended environmental fate and effect studies becomes mandatory which includes soil and sediment compartments and terrestrial organisms. PNEC: Predicted No Effect Concentration; PEC: Predicted Environmental Concentration; Koc: adsorption co-efficient; Kow: octanol–water co-efficient; PECsw: PEC surface water; PECgw: PEC ground water. | ||
There are two initial pre-screening steps – (a) if the predicted environment concentration of the pharmaceutical exceeds the threshold value of 0.01 μg L−1 (0.01 ppb), then it triggers the need for conducting few acute ecotoxicity tests on regulatory species to calculate the Predicted No Effect Concentration (PNEC) (b) if the octanol–water partition co-efficient (log Kow) is greater than 4.5, persistence, bioaccumulation and toxicity (PBT) assessment tests need to be conducted by following ECHA's guidance on chemical safety assessment. Firstly, the questions arise, how is the PEC calculated for nanomedicines? Apart from concerns which are similar to all NMs, should it to be based on the drug, the nanocarrier or on the nanocarrier–drug conjugate? How would the PEC be derived for more complex nanomedicines? As with all NMs, nanomedicines may present unique concerns and the applicability of such ERAs have not been fully demonstrated.
Secondly, it is well established that log Kow has deficiencies as a surrogate for determining mobility and partitioning of PPs or for application to NMs.238 For example, the antidepressant carbamazepine was found in fish tissues from effluent dominated streams though the drug has a log Kow of 2.67. Similarly, ciprofloxacin, an antibiotic, sorbs well onto active sludge or sediments,153,239 despite a log Kow of −1.74 (cited in ref. 240) and it was found to be persistent.241 It has been widely debated that log Kow for acidic and basic drugs is misleading, because the coefficient is dependent on solution pH, ionic strength, NOM and other factors. In the case of NMs, the inadequacy of the test protocol to determine log Kow has been discussed.238 A study performed on different generations of dendrimers showed that for dendrimers with terminal NH2 group, the log Kow of the polymer was negative for G1–G5 PAMAM dendrimers and G6–NH2 and G8–NH2 dendrimers partitioned at the octanol–water interface.242 The negative log Kow indicates that under the current ERA guidelines, there will not be any need to conduct a TIER 1 risk assessment. Similarly, PEG has negative log Kow and it doesn't change much with the chain length, furthermore, it is not easily biodegradable.
Although the action limit 0.01 μg L−1 is very low, it is based on acute rather than chronic toxicity tests and it has been widely discussed both for pharmaceuticals and nanomaterials, that chronic and sub-lethal toxicity end points are important to assess the environmental risks of the product and that the link between chronic and acute toxicity is not well established for NMs. Furthermore, the test protocols suggested in the ERA Guidelines for human pharmaceuticals for conducting the physical–chemical fate and effects studies is based on OECD test guidelines for chemicals. The recommended study types include adsorption–desorption using a batch equilibrium method, a ready biodegradability test, aerobic and anaerobic transformation in aquatic test, algae growth inhibition, daphnia reproduction test, etc. The drawbacks and the need for adaptability of the current OECD tests and protocols, originally meant for chemicals, with respect to NMs have been discussed and reviewed.243,244 Key issues are the influence of the test medium conditions on the NMs,245–247 the need to include benthic and filter feeding organisms as test species, the necessity of investigating chronic effects and finding novel toxicity end points, the need for extensive in situ physico-chemical characterisation, the limited applicability of the persistence and bioaccumulation tests. The applicability of testing to nanomedicines will include the same issues and perhaps other specific ones including role of the nanocarrier in increased uptake.
Knowledge gaps and uncertainties and conclusion
Despite much progress in recent years, knowledge and data gap exists for pharmaceuticals, including their metabolic products and excretion rate, and their environmental fate and behaviour, removal efficiencies in sewage treatment plants, chronic toxicity data and bioaccumulation.136 In nanotoxicology and nanoecotoxicology, the key gaps include environmental concentrations, environmental fate and behaviour, dynamic changes in physical and chemical properties both in vitro and in vivo, applicability of exposure assays, dose metrics for exposure assessment, biouptake and toxicity mechanisms and chronic/acute toxicity relationships.185–187,243 In nanomedicine, all these gaps in knowledge and data apply, along with others such potential discharges to the environment and medium term growth in applications. Given the significant potential benefits of nanomedicine such as potential for dose reduction, it is imperative to further understand any potential environmental risks to allow the long term safety, sustainability and development of the industry. However, further understanding of the impacts of nanomedicine in the environment can be provided by use of data from the pharmaceuticals, environmental and nanoscience fields. In addition, sophisticated models and techniques are available which can be applied and adapted to understanding the environmental implications of nanomedicine. Although caution needs to be exercised as there will be discipline-dependent differences, such methods, data and models provide a solid platform for future more specific studies which are urgently needed.Acknowledgements
The authors thank the School of Geography, Earth and Environmental Sciences, University of Birmingham which funded the studentship which supported IM. JRL would like to acknowledge financial support from the SmartState Center for Environmental Nanoscience and Risk, University of South Carolina.References
- G. Wang and J. Guan, J. Nanopart. Res., 2012, 14, 1–14 Search PubMed.
- M. L. Grieneisen, Nat. Nanotechnol., 2010, 5, 825 CrossRef CAS.
- A. E. Nel, L. Madler, D. Velegol, T. Xia, E. M. V. Hoek, P. Somasundaran, F. Klaessig, V. Castranova and M. Thompson, Nat. Mater., 2009, 8, 543–557 CrossRef CAS.
- S. Mitragotri and J. Lahann, Nat. Mater., 2009, 8, 15–23 CrossRef CAS.
- R. Duncan and R. Gaspar, Mol. Pharmaceutics, 2011, 8, 2101–2141 CrossRef CAS.
- D. P. Cormode, T. Skajaa, Z. A. Fayad and W. J. M. Mulder, Arterioscler., Thromb., Vasc. Biol., 2009, 29, 992–1000 CrossRef CAS.
- D. Ho, X. Sun and S. Sun, Acc. Chem. Res., 2011, 44, 875–882 CrossRef CAS.
- M. Swierczewska, G. Liu, S. Lee and X. Chen, Chem. Soc. Rev., 2012, 41, 2641–2655 RSC.
- M. J. Hawkins, P. Soon-Shiong and N. Desai, Adv. Drug Delivery Rev., 2008, 60, 876–885 CrossRef CAS.
- D. Rayson, T. M. Suter, C. Jackisch, S. van der Vegt, B. Bermejo, J. van den Bosch, G. L. Vivanco, A. M. van Gent, H. Wildiers, A. Torres, L. Provencher, M. Temizkan, J. Chirgwin, J. L. Canon, G. Ferrandina, S. Srinivasan, L. Zhang and D. J. Richel, Ann. Oncol., 2012, 23, 1780–1788 CrossRef CAS.
- U.S. Environment Protection Agency, Flexographic Ink Options: A Cleaner Technologies Substitutes Assessment, http://www.epa.gov/dfe/pubs/flexo/ctsa/frontv1-apr02.pdf, accessed 13 October 2012 Search PubMed.
- T. M. Allen and C. Hansen, Biochim. Biophys. Acta, Biomembr., 1991, 1068, 133–141 CrossRef CAS.
- W. J. Gradishar, S. Tjulandin, N. Davidson, H. Shaw, N. Desai, P. Bhar, M. Hawkins and J. O'Shaughnessy, J. Clin. Oncol., 2005, 23, 7794–7803 CrossRef CAS.
- S. Svenson, M. Wolfgang, J. Hwang, J. Ryan and S. Eliasof, J. Controlled Release, 2011, 153, 49–55 CrossRef CAS.
- M. E. Davis, J. E. Zuckerman, C. H. J. Choi, D. Seligson, A. Tolcher, C. A. Alabi, Y. Yen, J. D. Heidel and A. Ribas, Nature, 2010, 464, 1067–1070 CrossRef CAS.
- Y. Matsumura, Adv. Drug Delivery Rev., 2008, 60, 899–914 CrossRef CAS.
- O. Lőrincz, E. R. Tőke, E. Somogyi, F. Horkay, P. L. Chandran, J. F. Douglas, J. Szebeni and J. Lisziewicz, Nanomed.: Nanotechnol., Biol. Med., 2012, 8, 497–506 CrossRef.
- M. Perfezou, A. Turner and A. Merkoci, Chem. Soc. Rev., 2012, 41, 2606–2622 RSC.
- C. S. Thaxton, R. Elghanian, A. D. Thomas, S. I. Stoeva, J.-S. Lee, N. D. Smith, A. J. Schaeffer, H. Klocker, W. Horninger, G. Bartsch and C. A. Mirkin, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 18437–18442 CrossRef CAS.
- G. Peng, U. Tisch, O. Adams, M. Hakim, N. Shehada, Y. Y. Broza, S. Billan, R. Abdah-Bortnyak, A. Kuten and H. Haick, Nat. Nanotechnol., 2009, 4, 669–673 CrossRef CAS.
- L. M. Manus, D. J. Mastarone, E. A. Waters, X. Q. Zhang, E. A. Schultz-Sikma, K. W. MacRenaris, D. Ho and T. J. Meade, Nano Lett., 2010, 10, 484–489 CrossRef CAS.
- M. L. Etheridge, S. A. Campbell, A. G. Erdman, C. L. Haynes, S. M. Wolf and J. McCullough, Nanomed.: Nanotechnol., Biol. Med., 2012 DOI:10.1016/j.nano.2012.05.013.
- BCC Research, Nanotechnology in Medical Applications: The Global Market, 2010, http://www.bccresearch.com/report/HLC069A.html, accessed 22 December 2010 Search PubMed.
- BCC Research, Nanotechnology in Medical Applications: The Global Market, 2012, http://www.bccresearch.com/report/nanotechnology-medical-applications-global-market-hlc069b.html, accessed 4 May 2012 Search PubMed.
- G. Oberdörster, J. Intern. Med., 2010, 267, 89–105 CrossRef.
- C. Medina, M. J. Santos-Martinez, A. Radomski, O. I. Corrigan and M. W. Radomski, Br. J. Pharmacol., 2007, 150, 552–558 CrossRef CAS.
- W. H. De Jong and P. J. Borm, Int. J. Nanomed., 2008, 3, 133–149 CrossRef CAS.
- P. J. A. Borm and D. Müller-Schulte, Nanomedicine, 2006, 1, 235–249 CrossRef CAS.
- V. Murashov, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2009, 1, 203–213 CrossRef CAS.
- A. Baun and S. F. Hansen, Nanomedicine, 2008, 3, 605–608 CrossRef.
- European Medicines Agency, First International Workshop on Nanomedicine, 2–3 September 2010, http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/events/2009/12/event_detail_000095.jsp&mid=WC0b01ac058004d5c3, accessed 14 July 2012 Search PubMed.
- W. Sanchez, W. Sremski, B. Piccini, O. Palluel, E. Maillot-Maréchal, S. Betoulle, A. Jaffal, S. Aït-Aïssa, F. Brion, E. Thybaud, N. Hinfray and J.-M. Porcher, Environ. Int., 2011, 37, 1342–1348 CrossRef CAS.
- European Science Foundation, ESF Forward Look on Nanomedicine 2005, 2005, http://www.esf.org/publications/forward-looks.html, accessed 1 June 2012 Search PubMed.
- European Union, Commission Recommendation of 18 October 2011 on the definition of nanomaterial, http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ: L:2011:275:0038:0040:EN: PDF, accessed 10 July 2012.
- I. Canton and G. Battaglia, Chem. Soc. Rev., 2012, 41, 2718–2739 RSC.
- E. C. Dreaden, A. M. Alkilany, X. Huang, C. J. Murphy and M. A. El-Sayed, Chem. Soc. Rev., 2012, 41, 2740–2779 RSC.
- B. Thiesen and A. Jordan, Int. J. Hyperthermia, 2008, 24, 467–474 CrossRef CAS.
- C. G. Hadjipanayis, M. J. Bonder, S. Balakrishnan, X. Wang, H. Mao and G. C. Hadjipanayis, Small, 2008, 4, 1925–1929 CrossRef CAS.
- V. P. Torchilin, Nat. Rev. Drug Discovery, 2005, 4, 145–160 CrossRef CAS.
- C. Spuch and C. Navarro, J. Drug Delivery, 2011, 1–12 Search PubMed.
- B. Y. S. Kim, J. T. Rutka and W. C. W. Chan, N. Engl. J. Med., 2010, 363, 2434–2443 CrossRef CAS.
- J. Khandare, M. Calderon, N. M. Dagia and R. Haag, Chem. Soc. Rev., 2012, 41, 2824–2848 RSC.
- D. Q. McNerny, P. R. Leroueil and J. R. Baker, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2010, 2, 249–259 CrossRef CAS.
- S. Svenson, Eur. J. Pharm. Biopharm., 2009, 71, 445–462 CrossRef CAS.
- M. Elsabahy and K. L. Wooley, Chem. Soc. Rev., 2012, 41, 2545–2561 RSC.
- J. H. Adair, M. P. Parette, E. I. Altinoglu and M. Kester, ACS Nano, 2010, 4, 4967–4970 CrossRef CAS.
- R. A. Petros and J. M. DeSimone, Nat. Rev. Drug Discovery, 2010, 9, 615–627 CrossRef CAS.
- J. W. Singer, J. Controlled Release, 2005, 109, 120–126 CrossRef CAS.
- Y. Cu and W. M. Saltzman, Nat. Mater., 2009, 8, 11–13 CrossRef CAS.
- J. W. Singer, S. Shaffer, B. Baker, A. Bernareggi, S. Stromatt, D. Nienstedt and M. Besman, Anti-Cancer Drugs, 2005, 16, 243–254 CrossRef CAS.
- S. Jevševar, M. Kunstelj and V. G. Porekar, Biotechnol. J., 2010, 5, 113–128 CrossRef.
- R. Ionescu, Y. Broza, H. Shaltieli, D. Sadeh, Y. Zilberman, X. Feng, L. Glass-Marmor, I. Lejbkowicz, K. Müllen, A. Miller and H. Haick, ACS Chem. Neurosci., 2011, 2, 687–693 CrossRef CAS.
- http://clinicaltrials.gov/, NCT01386203, accessed 22 July 2012.
- http://clinicaltrials.gov/, NCT01420588, accessed 22 July 2012.
- http://clinicaltrials.gov/, NCT01465087, accessed 22 July 2012.
- http://clinicaltrials.gov/, NCT01292369, accessed 22 July 2012.
- http://clinicaltrials.gov/, NCT00299598, accessed 22 July 2012.
- http://clinicaltrials.gov/, NCT01007240, accessed 22 July 2012.
- http://clinicaltrials.gov/, NCT01050777, accessed 22 July 2012.
- Celator Pharmaceuticals, Products (CPX 351, CPX 1), http://www.celatorpharma.com/new/products.html, accessed 22 July 2012.
- http://clinicaltrials.gov/, NCT01416558, accessed 22 July 2012.
- http://clinicaltrials.gov/, NCT00944801, accessed 22 July 2012.
- T. Barnes and R. Moots, Int. J. Nanomed., 2007, 2, 3–7 CrossRef CAS.
- http://clinicaltrials.gov/, NCT01464593, accessed 22 July 2012.
- http://clinicaltrials.gov/, NCT00093444, accessed 22 July 2012.
- http://clinicaltrials.gov/, NCT01640847, accessed 22 July 2012.
- C. Oerlemans, W. Bult, M. Bos, G. Storm, J. F. Nijsen and W. E. Hennink, Pharm. Res., 2010, 27, 2569–2589 CrossRef CAS.
- NanoCarrier, Product Pipeline, http://www.nanocarrier.co.jp/en/research/pipeline/index.html, accessed 22 July 2012.
- Veridex LLC, http://www.veridex.com/CellSearch/CellSearchHCP.aspx, accessed 22 July 2012.
- Nanosphere Inc., http://www.nanosphere.us/products, accessed 22 July 2012.
- H. M. E. Azzazy and R. H. Christenson, Clin. Biochem., 2002, 35, 13–27 CrossRef CAS.
- Siemens Healthcare Diagnostics Inc., Stratus® CS Acute Care™ Diagnostic System, http://www.medical.siemens.com/webapp/wcs/stores/servlet/ProductDisplay~q_catalogId~e_-111~a_catTree~e_100001, 1023069, 1023067~a_langId~e_-111~a_productId~e_182056~a_storeId~e_10001.htm, accessed 22 July 2012.
- J. Lisziewicz, N. Bakare, S. A. Calarota, D. Bánhegyi, J. Szlávik, E. Újhelyi, E. R. Tőke, L. Molnár, Z. Lisziewicz, B. Autran and F. Lori, PLoS One, 2012, 7, e35416 CAS.
- D. Tyssen, S. A. Henderson, A. Johnson, J. Sterjovski, K. Moore, J. La, M. Zanin, S. Sonza, P. Karellas, M. P. Giannis, G. Krippner, S. Wesselingh, T. McCarthy, P. R. Gorry, P. A. Ramsland, R. Cone, J. R. A. Paull, G. R. Lewis and G. Tachedjian, PLoS One, 2010, 5, e12309 Search PubMed.
- Starpharma Holdings Limited, http://www.starpharma.com/vivagel, accessed 22 July 2012.
- Cytimmune Sciences Inc, http://www.cytimmune.com/go.cfm?do=page.view&pid=26, accessed 22 July 2012.
- Nanospectra Biosciences Inc., http://www.nanospectra.com/index.html, accessed 22 July 2012.
- http://clinicaltrials.gov/, NCT00660543, accessed 22 July 2012.
- http://clinicaltrials.gov/, NCT01296139, accessed 22 July 2012.
- Amag Pharmaceuticals Inc., GastroMark™, http://www.amagpharma.com/products/gastromark.php, accessed 22 July 2012.
- Amag Pharmaceuticals Inc., Feraheme, http://www.feraheme.com/, accessed 10 June 2012.
- Drugs@FDA, Feromoxytol, http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
- http://clinicaltrials.gov/, NCT01169935, accessed 22 July 2012.
- MagForce AG, http://www.magforce.de/en/produkte.html, accessed 22 July 2012.
- Smith&Nephew, ACTICOAT: Antimicrobial Barrier Dressing, http://global.smith-nephew.com/us/ACTICOAT_PRODUCT_RANGE_8803.htm, accessed 22 July 2012.
- http://clinicaltrials.gov/, NCT00659204, accessed 22 July 2012.
- http://clinicaltrials.gov/, NCT01598480, accessed 22 July 2012.
- http://clinicaltrials.gov/, NCT00337714, accessed 22 July 2012.
- J. E. Kim, J. Y. Shin and M. H. Cho, Arch. Toxicol., 2012, 86, 685–700 CrossRef CAS.
- A. Bumb, C. A. Regino, M. R. Perkins, M. Bernardo, M. Ogawa, L. Fugger, P. L. Choyke, P. J. Dobson and M. W. Brechbiel, Nanotechnology, 2010, 21, 175704 CrossRef CAS.
- C. G. Daughton and T. A. Ternes, Environ. Health Perspect., 1999, 107(suppl. 6), 907–938 CrossRef CAS.
- A. B. Boxall, EMBO Rep., 2004, 5, 1110–1116 CrossRef CAS.
- M. J. M. Bueno, M. J. Gomez, S. Herrera, M. D. Hernando, A. Agüera and A. R. Fernández-Alba, Environ. Pollut., 2012, 164, 267–273 CrossRef CAS.
- S. González Alonso, M. Catalá, R. R. Maroto, J. L. R. Gil, Á. G. de Miguel and Y. Valcárcel, Environ. Int., 2010, 36, 195–201 CrossRef.
- R. López-Roldán, M. L. de Alda, M. Gros, M. Petrovic, J. Martín-Alonso and D. Barceló, Chemosphere, 2010, 80, 1337–1344 CrossRef.
- T.-H. Fang, F.-H. Nan, T.-S. Chin and H.-M. Feng, Mar. Pollut. Bull., 2012, 64, 1435–1444 CrossRef CAS.
- Y. Yang, J. Fu, H. Peng, L. Hou, M. Liu and J. L. Zhou, J. Hazard. Mater., 2011, 190, 588–596 CrossRef CAS.
- P. Vazquez-Roig, R. Segarra, C. Blasco, V. Andreu and Y. Picó, J. Chromatogr., A, 2010, 1217, 2471–2483 CrossRef CAS.
- T. Eggen, M. Moeder and A. Arukwe, Sci. Total Environ., 2010, 408, 5147–5157 CrossRef CAS.
- M. S. Fram and K. Belitz, Sci. Total Environ., 2011, 409, 3409–3417 CrossRef CAS.
- C. Wang, H. Shi, C. D. Adams, S. Gamagedara, I. Stayton, T. Timmons and Y. Ma, Water Res., 2011, 45, 1818–1828 CrossRef CAS.
- European Medicines Agency, Guideline on the Environmental Risk Assessment of Medical Products for Human Use EMEA/CHMP/SWP/4447/00 corr 1*, Committee for Medicinal Products for Human Use, London, 2006, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500003978.pdf, accessed 10 June 2012 Search PubMed.
- A. J. Ramirez, R. A. Brain, S. Usenko, M. A. Mottaleb, J. G. O'Donnell, L. L. Stahl, J. B. Wathen, B. D. Snyder, J. L. Pitt, P. Perez-Hurtado, L. L. Dobbins, B. W. Brooks and C. K. Chambliss, Environ. Toxicol. Chem., 2009, 28, 2587–2597 CrossRef CAS.
- A. Lajeunesse, C. Gagnon, F. Gagné, S. Louis, P. Čejka and S. Sauvé, Chemosphere, 2011, 83, 564–571 CrossRef CAS.
- L. H. Santos, A. N. Araujo, A. Fachini, A. Pena, C. Delerue-Matos and M. C. Montenegro, J. Hazard. Mater., 2010, 175, 45–95 CrossRef CAS.
- A. Daneshvar, J. Svanfelt, L. Kronberg, M. Prévost and G. A. Weyhenmeyer, Chemosphere, 2010, 80, 301–309 CrossRef CAS.
- T. V. Madureira, J. C. Barreiro, M. J. Rocha, E. Rocha, Q. B. Cass and M. E. Tiritan, Sci. Total Environ., 2010, 408, 5513–5520 CrossRef CAS.
- A. Daneshvar, J. Svanfelt, L. Kronberg and G. A. Weyhenmeyer, J. Environ. Monit., 2012, 14, 596–603 RSC.
- H. Leknes, I. E. Sturtzel and C. Dye, Sci. Total Environ., 2012, 414, 632–638 CrossRef CAS.
- S. Kugathas, R. J. Williams and J. P. Sumpter, Environ. Int., 2012, 40, 15–23 CrossRef CAS.
- V. L. Cunningham, V. J. D'Aco, D. Pfeiffer, P. D. Anderson, M. E. Buzby, R. E. Hannah, J. Jahnke and N. J. Parke, Integr. Environ. Assess. Manage., 2012, 8, 530–542 CrossRef CAS.
- N. Vieno, Occurrence of Pharmaceuticals in Finnish Sewage Treatment Plants, Surface Waters, and Their Elimination in Drinking Water Treatment Processes, Tampere University of Technology, 2007 Search PubMed.
- P. Verlicchi, M. Al Aukidy, A. Galletti, M. Petrovic and D. Barceló, Sci. Total Environ., 2012, 430, 109–118 CrossRef CAS.
- WHO/UNICEF, Progress on Sanitation and Drinking-water: 2010, 2010, http://www.who.int/water_sanitation_health/publications/9789241563956/en/index.html, accessed 21 July 2012 Search PubMed.
- European Environment Agency, Urban waste water treatment (CSI 024) – Assessment published Dec 2010, http://www.eea.europa.eu/data-and-maps/indicators/urban-waste-water-treatment/urban-waste-water-treatment-assessment-2/#toc-2, accessed 21 July 2012.
- L. Wolf, C. Zwiener and M. Zemann, Sci. Total Environ., 2012, 430, 8–19 CrossRef CAS.
- S. Sauvé, K. Aboulfadl, S. Dorner, P. Payment, G. Deschamps and M. Prévost, Chemosphere, 2012, 86, 118–123 CrossRef.
- R. Owen and S. Jobling, Nature, 2012, 485, 441 CrossRef CAS.
- P. Verlicchi, M. Al Aukidy and E. Zambello, Sci. Total Environ., 2012, 429, 123–155 CrossRef CAS.
- C. E. West and S. J. Rowland, Environ. Sci. Technol., 2012, 46, 4749–4756 CrossRef CAS.
- D. Fatta-Kassinos, M. I. Vasquez and K. Kümmerer, Chemosphere, 2011, 85, 693–709 CrossRef CAS.
- A. Jelic, M. Gros, A. Ginebreda, R. Cespedes-Sánchez, F. Ventura, M. Petrovic and D. Barcelo, Water Res., 2011, 45, 1165–1176 CrossRef CAS.
- S. N. Mahnik, K. Lenz, N. Weissenbacher, R. M. Mader and M. Fuerhacker, Chemosphere, 2007, 66, 30–37 CrossRef CAS.
- S. Jobling and R. Owen, Ethinyl oestradiol in the aquatic environment: a bitter pill for the precautionary principle, in Late lessons from early warnings: science, precaution, innovation, ed. D. Gee, European Environment Agency, Copenhagen, 2013, ch. 13, pp. 199–226 Search PubMed.
- M. C. Perron and P. Juneau, Environ. Res., 2011, 111, 520–529 CrossRef CAS.
- W. Zhang, M. Zhang, K. Lin, W. Sun, B. Xiong, M. Guo, X. Cui and R. Fu, Environ. Toxicol. Pharmacol., 2012, 33, 344–352 CrossRef CAS.
- S. Dietrich, F. Ploessl, F. Bracher and C. Laforsch, Chemosphere, 2010, 79, 60–66 CrossRef CAS.
- J. C. Underwood, R. W. Harvey, D. W. Metge, D. A. Repert, L. K. Baumgartner, R. L. Smith, T. M. Roane and L. B. Barber, Environ. Sci. Technol., 2011, 45, 3096–3101 CrossRef CAS.
- Y. Guler and A. T. Ford, Aquat. Toxicol., 2010, 99, 397–404 CrossRef CAS.
- K. A. Kidd, P. J. Blanchfield, K. H. Mills, V. P. Palace, R. E. Evans, J. M. Lazorchak and R. W. Flick, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8897–8901 CrossRef CAS.
- C. Vannini, G. Domingo, M. Marsoni, F. De Mattia, M. Labra, S. Castiglioni and M. Bracale, Aquat. Toxicol., 2011, 101, 459–465 CrossRef CAS.
- F. Pomati, C. Orlandi, M. Clerici, F. Luciani and E. Zuccato, Toxicol. Sci., 2008, 102, 129–137 CrossRef CAS.
- T. V. Madureira, M. J. Rocha, C. Cruzeiro, I. Rodrigues, R. A. Monteiro and E. Rocha, Environ. Toxicol. Pharmacol., 2012, 34, 34–45 CrossRef CAS.
- J. L. Oaks, M. Gilbert, M. Z. Virani, R. T. Watson, C. U. Meteyer, B. A. Rideout, H. L. Shivaprasad, S. Ahmed, M. J. Iqbal Chaudhry, M. Arshad, S. Mahmood, A. Ali and A. Ahmed Khan, Nature, 2004, 427, 630–633 CrossRef CAS.
- ERAPharm, Environmental Risk Assessment of Pharmaceuticals (EU Sixth Framework Programme), http://www.erapharm.org/, accessed 17 June 2012.
- KNAPPE, Knowledge and Need Assessment of Pharmaceutical Products in Environmental Waters: Project Final Report (2008), http://environmentalhealthcollaborative.org/images/KNAPPE_REPORT_FINAL.pdf, accessed 21 July 2012.
- B. A. Boxall, M. A. Rudd, B. W. Brooks, D. J. Caldwell, K. Choi, S. Hickmann, E. Innes, K. Ostapyk, J. P. Staveley, T. Verslycke, G. T. Ankley, K. F. Beazley, S. E. Belanger, J. P. Berninger, P. Carriquiriborde, A. Coors, P. C. DeLeo, S. D. Dyer, J. F. Ericson, F. Gagné, J. P. Giesy, T. Gouin, L. Hallstrom, M. V. Karlsson, D. G. J. Larsson, J. M. Lazorchak, F. Mastrocco, A. McLaughlin, M. E. McMaster, R. D. Meyerhoff, R. Moore, J. L. Parrott, J. R. Snape, R. Murray-Smith, M. R. Servos, P. K. Sibley, J. O. Straub, N. D. Szabo, E. Topp, G. R. Tetreault, V. L. Trudeau and G. Van Der Kraak, Environ. Health Perspect., 2012, 120, 1221–1229 CrossRef.
- E. R. Cooper, T. C. Siewicki and K. Phillips, Sci. Total Environ., 2008, 398, 26–33 CrossRef CAS.
- V. Roos, L. Gunnarsson, J. Fick, D. G. J. Larsson and C. Ruden, Sci. Total Environ., 2012, 421, 102–110 CrossRef.
- A. Ginebreda, I. Muñoz, M. L. de Alda, R. Brix, J. López-Doval and D. Barceló, Environ. Int., 2010, 36, 153–162 CrossRef CAS.
- European Union, Press Release (Environment and Water: Proposal to Reduce Water Pollution Risks), http://europa.eu/rapid/pressReleasesAction.do?reference=IP/12/88, accessed 29 July 2012.
- W.-J. Sim, J.-W. Lee, E.-S. Lee, S.-K. Shin, S.-R. Hwang and J.-E. Oh, Chemosphere, 2011, 82, 179–186 CrossRef CAS.
- R. Rodil, J. B. Quintana, E. Concha-Graña, P. López-Mahía, S. Muniategui-Lorenzo and D. Prada-Rodríguez, Chemosphere, 2012, 86, 1040–1049 CrossRef CAS.
- C. Lacey, S. Basha, A. Morrissey and J. Tobin, Environ. Monit. Assess., 2012, 184, 1049–1062 CrossRef CAS.
- S. K. Behera, H. W. Kim, J.-E. Oh and H.-S. Park, Sci. Total Environ., 2011, 409, 4351–4360 CrossRef CAS.
- L. Shala and G. Foster, Arch. Environ. Contam. Toxicol., 2010, 58, 551–561 CrossRef CAS.
- K. Wille, H. Noppe, K. Verheyden, J. Vanden Bussche, E. De Wulf, P. Van Caeter, C. R. Janssen, H. F. De Brabander and L. Vanhaecke, Anal. Bioanal. Chem., 2010, 397, 1797–1808 CrossRef CAS.
- B. P. Chari and R. U. Halden, Water Res., 2012, 46, 4814–4824 CrossRef CAS.
- E. Gracia-Lor, J. V. Sancho, R. Serrano and F. Hernández, Chemosphere, 2012, 87, 453–462 CrossRef CAS.
- Y. Fang, A. Karnjanapiboonwong, D. A. Chase, J. F. Wang, A. N. Morse and T. A. Anderson, Environ. Toxicol. Chem., 2012, 31, 550–555 CrossRef CAS.
- V. Kumar, N. Nakada, M. Yasojima, N. Yamashita, A. C. Johnson and H. Tanaka, Chemosphere, 2011, 82, 1124–1128 CrossRef CAS.
- E. Vulliet, L. Wiest, R. Baudot and M.-F. Grenier-Loustalot, J. Chromatogr., A, 2008, 1210, 84–91 CrossRef CAS.
- J. Shen, Y. Zhu, X. Yang and C. Li, Chem. Commun., 2012, 48, 3686–3699 RSC.
- J. Yin, B. Shao, J. Zhang and K. Li, Bull. Environ. Contam. Toxicol., 2010, 84, 39–45 CrossRef CAS.
- L. Kovalova, C. S. McArdell and J. Hollender, J. Chromatogr., A, 2009, 1216, 1100–1108 CrossRef CAS.
- J.-U. Mullot, S. Karolak, A. Fontova, B. Huart and Y. Levi, Anal. Bioanal. Chem., 2009, 394, 2203–2212 CrossRef CAS.
- C. M. Coetsier, S. Spinelli, L. Lin, B. Roig and E. Touraud, Environ. Int., 2009, 35, 787–792 CrossRef CAS.
- J. C. Van De Steene, C. P. Stove and W. E. Lambert, Sci. Total Environ., 2010, 408, 3448–3453 CrossRef CAS.
- Y. Vystavna, F. Huneau, V. Grynenko, Y. Vergeles, H. Celle-Jeanton, N. Tapie, H. Budzinski and P. Le Coustumer, Water, Air, Soil Pollut., 2012, 223, 2111–2124 CrossRef CAS.
- M. Stuart, D. Lapworth, E. Crane and A. Hart, Sci. Total Environ., 2012, 416, 1–21 CrossRef CAS.
- J.-P. Besse, J.-F. Latour and J. Garric, Environ. Int., 2012, 39, 73–86 CrossRef CAS.
- J. O. Straub, Integr. Environ. Assess. Manage., 2010, 6, 540–566 CAS.
- V. Calisto and V. I. Esteves, Chemosphere, 2009, 77, 1257–1274 CrossRef CAS.
- European Union, Nanotechnology (CORDIS Archieves), ftp://ftp.cordis.europa.eu/pub/nanotechnology/docs/fp7_call_2007_nano.pdf, accessed 14 July 2014.
- A. D. Maynard, P. A. Baron, M. Foley, A. A. Shvedova, E. R. Kisin and V. Castranova, J. Toxicol. Environ. Health, Part A, 2004, 67, 87–107 CrossRef CAS.
- M. A. Kiser, P. Westerhoff, T. Benn, Y. Wang, J. Perez-Rivera and K. Hristovski, Environ. Sci. Technol., 2009, 43, 6757–6763 CrossRef CAS.
- S. A. Blaser, M. Scheringer, M. MacLeod and K. Hungerbühler, Sci. Total Environ., 2008, 390, 396–409 CrossRef CAS.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS.
- Y. Hong, R. J. Honda, N. V. Myung and S. L. Walker, Environ. Sci. Technol., 2009, 43, 8834–8839 CrossRef CAS.
- G. V. Lowry, K. B. Gregory, S. C. Apte and J. R. Lead, Environ. Sci. Technol., 2012, 46, 6893–6899 CrossRef CAS.
- S. A. Cumberland and J. R. Lead, J. Chromatogr., A, 2009, 1216, 9099–9105 CrossRef CAS.
- S. S. Khan, A. Mukherjee and N. Chandrasekaran, Water Res., 2011, 45, 5184–5190 CrossRef CAS.
- Z. Sheng and Y. Liu, Water Res., 2011, 45, 6039–6050 CrossRef CAS.
- J. Fabrega, S. R. Fawcett, J. C. Renshaw and J. R. Lead, Environ. Sci. Technol., 2009, 43, 7285–7290 CrossRef CAS.
- J. Fabrega, J. C. Renshaw and J. R. Lead, Environ. Sci. Technol., 2009, 43, 9004–9009 CrossRef CAS.
- Z. Q. Li, K. Greden, P. J. J. Alvarez, K. B. Gregory and G. V. Lowry, Environ. Sci. Technol., 2010, 44, 3462–3467 CrossRef CAS.
- J. Gao, K. Powers, Y. Wang, H. Zhou, S. M. Roberts, B. M. Moudgil, B. Koopman and D. S. Barber, Chemosphere, 2012, 89, 96–101 CrossRef CAS.
- H. Zhang, J. A. Smith and V. Oyanedel-Craver, Water Res., 2012, 46, 691–699 CrossRef CAS.
- A. Hitchman, Y. Ju-Nam, G. Sambrook-Smith, G. M. Sterling and J. R. Lead, Chemosphere, 2012 DOI:10.1016/j.chemosphere.2012.07.041.
- C. Coutris, E. J. Joner and D. H. Oughton, Sci. Total Environ., 2012, 420, 327–333 CrossRef CAS.
- H. Schwegmann, A. J. Feitz and F. H. Frimmel, J. Colloid Interface Sci., 2010, 347, 43–48 CrossRef CAS.
- S. Lee, K. Kim, H. K. Shon, S. D. Kim and J. Cho, J. Nanopart. Res., 2011, 13, 3051–3061 CrossRef CAS.
- A. A. Keller, H. Wang, D. Zhou, H. S. Lenihan, G. Cherr, B. J. Cardinale, R. Miller and Z. Ji, Environ. Sci. Technol., 2010, 44, 1962–1967 CrossRef CAS.
- J. Fabrega, S. N. Luoma, C. R. Tyler, T. S. Galloway and J. R. Lead, Environ. Int., 2011, 37, 517–531 CrossRef CAS.
- R. Handy, F. von der Kammer, J. R. Lead, M. Hassellöv, R. Owen and M. Crane, Ecotoxicology, 2008, 17, 287–314 CrossRef CAS.
- Y. Ju-Nam and J. R. Lead, Sci. Total Environ., 2008, 400, 396–414 CrossRef CAS.
- S. J. Klaine, P. J. J. Alvarez, G. E. Batley, T. F. Fernandes, R. D. Handy, D. Y. Lyon, S. Mahendra, M. J. McLaughlin and J. R. Lead, Environ. Toxicol. Chem., 2008, 27, 1825–1851 CrossRef CAS.
- A. M. El Badawy, R. G. Silva, B. Morris, K. G. Scheckel, M. T. Suidan and T. M. Tolaymat, Environ. Sci. Technol., 2010, 45, 283–287 CrossRef.
- S. C. Hayden, G. X. Zhao, K. Saha, R. L. Phillips, X. N. Li, O. R. Miranda, V. M. Rotello, M. A. El-Sayed, I. Schmidt-Krey and U. H. F. Bunz, J. Am. Chem. Soc., 2012, 134, 6920–6923 CrossRef CAS.
- J. L. Ferry, P. Craig, C. Hexel, P. Sisco, R. Frey, P. L. Pennington, M. H. Fulton, I. G. Scott, A. W. Decho, S. Kashiwada, C. J. Murphy and T. J. Shaw, Nat. Nanotechnol., 2009, 4, 441–444 CrossRef CAS.
- M.-N. l. Croteau, S. K. Misra, S. N. Luoma and E. Valsami-Jones, Environ. Sci. Technol., 2011, 45, 6600–6607 CrossRef CAS.
- J. B. Morrow, C. P. Arango and R. D. Holbrook, J. Environ. Qual., 2010, 39, 1934 CrossRef CAS.
- A. R. Stojak, T. Raftery, S. J. Klaine and T. L. Mcnealy, Nanotoxicology, 2011, 5, 730–742 CrossRef CAS.
- M. S. Hull, P. Chaurand, J. Rose, M. Auffan, J.-Y. Bottero, J. C. Jones, I. R. Schultz and P. J. Vikesland, Environ. Sci. Technol., 2011, 45, 6592–6599 CrossRef CAS.
- M. O. Montes, S. K. Hanna, H. S. Lenihan and A. A. Keller, J. Hazard. Mater., 2012, 225–226, 139–145 CrossRef CAS.
- A. Baun, S. N. Sørensen, R. F. Rasmussen, N. B. Hartmann and C. B. Koch, Aquat. Toxicol., 2008, 86, 379–387 CrossRef CAS.
- J. W. Park, T. B. Henry, S. Ard, F. M. Menn, R. N. Compton and G. S. Sayler, Nanotoxicology, 2011, 5, 406–416 CrossRef CAS.
- D. Zhang, H. Niu, X. Zhang, Z. Meng and Y. Cai, J. Hazard. Mater., 2011, 192, 1088–1093 CrossRef CAS.
- T. Walser, L. K. Limbach, R. Brogioli, E. Erismann, L. Flamigni, B. Hattendorf, M. Juchli, F. Krumeich, C. Ludwig, K. Prikopsky, M. Rossier, D. Saner, A. Sigg, S. Hellweg, D. Gunther and W. J. Stark, Nat. Nanotechnol., 2012, 7(8), 520–524 CrossRef CAS.
- L. Roes, M. K. Patel, E. Worrell and C. Ludwig, Sci. Total Environ., 2012, 417, 76–86 CrossRef.
- D. R. Taft, in Pharmacology: Principles and Practice, ed. M. P. Hacker, W. S. Messer and K. A. Bachmann, Academic Press/Elsevier, 2009, pp. 176–200 Search PubMed.
- J. Lipka, M. Semmler-Behnke, R. A. Sperling, A. Wenk, S. Takenaka, C. Schleh, T. Kissel, W. J. Parak and W. G. Kreyling, Biomaterials, 2010, 31, 6574–6581 CrossRef CAS.
- S. K. Balasubramanian, J. Jittiwat, J. Manikandan, C.-N. Ong, L. E. Yu and W.-Y. Ong, Biomaterials, 2010, 31, 2034–2042 CrossRef CAS.
- S. Hirn, M. Semmler-Behnke, C. Schleh, A. Wenk, J. Lipka, M. Schäffler, S. Takenaka, W. Möller, G. Schmid, U. Simon and W. G. Kreyling, Eur. J. Pharm. Biopharm., 2011, 77, 407–416 CrossRef CAS.
- V. Ferranti, C. Chabenat, H. Marchais, S. Menager, H. Hue, A. M. Orecchioni and O. Lafont, Drug Metab. Drug Interact., 2001, 18, 191–208 CrossRef CAS.
- V. L. Elliott, G. T. Edge, M. M. Phelan, L.-Y. Lian, R. Webster, R. F. Finn, B. K. Park and N. R. Kitteringham, Mol. Pharmaceutics, 2012, 9, 1291–1301 CAS.
- Drugs@FDA, Taxol, http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf, accessed 10 May 2012.
- Drugs@FDA, Abraxane, http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021660s025s026s029lbl.pdf, accessed 10 May 2012.
- H. P. Jarvie, H. Al-Obaidi, S. M. King, M. J. Bowes, M. J. Lawrence, A. F. Drake, M. A. Green and P. J. Dobson, Environ. Sci. Technol., 2009, 43, 8622–8628 CrossRef CAS.
- T. L. Kirschling, P. L. Golas, J. M. Unrine, K. Matyjaszewski, K. B. Gregory, G. V. Lowry and R. D. Tilton, Environ. Sci. Technol., 2011, 45, 5253–5259 CrossRef CAS.
- M. Zhang and M. Akbulut, Langmuir, 2011, 27, 12550–12559 CrossRef CAS.
- J.-M. Nam, C. S. Thaxton and C. A. Mirkin, Science, 2003, 301, 1884–1886 CrossRef CAS.
- J. Robbens, C. Vanparys, I. Nobels, R. Blust, K. Van Hoecke, C. Janssen, K. De Schamphelaere, K. Roland, G. Blanchard, F. Silvestre, V. Gillardin, P. Kestemont, R. Anthonissen, O. Toussaint, S. Vankoningsloo, C. Saout, E. Alfaro-Moreno, P. Hoet, L. Gonzalez, P. Dubruel and P. Troisfontaines, Toxicology, 2010, 269, 170–181 CrossRef CAS.
- T. C. K. Heiden, E. Dengler, W. J. Kao, W. Heideman and R. E. Peterson, Toxicol. Appl. Pharmacol., 2007, 225, 70–79 CrossRef.
- A. N. Petit, P. Eullaffroy, T. Debenest and F. Gagne, Aquat. Toxicol., 2010, 100, 187–193 CrossRef CAS.
- A. N. Petit, T. Debenest, P. Eullaffroy and F. Gagne, Nanotoxicology, 2012, 6, 315–326 CrossRef CAS.
- I. J. Suarez, R. Rosal, A. Rodriguez, A. Ucles, A. R. Fernandez-Alba, M. D. Hernando and E. García-Calvo, TrAC, Trends Anal. Chem., 2011, 30, 492–506 CrossRef CAS.
- H. Zhu, J. Han, J. Q. Xiao and Y. Jin, J. Environ. Monit., 2008, 10, 713–717 RSC.
- H. Wang, X. Kou, Z. Pei, J. Q. Xiao, X. Shan and B. Xing, Nanotoxicology, 2011, 5, 30–42 CrossRef CAS.
- J. Hu, D. Wang, J. Wang and J. Wang, Environ. Pollut., 2012, 162, 216–222 CrossRef CAS.
- V. Shah and I. Belozerova, Water, Air, Soil Pollut., 2009, 197, 143–148 CrossRef CAS.
- T. Sabo-Attwood, J. M. Unrine, J. W. Stone, C. J. Murphy, S. Ghoshroy, D. Blom, P. M. Bertsch and L. A. Newman, Nanotoxicology, 2012, 6, 353–360 CrossRef CAS.
- J. D. Judy, J. M. Unrine, W. Rao, S. Wirick and P. M. Bertsch, Environ. Sci. Technol., 2012, 46(15), 8467–8474 CrossRef CAS.
- R. Barrena, E. Casals, J. Colón, X. Font, A. Sánchez and V. Puntes, Chemosphere, 2009, 75, 850–857 CrossRef CAS.
- P. V. Asharani, Y. lianwu, Z. Gong and S. Valiyaveettil, Nanotoxicology, 2011, 5, 43–54 CrossRef CAS.
- B. Geffroy, C. Ladhar, S. Cambier, M. Treguer-Delapierre, D. Brethes and J. P. Bourdineaud, Nanotoxicology, 2012, 6, 144–160 CrossRef CAS.
- P. P. Pompa, G. Vecchio, A. Galeone, V. Brunetti, S. Sabella, G. Maiorano, A. Falqui, G. Bertoni and R. Cingolani, Nano Res., 2011, 4, 405–413 CrossRef CAS.
- G. Vecchio, A. Galeone, V. Brunetti, G. Maiorano, L. Rizzello, S. Sabella, R. Cingolani and P. P. Pompa, Nanomed.: Nanotechnol., Biol. Med., 2012, 8, 1–7 CrossRef CAS.
- S. Tedesco, H. Doyle, J. Blasco, G. Redmond and D. Sheehan, Aquat. Toxicol., 2010, 100, 178–186 CrossRef CAS.
- O. V. Tsyusko, J. M. Unrine, D. Spurgeon, E. Blalock, D. Starnes, M. Tseng, G. Joice and P. M. Bertsch, Environ. Sci. Technol., 2012, 46, 4115–4124 CrossRef CAS.
- V. Christen and K. Fent, Chemosphere, 2012, 87, 423–434 CrossRef CAS.
- C. Wei, Y. Zhang, J. Guo, B. Han, X. Yang and J. Yuan, J. Environ. Sci., 2010, 22, 155–160 CrossRef CAS.
- K. Van Hoecke, K. A. C. De Schamphelaere, P. Van der Meeren, S. Lcucas and C. R. Janssen, Environ. Toxicol. Chem., 2008, 27, 1948–1957 CrossRef CAS.
- European Medicines Agency, Orphan Medical Product Designation, http://www.ema.europa.eu/docs/en_GB/document_library/Brochure/2011/03/WC500104234.pdf, accessed 4 May 2012.
- D. B. Kramer, S. Xu and A. S. Kesselheim, N. Engl. J. Med., 2012, 366, 848–855 CrossRef CAS.
- B. H. Robinson, Sci. Total Environ., 2009, 408, 183–191 CrossRef CAS.
- European Union, Directive 2012/19/EU of the European Parliament and of the Council of 4 July 2012 on waste electrical and electronic equipment (WEEE), accessed 17 November 2012.
- E. J. Petersen, Q. Huang and W. J. Weber, Environ. Toxicol. Chem., 2010, 29, 1106–1112 CAS.
- W. Giger, A. C. Alder, E. M. Golet, H.-P. E. Kohler, C. S. McArdell, E. Molnar, H. Siegrist and M. J.-F. Suter, Chimia, 2003, 57, 485–491 CrossRef CAS.
- F. Stuer-Lauridsen, M. Birkved, L. P. Hansen, H. C. Holten Lützhøft and B. Halling-Sørensen, Chemosphere, 2000, 40, 783–793 CrossRef CAS.
- E. Walters, K. McClellan and R. U. Halden, Water Res., 2010, 44, 6011–6020 CrossRef CAS.
- J. Giri, M. S. Diallo, W. A. G. Iii, N. F. Dalleska, X. Fang and Y. Tang, Environ. Sci. Technol., 2009, 43, 5123–5129 CrossRef CAS.
- R. D. Handy, G. Cornelis, T. Fernandes, O. Tsyusko, A. Decho, T. Sabo-Attwood, C. Metcalfe, J. A. Steevens, S. J. Klaine, A. A. Koelmans and N. Horne, Environ. Toxicol. Chem., 2012, 31, 15–31 CrossRef CAS.
- K. Malkiewicz, M. Pettitt, K. A. Dawson, A. Toikka, S. O. Hansson, J. Hukkinen, I. Lynch and J. Lead, Nanomaterials in REACH, 2011, http://www.skep-network.eu/Libraries/Network_documents/SKEP_Nanomaterials_in_REACH_Report.sflb.ashx, accessed 10 June 2012 Search PubMed.
- I. Römer, T. A. White, M. Baalousha, K. Chipman, M. R. Viant and J. R. Lead, J. Chromatogr., A, 2011, 1218, 4226–4233 CrossRef.
- P. C. Naha, M. Davoren, A. Casey and H. J. Byrne, Environ. Sci. Technol., 2009, 43, 6864–6869 CrossRef CAS.
- M. Tejamaya, I. Römer, R. C. Merrifield and J. R. Lead, Environ. Sci. Technol., 2012, 46, 7011–7017 CrossRef CAS.
Footnotes |
| † The terms nanomedicine, nano-enabled medical products and nano-enabled medical applications have been used interchangeably in the text. |
| ‡ Systemic toxicity refers to adverse effects on any organ system following absorption and distribution of a chemical throughout the body (see ref. 11). |
| § Therapeutic agents can be small molecule drugs, biotechnology derived products e.g., cell, oligonucleotides, monoclonal antibodies, polymers, and vaccines. The terms therapeutics agents, pharmaceuticals and drugs has been used interchangeably in the text. |
| ¶ Sometimes called nanovectors or drug delivery systems/vehicles or nanoconstructs. |
| || The term ‘pharmaceuticals products’ (PPs) used in the text in this section includes prodrugs, pharmaceuticals, metabolites and transformation products. Unless otherwise mentioned, pharmaceuticals mean pharmaceuticals for human use. |
| ** PECsw is Predicted Environmental Concentration in surface waters. |
| †† In this review, nanomaterials (NMs) refer to all engineered or manufactured nanomaterials and do not cover incidental or natural nanomaterials. There is some understanding of manufactured NM fate and behaviour and nanomedicines, where there is little direct information, are a sub-set of NMs; therefore we focus on engineered NMs in this section to highlight potential nanomedicine behavior. |
| ‡‡ See Janez Potočnik's, European Commissioner for Science & Research, statement at http://cordis.europa.eu/nanotechnology/ |
| §§ Bile formation in the liver helps to bio-transform the drug and helps in drug metabolism. Excretion is primarily in the form of faeces. Other routes of drug excretion are via saliva, breast milk, intestinal exosorption (see ref. 201). |
¶¶ To qualify for orphan designation, a medicine must meet one of these criteria (as defined by the EMA, ref. 234): (1) it is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting no more than 5 in 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people in the EU at the time of submission of the designation application; (2) it is intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition and without incentives it is unlikely that the revenue after marketing of the medicinal product would cover the investment in its development. 000 people in the EU at the time of submission of the designation application; (2) it is intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition and without incentives it is unlikely that the revenue after marketing of the medicinal product would cover the investment in its development. |
| This journal is © The Royal Society of Chemistry 2013 |
