The biophysicochemical interactions at the interfaces between nanoparticles and aquatic organisms: adsorption and internalization
Si
Ma
a and
Daohui
Lin
*ab
aDepartment of Environmental Science, Zhejiang University, Hangzhou 310058, China. E-mail: lindaohui@zju.edu.cn; Fax: +86 571 88982590; Tel: +86 571 88982582
bZhejiang Provincial Key Laboratory of Organic Pollution Process and Control, Zhejiang University, Hangzhou 310058, China
First published on 11th December 2012
Abstract
Nano–bio interfacial interactions that can likely regulate the potential toxicity of nanoparticles (NPs) toward aquatic organisms are receiving increasing research interest worldwide and warrant more investigation. This review presents an overview of already-known nano–bio interactions and some speculations on the interfaces between NPs and aquatic organisms, in order to gain a new insight into the biological effects of NPs in the aquatic environment. The fundamental interfaces between NPs and organism cells and the main biophysicochemical interactions that occur at the nano–bio interfaces are described. The interfacial interactions, focused on adsorption and internalization, during the contact of NPs with microorganisms, hydrophytes, invertebrates and fish were reviewed. The effects of NP properties and suspending states as well as environmental conditions including pH, ionic strength, natural organic matter and other factors on the interfacial interactions were elucidated. Furthermore, the analytical methods employed in the interfacial interaction investigations were also briefly introduced. Future research directions of nano–bio interactions were prospected.
 Daohui Lin | Dr Daohui Lin is Professor of Environmental Chemistry (since 2009) at Zhejiang University, where he has been involved in teaching and research since 1998. He received his Ph.D. from Zhejiang University (2005), M.Sc. degree from Beijing Normal University (1998) and B.Sc. degree from Hangzhou University (1995). He was a visiting scholar (2006–2008) at the University of Massachusetts at Amherst and was selected as a New Century Excellent Talent in University by Ministry of Education of China in 2010. His research interests focus on environmental behavior and risk of contaminants, especially of engineered nanomaterials. He has published about 50 peer-reviewed articles. |
Environmental impactAs an increasing number of nanoparticles (NPs) are being released into the aquatic environment, their nanotoxicity to aquatic organisms has aroused research interest, but studies on the interfacial interactions from a micro perspective are really limited. In this review, we provide an overview of the biophysicochemical interactions between NPs and cells, and then demonstrate the nano–bio interactions at the interfaces when NPs meet microorganisms, hydrophytes, invertebrates and fish. Besides, the influencing factors (including the NP properties and the environmental conditions) affecting the interfacial interactions were stated. Analytical methods employed to study the nano–bio interfaces were briefly introduced, and the perspectives were also put forward. We believe this review will shed light on the ecological effect of NPs. |
1 Introduction
Nowadays, it is known that nanoparticles (NPs) will be increasingly released into the aquatic environment and their behaviors greatly depend on the environmental conditions, such as natural organic matter, pH, and ionic strength.1,2 As long as NPs are released into the aquatic environment, they may inevitably interact with microorganisms, hydrophytes, invertebrates, and/or fish, and thereby exhibit potential toxicity to these aquatic organisms and consequently human beings through food chains. Increasing research has therefore been performed to investigate the nanotoxicity of NPs to aquatic organisms;3 however, the nano–bio interfacial interactions that can likely determine the potential nanotoxicity are still waiting to be fully understood.Unlike other contaminants, with unique physicochemical properties, NPs exhibit quite different toxicities when exposed to aquatic organisms during their existence in the aquatic environment. Oxidative stress, released metal ions, physical damage, and shading effects are the four mainly addressed toxic mechanisms of NPs toward aquatic organisms, especially microorganisms.3 Increasing evidence indicates that NPs can generate reactive oxygen species (ROS), and consequently induce oxidative injury, cell damage, and even apoptosis.4–8 Toxic metal ions released from metal NPs9 and carbon nanomaterials10 can cause toxicity toward aquatic organisms. Physical contact, including adsorption and internalization, is another mechanism contributing to the nanotoxicity.11,12 Shading and agglomeration can play an important role in the nanotoxicity toward phototrophic organisms.13–15 Each mechanism is not supposed to work alone. As to larger aquatic organisms, such as invertebrates and fish that contain complex tissues and organs, accumulation and distribution of the NPs inside the organisms, as well as the nano–bio interactions at the outer-surfaces of the organisms are of vital importance; and the toxic mechanisms may become much more sophisticated than that between unicellular animals and NPs. Besides the release of toxic metal ions, the other toxic mechanisms all involve a crucial precondition of close contact between NPs and aquatic organisms.16
It is worth noting that there are various microcosmic interfaces where biophysicochemical interactions occur between NPs and aquatic organisms, and in most cases the interactions can cause subsequent toxicity towards the organisms. A few researchers have already focused on nano–bio interfaces in order to fully understand the interactions between NPs and organisms.17 The main forces acting at the nano–bio interfaces include hydrodynamic, electrodynamic, electrostatic, solvent, steric and polymer bridging interactions.17 NPs may be adsorbed and thereupon be internalized by the aquatic organisms due to these interactions. However, there are many questions regarding the nano–bio interfaces and interactions which remain to be answered: How do NPs attach to organism surfaces and be internalized? What interfaces and interactions between NPs and aquatic organisms should be of importance? What factors will influence these interfaces and interactions? Therefore, this review is aimed at providing an overview of details from documented studies and introducing available knowledge on the interfacial interactions between NPs and aquatic organisms. The interfaces between cells and NPs as well as the interfacial interactions are described, and then the specific details of interfaces between NPs and microorganisms, hydrophytes, invertebrates and fish are elaborated. Furthermore, the influencing factors governing the interfacial interactions and some analytical methods were delineated and several future research needs are put forward.
2 Biophysicochemical interactions at NP–cell interfaces
2.1 NP–cell interfaces
In the aquatic environment, there are complex food webs supporting the whole ecosystem (Fig. 1). Micro algae and hydrophytes are regarded as the first trophic level as they are the primary producers. Bacterium is the decomposer, but also becomes the beginning of the food chain together with algae and hydrophytes after being eaten by plankton. Invertebrates, both zooplankton and zoobenthos, form the second trophic level as they feed on microorganisms, especially algae. Fish that eat invertebrates are in the higher trophic level. The released NPs may be suspended in the water phase or deposited in the sediment. The suspended NPs can likely interact with zooplankton, such as daphnia, while the NPs in the sediment may react with zoobenthos, including oyster, for instance.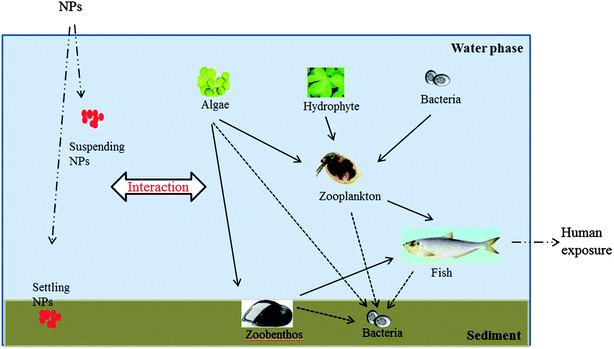 | ||
| Fig. 1 The food web in the aquatic environment. | ||
When NPs come into contact with aquatic organisms, the interface between these two objects refers to the micro region existing on the surfaces of both of them. The most important and fundamental interface is between the NPs and cells. Alga, bacterium, and hydrophyte cells generally have cell walls, which are the outermost layers of the cells when meeting NPs. Cellulose and pectin are the main compositions of the cell walls of algae and plants, while for bacteria the major component is peptidoglycan. In spite of this slight difference, the tough cell walls of these organisms can play a protective role in supporting the cell structure and resisting potential harmful substances including NPs. To date, however, research aimed at the interactions between NPs and cell walls is really scarce. Animal cells have no cell wall, so the membrane consisting of a phospholipid bilayer is the concerned interface for invertebrates and fish in the aquatic environment. Generally, there is a hydrated sheath on the interfaces in water, the thickness of which depends on various factors including the surface properties of the two contacting objects and the environmental conditions.
2.2 Interfacial interactions
Once NPs come into contact with cell surfaces, they may be adsorbed on the cell walls or membranes by multiple forces, and then it is likely that they will enter the cells through various routes. The biophysicochemical interactions at the interfaces between the NPs and cells, in conclusion, mainly include adsorption and internalization.Fig. 2 schematically illustrates the main adsorption mechanisms at the NP–cell interfaces. NPs with bio interfaces can be combined by van der Waals forces, a common attracting force. This force was confirmed to determine the interactions of SiO2 NPs with a dioleoyl phosphatidylcholine monolayer.18 NPs with hydrophobic surfaces would be adsorbed on the hydrophobic surface zones of the cells through hydrophobic forces. Electrostatic attraction, as another important and general adsorption mechanism, that can cause the charged NPs to become adsorbed on the cell surfaces with opposite charges, which has been documented in many studies.19 Moreover, some specific interactions exist, e.g. hydrogen bonding and receptor–ligand interactions, depending on the surface properties of both NPs and cells.17
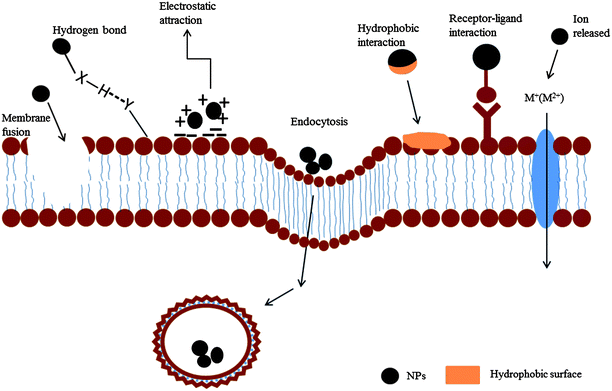 | ||
| Fig. 2 The interactions between NPs and a cell membrane. NPs can be adsorbed on the membrane (phospholipid bilayer) through receptor–ligand interactions, hydrophobic interactions, electrostatic attractions and hydrogen bonds. When membrane fusion occurs, the NPs can cross the membrane and enter the cells directly. Metal ions released from the NPs can be transported into the cells via certain membrane channels. Endocytosis occurs directly across the membrane and the NPs are wrapped inside. | ||
The cell-adsorbed NPs may be internalized via different routes. Rigid NPs with sharp shapes may directly penetrate through cell wall and membrane and then enter into cells. For cells without cell walls, NPs would interact directly with the membranes (Fig. 2). NPs may disrupt the cell membrane and directly enter the cells, e.g. cationic NPs20 and polycationic polymers21 could induce the formation of nanoscale holes, membrane permeability alterations, membrane thinning, and/or membrane erosion in the lipid bilayers of the cells and thus be internalized. On most occasions, internalization can not occur independently. A study has shown that the internalization of polymer iron oxide NPs into rat pheochromocytoma cells involves three distinct steps: plasma membrane attachment, endocytosis, and gradual accumulation in the membrane-bound intracellular vesicles.22 Endocytosis is the most accepted route for NP uptake. It was demonstrated that single-walled carbon nanotubes (SWCNTs) could enter cells by both penetration and endocytosis.23 PAA-coated Au nanospheres were also implied to enter cells via receptor-mediated endocytosis or membrane fusion.19 Interestingly, in contrast, CdTe nanocrystals capped by thiolated methoxypolyethyleneglycol were shown to penetrate through the lipid bilayer of giant unilamellar vesicles and giant plasma membrane vesicles which constitute basic endocytosis-free model membrane systems.24 Obviously, for different NPs and cells, endocytosis plays a different role. In addition, internalization of NPs could occur without overt membrane disruption, e.g., cationic nano-objects were observed to pass through cell membranes by generating transient holes.25 Besides, metal ions released from the NPs could pass through the membrane via specific channels and enter the cells.26 Hence, under different circumstances, the mechanisms of internalization may be quite different or even be contradictory – the specific reasons of which remain unknown and deserve further and systematic research. Most of the recorded studies concerning NP–cell interactions were focused on mammalian or model cells. We suppose that the internalization would be quite specific when NPs meet aquatic organisms as mentioned later.
Besides the interactions between NPs and membranes as well as cell walls, from a more detailed perspective, the association between NPs and biomolecules should be taken into consideration. Proteins, lipids, and polysaccharides are common components existing on the inner or outer interfaces of the organisms, thus they would inevitably come into contact with NPs at the nano–bio interfaces. The interactions between NPs and biomolecules have therefore received increasing research attention.27,28 It was indicated that proteins can associate with NPs as a “corona” and thereupon bind the NPs and cells together.29–32 Hydrogen bonding, ionic interactions, and dehydration of polar groups may be the main forces connecting NPs and polysaccharides.33 Both the specific and nonspecific NP–biomolecule interactions may facilitate cell internalization of the NPs. It was indicated that nonspecific adsorption of serum proteins mediated the uptake of gold NPs into mammalian cells,34 whereas strong specific ligand–receptor interactions, between folate on silicon nanowires and folate receptors on the cell membranes, were suggested to expedite the internalization of the nanowires.35
3 Biophysicochemical interactions at the interfaces between NPs and microorganisms
3.1 Adsorption of NPs on microorganisms
Table 1 shows the selected research results at the nano–bio interactions between NPs and microorganisms. Alga and bacterium are the two common target aquatic microorganisms in nanotoxicity studies. In general, microorganisms have negative charges on their surfaces, thus adsorption may occur as soon as they meet NPs with positive charges. Positively charged CeO2 NPs51 and alumina coated SiO2 NPs52 were observed to be adsorbed (driven by electrostatic attraction) on the algal cell surfaces. This kind of purely physical process was also reported between positively charged Fe0 NPs and negatively charged cyanobacterial cells,39 and between oxide NPs [Al2O3 (ref. 43) and CeO2 (ref. 12)] and bacterial cells. NPs may not directly attach to the cell walls of microorganisms, but could interact with the extracellular substances such as proteins and polysaccharides. It was confirmed that Ag NPs could bind bacterial extracellular proteins through electrostatic attraction.53| NPs | Microorganisms | Toxicity | Adsorption | Internalization | Ref. | ||
|---|---|---|---|---|---|---|---|
| Yes/no | Mechanism | Yes/no | Mechanism | ||||
| a Note: “yes” refers to the occurrence of the interaction; “no” refers to the absence of the interaction; “/” refers to the fact that the interaction and the mechanism were not addressed in the references. | |||||||
| Si | Pseudokirchneriella subcapitata | Growth inhibition | Yes | / | No | / | 36 |
| Ag | Chlorella vulgaris, Dunaliella tertiolecta | Oxidative stress | Yes | Electrostatic attraction | No | / | 37 |
| Pseudomonas putida biofilms | Growth inhibition | Yes | / | Yes | / | 38 | |
| Fe0 | Cyanobacteria | Oxidative stress, shading effect | Yes | Electrostatic attraction | / | / | 39 |
| CdTe/CdS QDs | Chlamydomonas reinhardtii | Oxidative stress, genotoxicity | Yes | / | Yes | Ion released | 9 |
| NiO | Chlorella vulgaris | Growth inhibition, morphological alteration | Yes | / | Yes | Ion released | 14 |
| CuO | Chlamydomonas reinhardtii | Oxidative stress | Yes | / | Yes | Penetration | 40 |
| Microcystis aeruginosa | Oxidative stress, genotoxicity | Yes | / | Yes | Endocytosis, ion released | 41 | |
| ZnO | Chlorella sp. | Shading, growth inhibition, ion released | Yes | / | Yes | Ion released, cell surface damaged | 42 |
| Bacillus subtilis, Escherichia coli, Pseudomonas fluorescens | Physical damage, ion released | Yes | Electrostatic attraction, receptor–ligand interaction | / | / | 43 | |
| Escherichia coli | Ion released, growth inhibition | Yes | / | Yes | / | 44 | |
| TiO2 | Chlorella sp., Scenedesmus sp. | Cell damage, growth inhibition | Yes | Specific interactions between functional groups | No | / | 45 |
| Anabaena variabilis | Oxidative stress | Yes | / | Yes | Membrane damage | 46 | |
| Salmonella typhimurium | Genotoxicity | Yes | / | Yes | Non-specific membrane damage and specific uptake | 47 | |
| SiO2 | Scenedesmus obliquus | Shading, photosynthetic activity inhibition | Yes | / | No | / | 48 |
| CeO2 | Pseudokirchneriella subcapitata | Oxidative stress, physical damage | Yes | / | Yes | Membrane damage | 49 |
| Escherichia coli | Oxidative stress, growth inhibition | Yes | Electrostatic attraction | / | / | 12 | |
| Al2O3 | Cupriavidus Metallidurans, Escherichia coli | Oxidative stress | Yes | / | Yes | Transfer across peptidoglycan layer | 50 |
| MWCNTs | Chlorella vulgaris, Pseudokirchneriella subcapitata | Shading effect, growth inhibition | Yes | Hydrogen bonds | No | / | 13 |
| Chlorella sp. | Oxidative stress, shading, physical damage | Yes | / | Yes | Physical penetration | 16 | |
Specific interactions may occur between surface functional groups on the NPs and microorganisms. Carboxyl, amide, phosphate and hydroxyl functional groups on the bacterial cell walls and of the extracellular polymer substances may provide active sites for molecular-scale interactions with the oxide NPs.54 Schwab et al.13 suggested that hydrogen bonds between the algal cell surfaces and oxygen defects of carbon nanotubes (CNTs) could form to coagulate them. Furthermore, the majority of metal NPs can likely release metal ions that may bind with cationic and anionic exchange sites on the algal cell surfaces and thus provide binding sites for the NPs.53 The lipopolysaccharide chain molecules that form on the outer membrane of a Gram-negative bacterium, together with phospholipids, peptidoglycan, and exopolyscaccharide layers have been indicated to contain a large number of metal-complexing sites.55
From a kinetics point of view, the generation of adsorption largely depends on the interaction energy. Zhang et al.56 explored the adsorption pattern between hematite NPs and Escherichia coli using a series of adsorption experiments and data analysis. It was indicated that the interaction energy is rather complex because the interactions may concurrently include van der Waals forces, electrostatic interactions, acid–base forces, and specific interactions.56,57 The force distribution varies under different circumstances, which can result in a change of the interaction energy of the interfaces and subsequently influence the adsorption.
The adsorption of NPs on microorganisms could result in a series of subsequent toxicity, including physical damage and biochemical injury.12,48,58,59 Based on the observation of SiO2 NPs adsorbed on algal cells and no evidence of uptake into the cells, Hoecke et al.36 concluded that the apparent toxicity of SiO2 NPs occurred through the surface interactions. Algal surface accumulated CNTs could inhibit the algal growth by enhanced shading and agglomeration effects.13 Microorganism-adsorbed NPs were also observed to result in toxicity by pitting the cell walls,58 inducing the intracellular leakage via damaged cell membranes,46,59 and disturbing the permeability and respiration functions of the cells.60 The physical characteristics of microorganism cells, including adhesiveness and hardness or elasticity, would change due to NP-adsorption.61 The adsorbed NPs may also block the nutrient uptake and influence the cell metabolism. It was suggested in a research study that the direct interaction of Ag NPs with bacteria could lead to impaired metabolism, because the protein on the bacterial surface that binds to the NP surfaces alters the conformation or shields the active sites of the essential enzymes on the surfaces of the bacterial cells.62 Furthermore, oxidative injury is another highly concerning toxicity which follows the adsorption of NPs.63 It is known that cellular uptake and intracellular ROS production requires close contact with the NPs.64,65
3.2 Internalization of NPs into microorganisms
An increasing amount of evidence has been provided, that shows that many different kinds of NPs are capable of entering microorganism cells,46,47,50,59 however, the specific mechanism remains to be explored. For most algae and bacteria, the cell wall is a protective barrier that forbids NPs to enter the cells by blocking the possible entrances, such as endocytosis.48 For example, the physical properties of the bacterial peptidoglycan layer could resist the access of NPs into the cells.50 The most common ingress is the natural pores on the cell walls, the size of which decides the size of NPs that can enter the cells directly.48,66 However, sometimes internalization immediately occurs after adsorption of the NPs on the cell surfaces, because, as mentioned above, adsorption could induce cell wall pitting or membrane damage. For example, Ag NPs could be transported into microorganism cells because of the impaired cell walls and increase in cell membrane permeability.67,68 There are other possible specific internalizations of NPs. A specific uptake of ZnO and TiO2 NPs through porins existing on the surface of the bacterium S. typhimurium has been suggested.47 Under some circumstances, it is the metal ions that are released from NPs, rather than the NPs themselves, that enter the microorganism cells.55,68After entering the cells, the NPs will disperse inside the cells and locate in the cytoplasm and organelles, such as vacuole, chloroplast (for algal cells) and nuclear body. In this way, NPs would become accumulated in the cells. As a consequence, the intracellular interactions between NPs and these cellular substances may lead to nanotoxicity.40
4 Biophysicochemical interactions at the interfaces between NPs and hydrophytes
Hydrophytes, including emergent plants, floating-leaved plants and submerged hydrophytes, can likely meet the increasing number of NPs being released into the aquatic environment. There have been plenty of studies focusing on the phytotoxicity of NPs towards terrestrial plants,69,70 however, those toward hydrophytes are really limited. Despite the difference between terrestrial and aquatic plants, the biophysicochemical interactions between NPs and hydrophytes are still mainly reviewed based on phytotoxicity studies on the terrestrial plants, since a majority of these studies were performed in hydroponic culture systems. There is usually a layer of ceraceous substances or cuticles on the surfaces of leaves; the adsorption of NPs would therefore be difficult but could still occur. As pointed out in a review, small and lipophilic NPs could be incorporated into the apolar zones of the cuticles through apolar and/or polar pathways.69 The root is vulnerable when exposed to NPs. It has been widely observed that plant roots can accumulate and internalize various NPs such as ZnO NPs,71,72 CuO NPs,71 water-soluble fullerenes,73 and CdSe/ZnS QDs.74 The irreversible adsorption of fullerenes on the aquatic plant Ceratophyllum has been reported, and it was pointed out that C60 molecules could bind to cellulose in the plant structure via hydrogen bonds.75 After being adsorbed on the plant surfaces, NPs can likely be internalized into the plant tissues. Compared with terrestrial plants, aquatic plants generally have much thinner cuticle layers and their surfaces are much softer; furthermore, there are more pores on the stems and leaves of aquatic plants, which provide more uptake routes of NPs, namely allowing NPs with specific sizes to enter into the cells.76 It was revealed that multi-walled carbon nanotubes (MWCNTs) could penetrate the cell wall and enter the cytoplasm after uptake through the roots.77 It was indicated that CNTs could enter living walled plant cells through cellulose-induced nanoholes.78 Once uptake occurs, the NPs would be transported and translocated to various tissues of the plants, such as shoots, epidermis, vacuoles, plasmodesmos, mesophylls, and gas spaces.69 Considering the important position of hydrophytes as the primary producer in the aquatic environment, the adsorption and internalization of NPs may affect the aquatic biosphere through the trophic levels, and even influence the entire aquatic ecosystem owing to the potential impact on photosynthesis.5 Biophysicochemical interactions at the interfaces between NPs and invertebrates
Invertebrates, including zooplankton and zoobenthos, are regarded as the largest community in water environments. Zooplankton, such as daphnia, shrimp and some snails, feed on micro algae, and would undoubtedly ingest NPs because of the adsorption of NPs on the algal cells. Even though some zooplanktons do not feed on algae, it is quite easy for the suspending NPs to enter the organisms via the water phase through certain dietary pathways. On the other hand, zoobenthos, like oysters and mussels, would obviously come into contact with the settled NPs. Recent research results on the nano–bio interactions between NPs and invertebrates are summarized in Table 2.| NPs | Invertebrates | Toxicity | Adsorption | Internalization | Involved organs (parts) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Yes/no | Mechanism | Yes/no | Mechanism | |||||
| a Note: “yes” refers to the occurrence of the interaction; “no” refers to the absence of the interaction; “/” refers to the fact that the interaction and the mechanism were not addressed in the references. | ||||||||
| Ag | Daphnia magna | Chronic toxicity, bioaccumulation | Yes | / | Yes | Endocytosis | Branchia, guts | 79 |
| Au | Mytilus edulis | Oxidative stress | / | / | Yes | Digestion and endocytosis | Guts, digestive gland | 4 |
| CuO | Mytilus galloprovincialis | Oxidative stress | Yes | / | Yes | Endocytosis, ion released | Gill | 80 |
| ZnO | Daphnia magna | Molting inhibition, oxidative stress, genotoxicity | / | / | Yes | Endocytosis | / | 81 |
| TiO2 | Daphnia magna | Molting inhibition, physical damage | Yes | / | Yes | / | Shell, exoskeleton | 11 |
| C60 | Crassostrea virginica | Lysosomal destabilization, oxidative stress | / | / | Yes | Endocytosis, phagocytosis | Hepatopancreas | 82 |
| CNTs | Daphnia magna | Movement inhibition | Yes | / | Yes | / | Guts | 83 |
| MWCNTs | Ceriodaphnia dubia | Bioaccumulation | Yes | Specific connection by functional groups | / | / | Digestive tract, brood chamber | 84 |
| Carbon black | Bivalve mollusc mytilus | Inflammation, oxidative stress, genotoxicity | / | / | Yes | Endocytosis | Gills and digestive gland, the immune cells, the hemocytes | 85 |
| Polystyrene NPs | Mytilus edulis, Crassostrea virginica | Bioaccumulation | / | / | Yes | Endocytosis | Guts, digestive gland | 86 |
Adsorption of NPs on the surfaces of aquatic invertebrates has been documented. It should be noted that there are setae on the surfaces of some invertebrates such as daphnia; NPs can likely be adsorbed onto these setae. It was observed that NPs could gather on the surfaces of daphnia probably due to the adhesion to the exoskeleton of daphnia.11,87 Direct adherence of TiO2 aggregates to the outer envelope (chorion) of abalone embryos was also observed.88 As a matter of fact, the interface between invertebrates and NPs is not as simple as just a layer or flat surface. It was pointed out that oysters could produce an organic–inorganic adhesive displaying a cross-linked organic matrix, and elevated protein content.89 Thus, when considering the interfacial interactions, nano–protein interactions should be taken into account. It was demonstrated that Au NPs were able to bind thiol-containing proteins in the digestive glands of mussels.4 For invertebrates with shells, such as mussels and oysters, the adhesion of NPs on the shell surfaces deserves attention, and indeed it has already aroused research interest. It was confirmed that Ag NPs could be incorporated into the surfaces of the nacreous layer of the marine bivalves Mytilus edulis, and were transported to the mollusk extrapallial fluid (EPF) between the soft tissues and shell of blue mussels.90 In addition, due to their filter feeding habits, the bivalves gill epithelium was the first contact interface between the organism and NPs.80 For some invertebrates, besides the gills, mantle and labial palps were also the exposure organs contributing to the internalization of NPs,87 though they were not the main entrance compared to the ingestion route.
As mentioned above, NPs can enter invertebrates, and interact with the internal organs, therefore the interfaces formed when NPs meet invertebrates not only include the outer surfaces but also contain the inner interfaces of the organisms (Fig. 3). Different entrances and routes for the uptake of NPs can lead to various distributions of the NPs inside the invertebrates. For instance, it was suggested that CdSe/ZnS quantum dots (QDs) were observed in the lower intestine of daphnia because such crustacea were capable of pumping water through the anus into the intestine.91 However, since ingestion is the most common uptake route of NPs, the digestive system likely becomes the vulnerable target inside the invertebrates. The guts are the most concerned organs during the localization and distribution of the NPs. Heinlaan et al.92 have paid special attention to the interaction between CuO NPs and the midgut epithelial cells of daphnia, and concluded that the CuO NPs could be located between the midgut epithelium microvillies, indicating the potential internalization. Actually, during the ingestion, the state of some NP aggregates may be altered by the action of cilia on the gills and labial palps,87 which would facilitate the dispersion and distribution of the NPs inside the organisms. Moreover, the interaction between NPs and hemocytes cannot be ignored, because NPs could be transferred from the digestive system to the hemolymph and circulating hemocytes.87
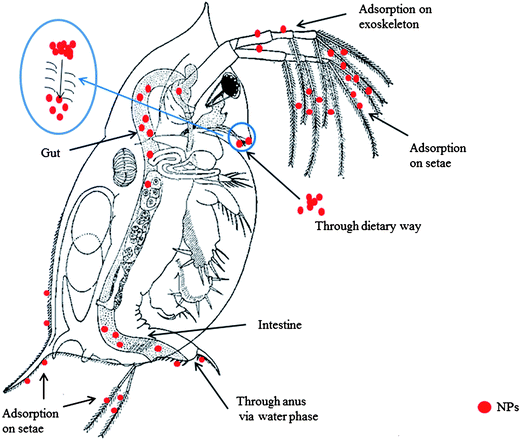 | ||
| Fig. 3 Uptake routes and distributions of NPs in daphnia. The figure shows both the outer nano–bio interface and inner distribution of NPs. The NPs could be adsorbed on the exoskeleton and setae. The NPs can enter the daphnia through dietary routes or through the anus via water phase – they then mainly accumulate in the digestive system. The NP aggregates could be dispersed by the action of cilia on the gills when entering the organism. | ||
Endocytosis could be the main internalization mechanism for NPs to cross the membrane and finally reach the inner part of animal cells.93 Polystyrene NPs were observed entering the tubules of the digestive glands and being internalized by the digestive cells of oysters via endocytosis.94 Fullerene particles could be readily accumulated inside the hepatopancreas tissues of Crassostrea virginica, which have rich lysosomes cells due to endocytosis.82 Interestingly, on the contrary, it was reported that the internalization of NPs did not occur inside the midgut of daphnia, even though the NPs were observed around the microvilli of the midgut epithelium cells.95 Therefore, more research is required for the actual internalization mechanisms.
These interactions that occur in the microworld between NPs and invertebrates can potentially cause either acute or chronic toxicity. The adsorption of NP clusters on the outer surfaces may affect the movement of zooplankton and thus have an impact on their predation, and subsequently influences the whole food chain performance.96 Exposure of daphnia to CuO NPs could lead to ultrastructural changes of the midgut epithelial cells.92 For molluscs with a protective shell, interfacial interactions with NPs can probably alter the nacre surface micromorphology.97 After entering the digestive system, NPs may impact the inner organs, like the gut, through inducing lysosome perturbations and oxidative injury.98
6 Nano–bio interactions at the interfaces during the exposure of fish to NPs
Being in the higher trophic level of the aquatic ecosystem, fish are vulnerable to contaminants in their surroundings. NPs can not only come into contact with fish directly, but can also enter the body via the fish predation of NP-exposed microorganisms and invertebrates. Nano–bio interactions at the interfaces during the exposure of fish to NPs have been concerned in some studies on the toxicity of NPs toward fish (Table 3).| NPs | Fish | Toxicity | Adsorption | Internalization | Involved organs (parts) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Yes/no | Mechanism | Yes/no | Mechanism | |||||
| a Note: “yes” refers to the occurrence of the interaction; “no” refers to the absence of the interaction; “/” refers to the fact that the interaction and the mechanism were not addressed in the references. | ||||||||
| Ag | Sheepshead minnows (Cyprinodon variegatus) | Ion released, chronic toxicity | Yes | Receptor–ligand interaction | Yes | / | Gill | 99 |
| Medaka embryo | Oxidative stress, genotoxicity | Yes | / | Yes | Penetration | Chorion | 100 | |
| Al | Zebrafish (Danio rerio) | Genotoxicity | Yes | / | / | / | Gills | 101 |
| ZnO | Trout hepatocytes | Oxidative stress | / | / | Yes | / | Liver | 102 |
| TiO2 | Rainbow trout (Oncorhyncusmykiss) gonadal tissue (RTG-2 cells) | Oxidative stress, genotoxicity | Yes | / | Yes | Membrane damage | Gonad | 103 |
| Larval zebrafish (Danio rerio) | Histopathological injuries and oxidative stress | Yes | / | No | / | Embryo | 104 | |
| Juvenile rainbow trout (Oncorhynchus mykiss) | Oxidative | / | / | Yes | / | Gill, gut, liver, brain and spleen | 105 | |
| CuO | Juvenile carp (Cyprinus carpio) | Neurotoxicity | Yes | Interaction with mucus and protein | Yes | Ion released, across mouth and gill | Skin, scale, gill, intestine, liver, brain, muscle | 106 |
| CeO2 | Carp (Cyprius carpio) | Cytotoxicity | / | / | Yes | / | Gastrointestinal tract, hepatocytes | 107 |
| C60 | Carp (Cyprinus carpio) | Oxidative stress | Yes | Protein–NPs binding | Yes | / | Gill, brain | 108 |
| SWCNTs | Rainbow trout | Respiratory toxicity, oxidative stress, organ pathologies | Yes | Trapped by gill mucus | Yes | / | Gill, intestine, brain, liver | 109 |
Fig. 4 schematically shows the possible adsorption/accumulation sites and internalization pathways of NPs for fish. Generally, the skin is the outermost layer of the surface of fish, containing an epidermis and dermis. The epidermis secretes a mucus that covers the fish’s body, which may embed NPs. It has been found that NPs can penetrate into the fish’s mucous layer by peri-kinetic forces, and are subsequently electrostatically attracted to and bind with the mucoproteins; NPs with a non-spherical shape would be more likely to tangle with strands of mucus.110 In fact, the epidermis of fish consists of multilayers of epithelial cells. Accordingly, the adsorption of NPs on these epithelial cells plays an important role in the interfaces that form when fish meet NPs. Besides the skin, fins and scales are also present on the surfaces of fish, which can also become adsorption sites attracting NPs. Cu was detected in the skin and scales of juvenile carp, and the mechanism was ascribed to the adsorption of CuO NPs and binding of Cu ions.106 Fish have gills as their respiratory organ which provides an access route for NPs, as water and air would pass through this organ. There are also protective covers, like a mucus layer on the gills of some species of fish, in which NPs may be trapped,111 as in the case of the association of SWCNTs with the gill mucus in trout.109
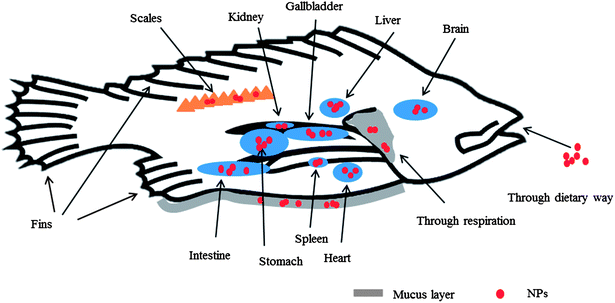 | ||
| Fig. 4 Adsorption of NPs on the outer surfaces and distribution in the main internal organs of fish. The involved outer interfaces contain scales, mucus layers, fins, and skin. NPs inside the fish may group in some of the organs, including gill, stomach, intestine, gallbladder, liver, kidney, spleen, heart, and brain. | ||
Owing to their much larger body, compared to microorganisms or invertebrates, fish are more likely to accumulate NPs in the body. It has been observed that CuxTiOy and pure TiO2 NPs were able to penetrate into the embryo cells as well as skin, nerve and yolk sac epithelium cells of zebrafish larvae as aggregated particles.112 Ag NPs were observed to be adsorbed on and transported through zebrafish embryos113 as well as fathead minnow (Pimephales promelas) embryos,114 penetrating the chorion via pore canals, with uptake kinetics characteristic of diffusion instead of active transport. Ag NPs were also found adhered to the surfaces and insides of the chorion of medaka fish.100 As stated above, the NPs on the boundary between fish and the surrounding water may be trapped in the mucus layer formed around the gill epithelial cells, and will then probably enter the cells via endocytosis.110 The gill is regarded as the first organ to be affected by NPs. NPs can enter a fish through respiration (via gills) and access through the mouth with dietary exposure, as a result, the concentrations of NPs in the gills and intestine were the highest.106 Limited uptake of NPs can occur directly from water and across the epithelial membrane in the gill.115 After entering the gastrointestinal system, the NPs can be translocated to internal organs, including the gut, liver, spleen, kidneys, gallbladder, and even brain.105,107 The accumulation of NPs inside the fish body causes inevitable interactions with various fish cells. Once the NPs enter the cells, they are likely to be stored in some organelles, such as lysosomes, endoplasmatic reticulum, or Golgi apparatus.116 The internalized NPs would also interact with the biomacromolecules in the cells and thereupon affect the cell functions. Griffitt et al.101 indicated that the internalization of Al NPs could result in a concentration-dependent decrease in sodium potassium ATPase activity in the freshwater fish.
The accumulated NPs could be excreted from or transformed in the fish. Desorption of Ce NPs from the gill surfaces and excretion from the alimentary tract occurred through respiration and digestion activity.117 Macrophages in vertebrates may phagocytose NPs.118 NPs in the blood of fish may form aggregates with thrombocyte, which can also be phagocytized by macrophages.119 After entering a biological fluid (such as blood and plasma), the NPs will become coated with proteins that may undergo conformational changes, leading to the exposure of new epitopes and the formation of protein corona at the nano–bio interface.17
The nano–bio interactions stated above can certainly cause diverse toxicity toward fishes. The adsorbed NPs will increase the living burden and stress of fish, inhibiting the movement and predation. The interactions that occur in the internal organs may result in various adverse effects. Edema, fusion and hyperplasia in the gill lamellae and filaments were observed when TiO2 NPs were accumulated in the gills; no matter whether the interaction was via uptake or just adhesion.120 TiO2 NPs in the kidney of a fathead minnow caused a reduction in neutrophil function, which is probably due to the potential interactions with neutrophils in the kidney.119 Furthermore, liver pathologies of juvenile carp, including necrotic and apoptosis hepatocytes, occurred due to the accumulation of TiO2 NPs.120 Overall, NPs on the surfaces or in various internal organs of the fish, would induce sub-lethal toxicity due to many micro interfacial interactions that are still lacking thorough research.
7 Influencing factors of the interfacial interactions
7.1 Properties of NPs and their state in water
The size and shape of NPs are important factors that can likely regulate the nano–bio interactions. From an adsorption kinetics point-of-view, smaller NPs may have a faster adsorption rate than larger NPs, which may be attributed to their faster particle mobility and lower energy barriers to the target organism surfaces.56 Nano-sized TiO2 particles were confirmed to be adsorbed much more easily on the daphnia exoskeleton, resulting in higher toxicity than the larger particles.11 Adsorption rates expressed as the number of NPs per unit cell surface area and unit time were much greater for small hematite NPs (26 and 53 nm) than large ones (76 and 98 nm), though the large NPs with a greater mass weight had a higher affinity toward the Caco-2 human intestinal cells according to the adsorption rate of the adsorbed mass of NPs.121Regarding the internalization of NPs, it was suggested that nanoparticle size and shape can mediate receptor–ligand binding constants, receptor recycling rates, and exocytosis during the uptake of gold NPs into mammalian cells.34 When pores on the cell surfaces are the entrances, size plays a vital role, because only NPs with a specific size can fit in the pores and pass through them. It was observed that QD aggregates with a certain hydrodynamic diameter range were too large to be internalized, while those with a much smaller diameter could enter the bacterial cells.122 NPs with larger surface areas undoubtedly have more opportunities and sites to come into contact with cells. SWCNTs exhibited higher toxicity to the cell membrane of bacteria than MWCNTs due to the much larger surface area.123 Moreover, a research study indicated that smaller Ag NPs with a higher surface area could react with cell membranes more easily and thus become more toxic to the bacteria than any other larger NPs.124 Theoretically, NPs with different shapes could exhibit diverse interactions with aquatic organisms. Rigid and sharp CNTs could likely penetrate into cells easily.125 Ag nanoplates were considered to be more toxic than nanospheres and nanowires due to their stronger reactivity.126
From the above description, it can be concluded that NPs with a smaller size have a higher potential to be adsorbed and internalized by aquatic organisms. However, small-sized NPs are prone to aggregation in water and the aggregation can likely influence the nano–bio interactions. When the NPs aggregate in water, the apparent particle size becomes large; this makes it difficult or even impossible for the NPs to pass through the cell wall via the natural pores.76 Aggregation of Ag NPs can change the organism exposure levels by reducing the nominal (added) dose to which the organisms are exposed, while the specific surface area available to interact with biota will be larger in the dispersed form.127 In addition, dosimetric selection is another factor to be considered, because different concentrations or particle number density caused by different dosages is supposed to impact the particulate gathering state and the interpretation of the size effect of NPs.34 It was suggested that the increasing number of nanometer particulates in air pollution may enhance the interfacial interactions between reactive particle surfaces and epithelial cells.128 However, there is very limited information available to date on the size effect of NPs as influenced by NP dose, which should be an area of focus for future nanotoxicity research, especially on the microcosmic interactions.129
The adsorption of NPs on the organisms by electrostatic forces can be regulated by the surface charges of the organisms and the NPs. Therefore, the surface charge or zeta potential of the NPs is one of the key characteristics of the NPs that may dominate the nano–bio interactions as addressed in Section 3.1. The functional groups on the NP surfaces are also very important to the nano–bio interactions because they decide the potential specific interactions (like hydrogen bonding) and the active sites on the nano–bio interfaces; they may also change the surface charges of the NPs and influence the electrostatic interactions.84 A study indicated that cationic Au NPs were moderately toxic, while anionic ones were nontoxic.130
Surface coating by surfactants or polymers would change the surface properties of the NPs, and thereupon change the environmental behaviors of the NPs and the nano–bio interactions.1 It was observed that an organic polymer coating on carboxyl QDs provided protection from the metal toxicity during laboratory exposure to freshwater organisms.131 However, on the contrary, it was also reported that polymer-coated CuO NPs were more toxic than the uncoated NPs toward a green alga, which was mainly attributed to the increased capacity of the polymer-coated CuO NPs to penetrate the algal cell.40
7.2 Environmental conditions
Environmental conditions (such as pH, ionic strength, and coexisting substances) can change the surface properties of both NPs and aquatic organisms, and thereupon influence the nano–bio interactions. Solution pH and ionic strength are key parameters that can determine the surface charge properties of the NPs,1,132 and can therefore regulate the electrostatic interactions between NPs and organisms. However, very few studies have been specifically performed to examine the effects of solution pH and ionic strength on the nano–bio interactions. A study53 revealed that a low solution pH (<8) favored the adsorption of positively charged Ag NPs (point of zero charge = 8) on algal surfaces due to electrostatic attraction, whereas a high pH (>8) inhibited the adsorption because of the electrostatic repulsion between the negatively charged NPs and algae. Moreover, it was indicated that a low pH could facilitate the accumulation of Cd from CdTe/CdS QDs in Chlamydomonas reinhardtii.9 Counter-ions can depress the electric double-layer and lower the absolute zeta potential of the colloidal NPs,1 and thereby affect the electrostatic interaction between NPs and organisms. The depressed electric double-layer will facilitate the aggregation of NPs,1 which can also influence the nano–bio interactions.Ubiquitous natural organic matter (NOM) can coat the released NPs, and change their surface properties and environmental behaviors,1,133,134 and therefore can definitely influence the nano–bio interactions to a great extent. NOM-coating can deliver negative charges to the NPs; therefore, theoretically it can inhibit the adsorption of the NPs to the negatively charged organisms. It has been confirmed that both dissolved and surface bound humic acid could increase the negativity of the surface charges of TiO2 NPs, and thereby limit their adsorption and toxicity to algal cells.65 Actually, mitigation of the nanotoxicity has been widely observed in the presence of NOM, such as the toxicity of nC60 to bacteria,135 Ag NPs to bacteria136 and early life stage zebrafish (Danio rerio) and Daphnia magna,137 Fe0 NPs to bacteria,64,138 CeO2 NPs to algae,139 and CdSe/ZnS QDs to Daphnia magna.41 However, it has also been reported that dissolved organic matter could increase the toxicity of CuO NPs toward algae because of the higher Cu2+ release, decreased degree of NP aggregation and enhanced NP internalization.140 The effect of NOM on the nano–bio interaction and nanotoxicity warrants more specific investigation.
NPs are excellent sorbents for both organic and inorganic chemicals due to their huge specific surface areas. Therefore, coexisting contaminants in the aquatic environment are likely to be adsorbed by the NPs, which may alter the surface properties of the NPs and the potential interactions with organisms. To date, however, no study has been focused on the effect of the coexisting contaminants on the nano–bio interactions or the nanotoxicity; while, a few researchers have examined the effect of NPs on the bioavailability of the coexisting contaminants. In these stuidies, NPs were observed to act as the “Trojan horse”141 for delivery of the adsorbed contaminants into the aquatic organisms, such as C60 facilitating the internalization and accumulation of arsenic in zebrafish hepatocytes,142 TiO2 NPs enhancing the accumulation of cadmium in viscera and gills of carp,143 and nano-charcoal favoring the uptake of tributyltin and dibutyltin in Daphnia magna.144
Temperature and light can influence the interfacial interactions to some degree. Concerning global warming, the temperature of the earth and the sensitive water keeps changing; although it is only slightly, the effect on the whole aquatic environment cannot be ignored. It was concluded that temperature had different effects on the formation and zeta potential of Ag NP agglomerates, and the increase of temperature tended to enhance the toxic effects on two green algae (Chlorella vulgaris and Dunaliella tertiolecta).145 In addition, temperature fluctuation may induce a change in the suspending conditions of NPs, the habitat and some properties of the aquatic organisms, and the adsorption energy of the nano–bio interface.146 The nano–bio interactions and nanotoxicity of some photoactive NPs (like TiO2) could be affected in the presence of ultraviolet radiation.147
8 Analytical methods
To date, there are many methods for the characterization of the surface properties of NPs and organisms.148–150 However, it is still a big challenge to correctly and precisely analyze the nano–bio interfaces and interactions. Microscopic observations were generally employed to analyze the nano–bio interfaces and interactions (Table 4). The used microscopes include a light microscope (LM), fluorescence microscope, transmission electron microscope (TEM), scanning electron microscope (SEM), scanning tunnel microscope (STM), atomic force microscope (AFM), laser scanning confocal microscope (LSCM), and coherent anti-Stokes Raman scattering microscope (CARS). The majority of these microscopes can be used to directly observe the apparent surface morphology of the objects, distribution of the NPs, and the association relationship between the NPs and organisms, all of which are substantially helpful for the analysis of the nano–bio interfaces and interactions. Gilbert et al.159 complemented X-ray 2D imaging methods with AFM to differentiate NPs transported inside the cells with those adhered to the cell surface. Reuel et al.160 detected the association of SWCNTs with living cells using 3D tracking with an orbital tracking microscope. Some fluorescent QDs can be used in LED systems and flat screen computer displays, thus synchrotron X-ray 2D and 3D elemental imaging was performed to investigate the interfacial interactions between these QDs and daphnia.91 Flow cytometry was also an effective method used for the rapid detection of the internalization of NPs in live bacteria.47,161 However, direct observation is not enough to perform precise research. Adsorption experiments can be performed to study the association of NPs with cells and biomolecules.162–164 There are still many problems concerning the methodology used for the investigation of nano–bio interfacial interactions which remain to be solved. Sometimes, it is really difficult to distinguish whether the metal accumulated in the organisms is the NPs or just the corresponding released ions,99 though a study reported that synchrotron X-ray microspectroscopy could be used to analyze Au particles versus Au ions in soil.165 Therefore, the methods used to define the interfaces and explore the interactions, especially the observation technology and comprehensive methods concerning microcosmic world, still need to be developed.| Observation technique | NPs | Organisms | Interactions | Ref. |
|---|---|---|---|---|
| CARS | ZnO, CeO2, TiO2 | Zebrafish (Danio rerio), rainbow trout (Oncorhynchus mykiss) | Internalization and localization | 115,151 |
| CeO2, TiO2, Ag | Trout hepatocytes | Internalization | 102 | |
| TEM | C60 | Daphnia magna | Attachment and internalization | 152 |
| TiO2 | Nitrogen-fixing cyanobacteria (Anabaena variabilis) | Internalization and distribution | 46 | |
| TiO2 | Abalone (Haliotis diversicolor supertexta) embryos | Internalization | 88 | |
| Au | Escherichia coli, Bacillus subtilis | Attachment | 153 | |
| SEM | Cu, Ag, TiO2 | Zebrafish | The change of gill filament after NPs exposure | 111 |
| LM | MWCNTs | Ceriodaphnia dubia | Accumulation and distribution | 84 |
| TiO2 | Daphnia magna | Uptake | 154 | |
| CLSM | QDs | Ciliate (T. pyriformis) | Uptake and depuration | 155 |
| Epifluorescence microscopy | QDs | Ceriodaphnia dubia, Pseudokirchneriella subcapitata | Internalization, distribution and adsorption | 131 |
| Transmission and confocal fluorescence microscopy | Fluorescent core–shell silica nanoparticles | Zebrafish (Danio rerio) | Adsorption | 156 |
| Bright-field and fluorescent microscopy | CuO, ZnO, NiO, and Co3O4 | Zebrafish embryos and larvae | Adsorption and distribution | 157 |
| Fluorescence stereomicroscope | Nano-sized latex particles | Medaka (Oryzias latipes) embryos and larvae | Internalization and distribution | 158 |
9 Summary and perspectives
The interactions at the nano–bio interfaces are regarded as a prerequisite and of vital importance to the nanotoxicity, but they still lack thorough research to date. We have briefly described the NP–cell interactions at the nano–bio interfaces, reviewed the research advances on the nano–bio interactions between NPs and aquatic organisms, and introduced the recorded methods for the interfacial interaction research. NPs may be adsorbed on the cell surfaces mainly through hydrophobic, electrostatic, receptor–ligand and hydrogen bonding interactions, and thereafter could be internalized into the cells via penetration, endocytosis, transient holes or membrane disruption. The NPs adsorbed or internalized by the microorganisms could be transferred through the food chain and risk the whole ecosystem. The surface properties and suspending state of the NPs and the conditions of the aquatic environment have profound influences on the interfacial interactions. By controlling the environmental conditions, such as pH, ionic strength, NOM, and temperature as well as the NP surface properties, the nano–bio interactions can be mastered.However, there are many uncertainties concerning nano–bio interfacial interactions that merit more specific investigations, with a few summarized below as research directions for the future.
(1) The mechanisms of the interfacial interactions between NPs and aquatic organisms have not been well illustrated. The adsorption, internalization, and NP–biomolecule interactions mentioned in the present paper are just the tip of the iceberg, and more research into nano–bio interfaces is needed to fully understand the nano–bio interactions. Conditions (such as solution pH, ionic strength, and especially NOM) can influence the nano–bio interaction and therefore can be used to investigate the interaction mechanism. Research on the NP–biomolecule interaction should be strengthened to probe the molecular mechanisms of the interfacial interactions.
(2) The interfaces and interfacial interactions vary from one species to another. Different algae or bacteria exhibit quite different surface properties and contain diverse chemical substances; invertebrates and fish have many kinds of outer and inner interfaces that can meet NPs. Therefore, the interspecies differences deserve more attention to improve the knowledge of the nano–bio interactions. Future research should also be directed to the contribution of extracellular substances that may govern the nano–bio interfaces and the interfacial interactions.
(3) Though there are a few studies providing evidence of the transfer of NPs through aquatic food chains, the detailed pathway and the interfacial interactions involved are still unclear. It is really crucial to study the nano–bio interactions on a series of interfaces concerning the aquatic food chains. The relationship between the nano–bio interaction and the nanotoxicity should be elucidated. Methods modulating the nano–bio interaction are warranted to control the nanotoxicity. Manufacturers are encouraged to produce environmentally benign NPs that have a lower potential of being accumulated by the organisms and transported through the food chains.
(4) The methodology used for nano–bio interfacial interaction research is still in its infancy. The commonly adopted microscope observations are helpful to visualize the location and distribution of the NPs at the bio interfaces and in the organisms, however, they could not be used to quantitatively or qualitatively analyze the nano–bio interaction forces. Novel techniques and/or methods are demanded to characterize the nanoscale interfaces, quantify and locate the adsorption of NPs, reveal the transmembrane internalization process, and trace NP distribution in the organism and their transport in the food chain.
Acknowledgements
This work was supported by National Natural Science Foundation of China (no. 21077089), Zhejiang Provincial Natural Science Foundation of China (LR12B07001), Zhejiang Provincial “Qianjiang Talent Program” (no. 2010R10041), Program for New Century Excellent Talents in University (NCET-10-0731), Fundamental Research Funds for the Central Universities, and Zhejiang Provincial Innovative Research Team of Water Treatment Functional Materials and their Application.References
- D. H. Lin, X. L. Tian, F. C. Wu and B. S. Xing, J. Environ. Qual., 2010, 39, 1896–1908 CrossRef.
- Y. Ju-Nam and J. R. Lead, Sci. Total Environ., 2008, 400, 396–414 CrossRef CAS.
- T. M. Scown, R. van Aerle and C. R. Tyler, Crit. Rev. Toxicol., 2010, 40, 653–670 CrossRef CAS.
- S. Tedesco, H. Doyle, J. Blasco, G. Redmond and D. Sheehan, Aquat. Toxicol., 2010, 100, 178–186 CrossRef CAS.
- L. H. Hao and L. Chen, Ecotoxicol. Environ. Saf., 2012, 80, 103–110 CrossRef CAS.
- G. Marcone, A. C. Oliveira, G. Almeida, G. A. Umbuzeiro and W. F. Jardim, J. Hazard. Mater., 2012, 211, 436–442 CrossRef.
- X. S. Zhu, L. Zhu, Y. P. Lang and Y. S. Chen, Environ. Toxicol. Chem., 2008, 27, 1979–1985 CrossRef CAS.
- J. E. Choi, S. Kim, J. H. Ahn, P. Youn, J. S. Kang, K. Park, J. Yi and D. Y. Ryu, Aquat. Toxicol., 2010, 100, 151–159 CrossRef CAS.
- R. F. Domingos, D. F. Simon, C. Hauser and K. J. Wilkinson, Environ. Sci. Technol., 2011, 45, 7664–7669 CrossRef CAS.
- M. S. Hull, A. J. Kennedy, J. A. Steevens, A. J. Bednar, C. A. Weiss and P. J. Vikesland, Environ. Sci. Technol., 2009, 43, 4169–4174 CrossRef CAS.
- A. Dabrunz, L. Duester, C. Prasse, F. Seitz, R. Rosenfeldt, C. Schilde, G. E. Schaumann and R. Schulz, PLoS One, 2011, 6, e20112 CAS.
- A. Thill, O. Zeyons, O. Spalla, F. Chauvat, J. Rose, M. Auffan and A. M. Flank, Environ. Sci. Technol., 2006, 40, 6151–6156 CrossRef CAS.
- F. Schwab, T. D. Bucheli, L. P. Lukhele, A. Magrez, B. Nowack, L. Sigg and K. Knauer, Environ. Sci. Technol., 2011, 45, 6136–6144 CrossRef CAS.
- N. Gong, K. S. Shao, W. Feng, Z. Z. Lin, C. H. Liang and Y. Q. Sun, Chemosphere, 2011, 83, 510–516 CrossRef CAS.
- J. Ji, Z. F. Long and D. H. Lin, Chem. Eng. J., 2011, 170, 525–530 CrossRef CAS.
- Z. F. Long, J. Ji, K. Yang, D. H. Lin and F. C. Wu, Environ. Sci. Technol., 2012, 46, 8458–8466 CrossRef CAS.
- A. E. Nel, L. Madler, D. Velegol, T. Xia, E. Hoek, P. Somasundaran, F. Klaessig, V. Castranova and M. Thompson, Nat. Mater., 2009, 8, 543–557 CrossRef CAS.
- A. Vakurov, R. Brydson and A. Nelsont, Langmuir, 2012, 28, 1246–1255 CrossRef CAS.
- E. C. Cho, J. W. Xie, P. A. Wurm and Y. N. Xia, Nano Lett., 2009, 9, 1080–1084 CrossRef CAS.
- J. M. Chen, J. A. Hessler, K. Putchakayala, B. K. Panama, D. P. Khan, S. Hong, D. G. Mullen, S. C. DiMaggio, A. Som, G. N. Tew, A. N. Lopatin, J. R. Baker, M. Holl and B. G. Orr, J. Phys. Chem. B, 2009, 113, 11179–11185 CrossRef CAS.
- P. R. Leroueil, S. A. Berry, K. Duthie, G. Han, V. M. Rotello, D. Q. McNerny, J. R. Baker, B. G. Orr and M. Holl, Nano Lett., 2008, 8, 420–424 CrossRef CAS.
- C. W. Evans, M. Fitzgerald, T. D. Clemons, M. J. House, B. S. Padman, J. A. Shaw, M. Saunders, A. R. Harvey, B. Zdyrko, I. Luzinov, G. A. Silva, S. A. Dunlop and K. S. Iyer, ACS Nano, 2011, 5, 8640–8648 CrossRef.
- A. A. Skandani, R. Zeineldin and M. Al-Haik, Langmuir, 2012, 28, 7872–7879 CrossRef CAS.
- A. Dubavik, E. Sezgin, V. Lesnyak, N. Gaponik, P. Schwille and A. Eychmuller, ACS Nano, 2012, 6, 2150–2156 CrossRef CAS.
- A. Verma, O. Uzun, Y. H. Hu, Y. Hu, H. S. Han, N. Watson, S. L. Chen, D. J. Irvine and F. Stellacci, Nat. Mater., 2008, 7, 588–595 CrossRef CAS.
- R. Choudhury and S. Srivastava, World J. Microbiol. Biotechnol., 2001, 17, 149–153 CrossRef CAS.
- M. S. Lord, M. Foss and F. Besenbacher, Nano Today, 2010, 5, 66–78 CrossRef CAS.
- P. Desai, R. R. Patlolla and M. Singh, Mol. Membr. Biol., 2010, 27, 247–259 CrossRef CAS.
- G. F. Audette, S. Lombardo, J. Dudzik, T. M. Arruda, M. Kolinski, S. Filipek, S. Mukerjee, A. M. Kannan, V. Thavasi, S. Ramakrishna, M. Chin, P. Somasundaran, S. Viswanathan, R. S. Keles and V. Renugopalakrishnan, Mater. Today, 2011, 14, 360–365 CrossRef CAS.
- I. Lynch and K. A. Dawson, Nano Today, 2008, 3, 40–47 CrossRef CAS.
- S. Milani, F. B. Bombelli, A. S. Pitek, K. A. Dawson and J. Radler, ACS Nano, 2012, 6, 2532–2541 CrossRef CAS.
- M. Lundqvist, J. Stigler, T. Cedervall, T. Berggard, M. B. Flanagan, I. Lynch, G. Elia and K. Dawson, ACS Nano, 2011, 5, 7503–7509 CrossRef CAS.
- Z. Y. Zeng, J. Patel, S. H. Lee, M. McCallum, A. Tyagi, M. D. Yan and K. J. Shea, J. Am. Chem. Soc., 2012, 134, 2681–2690 CrossRef CAS.
- B. D. Chithrani, A. A. Ghazani and W. Chan, Nano Lett., 2006, 6, 662–668 CrossRef CAS.
- W. X. Zhang, L. Tong and C. Yang, Nano Lett., 2012, 12, 1002–1006 CrossRef CAS.
- K. Van Hoecke, K. De Schamphelaere, P. Van der Meeren, S. Lucas and C. R. Janssen, Environ. Toxicol. Chem., 2008, 27, 1948–1957 CrossRef CAS.
- A. Oukarroum, S. Bras, F. Perreault and R. Popovic, Ecotoxicol. Environ. Saf., 2012, 78, 80–85 CrossRef CAS.
- J. Fabrega, J. C. Renshaw and J. R. Lead, Environ. Sci. Technol., 2009, 43, 9004–9009 CrossRef CAS.
- B. Marsalek, D. Jancula, E. Marsalkova, M. Mashlan, K. Safarova, J. Tucek and R. Zboril, Environ. Sci. Technol., 2012, 46, 2316–2323 CrossRef CAS.
- F. Perreault, A. Oukarroum, S. P. Melegari, W. G. Matias and R. Popovic, Chemosphere, 2012, 87, 1388–1394 CrossRef CAS.
- S. Lee, K. Kim, H. K. Shon, S. D. Kim and J. Cho, J. Nanopart. Res., 2011, 13, 3051–3061 CrossRef CAS.
- J. Ji, Z. F. Long and D. H. Lin, Chem. Eng. J., 2011, 170, 525–530 CrossRef CAS.
- W. Jiang, H. Mashayekhi and B. S. Xing, Environ. Pollut., 2009, 157, 1619–1625 CrossRef CAS.
- M. Li, L. Z. Zhu and D. H. Lin, Environ. Sci. Technol., 2011, 45, 1977–1983 CrossRef CAS.
- I. M. Sadiq, S. Dalai, N. Chandrasekaran and A. Mukherjee, Ecotoxicol. Environ. Saf., 2011, 74, 1180–1187 CrossRef CAS.
- C. Cherchi, T. Chernenko, M. Diem and A. Z. Gu, Environ. Toxicol. Chem., 2011, 30, 861–869 CrossRef CAS.
- A. Kumar, A. K. Pandey, S. S. Singh, R. Shanker and A. Dhawan, Chemosphere, 2011, 83, 1124–1132 CrossRef CAS.
- C. X. Wei, Y. B. Zhang, J. Guo, B. Han, X. Yang and J. L. Yuan, J. Environ. Sci., 2010, 22, 155–160 CrossRef CAS.
- N. J. Rogers, N. M. Franklin, S. C. Apte, G. E. Batley, B. M. Angel, J. R. Lead and M. Baalousha, Environ. Chem., 2010, 7, 50–60 CrossRef CAS.
- A. Simon-Deckers, S. Loo, M. Mayne-L'Hermite, N. Herlin-Boime, N. Menguy, C. Reynaud, B. Gouget and M. Carriere, Environ. Sci. Technol., 2009, 43, 8423–8429 CrossRef CAS.
- I. Rodea-Palomares, K. Boltes, F. Fernandez-Pinas, F. Leganes, E. Garcia-Calvo, J. Santiago and R. Rosal, Toxicol. Sci., 2011, 119, 135–145 CrossRef CAS.
- K. Van Hoecke, K. De Schamphelaere, S. Ramirez-Garcia, P. Van der Meeren, G. Smagghe and C. R. Janssen, Environ. Int., 2011, 37, 1118–1125 CrossRef CAS.
- S. S. Khan, P. Srivatsan, N. Vaishnavi, A. Mukherjee and N. Chandrasekaran, J. Hazard. Mater., 2011, 192, 299–306 CAS.
- I. M. Sadiq, S. Pakrashi, N. Chandrasekaran and A. Mukherjee, J. Nanopart. Res., 2011, 13, 3287–3299 CrossRef CAS.
- M. H. Li, S. Pokhrel, X. Jin, L. Madler, R. Damoiseaux and E. Hoek, Environ. Sci. Technol., 2011, 45, 755–761 CrossRef CAS.
- W. Zhang, B. Rittmann and Y. S. Chen, Environ. Sci. Technol., 2011, 45, 2172–2178 CrossRef CAS.
- D. Snoswell, J. M. Duan, D. Fornasiero and J. Ralston, J. Colloid Interface Sci., 2005, 286, 526–535 CrossRef CAS.
- O. Choi, K. K. Deng, N. J. Kim, L. Ross, R. Y. Surampalli and Z. Q. Hu, Water Res., 2008, 42, 3066–3074 CrossRef CAS.
- S. Dalai, S. Pakrashi, R. S. Suresh Kumar, N. Chandrasekaran and A. Mukherjee, Toxicol. Res., 2012, 1, 116–130, 10.1039/c2tx00012a.
- V. K. Sharma, R. A. Yngard and Y. Lin, Adv. Colloid Interface Sci., 2009, 145, 83–96 CrossRef CAS.
- W. Zhang, J. Hughes and Y. S. Chen, Appl. Environ. Microbiol., 2012, 78, 3905–3915 CrossRef CAS.
- N. S. Wigginton, A. De Titta, F. Piccapietra, J. Dobias, V. J. Nesatty, M. Suter and R. Bernier-Latmani, Environ. Sci. Technol., 2010, 44, 2163–2168 CrossRef CAS.
- A. Nel, T. Xia, L. Madler and N. Li, Science, 2006, 311, 622–627 CrossRef CAS.
- Z. Q. Li, K. Greden, P. Alvarez, K. B. Gregory and G. V. Lowry, Environ. Sci. Technol., 2010, 44, 3462–3467 CrossRef CAS.
- J. Ji, Z. F. Long and D. H. Lin, Water Res., 2012, 46, 4477–4487 CrossRef.
- A. L. Neal, Ecotoxicology, 2008, 17, 362–371 CrossRef CAS.
- A. J. Miao, Z. P. Luo, C. S. Chen, W. C. Chin, P. H. Santschi and A. Quigg, PLoS One, 2010, 5, e15196 CAS.
- C. Marambio-Jones and E. Hoek, J. Nanopart. Res., 2010, 12, 1531–1551 CrossRef CAS.
- K. J. Dietz and S. Herth, Trends Plant Sci., 2011, 16, 582–589 CrossRef CAS.
- D. H. Lin and B. S. Xing, Environ. Pollut., 2007, 150, 243–250 CrossRef CAS.
- S. Kim, S. Lee and I. Lee, Water, Air, Soil Pollut., 2012, 223, 2799–2806 CrossRef CAS.
- D. H. Lin and B. S. Xing, Environ. Sci. Technol., 2008, 42, 5580–5585 CrossRef CAS.
- Q. L. Liu, Y. Y. Zhao, Y. L. Wan, J. P. Zheng, X. J. Zhang, C. R. Wang, X. H. Fang and J. X. Lin, ACS Nano, 2010, 4, 5743–5748 CrossRef CAS.
- D. A. Navarro, M. A. Bisson and D. S. Aga, J. Hazard. Mater., 2012, 211, 427–435 CrossRef.
- S. T. Mukherji, J. Leisen and J. B. Hughes, Chemosphere, 2011, 84, 390–396 CrossRef CAS.
- E. Navarro, A. Baun, R. Behra, N. B. Hartmann, J. Filser, A. J. Miao, A. Quigg, P. H. Santschi and L. Sigg, Ecotoxicology, 2008, 17, 372–386 CrossRef CAS.
- X. P. Wang, H. Y. Han, X. Q. Liu, X. X. Gu, K. Chen and D. L. Lu, J. Nanopart. Res., 2012, 14, 841 CrossRef CAS.
- M. F. Serag, N. Kaji, M. Tokeshi and Y. Baba, RSC Adv., 2012, 2, 398–400 RSC.
- C. M. Zhao and W. X. Wang, Environ. Sci. Technol., 2010, 44, 7699–7704 CrossRef CAS.
- T. Gomes, J. P. Pinheiro, I. Cancio, C. G. Pereira, C. Cardoso and M. J. Bebianno, Environ. Sci. Technol., 2011, 45, 9356–9362 CrossRef CAS.
- H. C. Poynton, J. M. Lazorchak, C. A. Impellitteri, M. E. Smith, K. Rogers, M. Patra, K. A. Hammer, H. J. Allen and C. D. Vulpe, Environ. Sci. Technol., 2011, 45, 762–768 CrossRef CAS.
- A. H. Ringwood, N. Levi-Polyachenko and D. L. Carroll, Environ. Sci. Technol., 2009, 43, 7136–7141 CrossRef CAS.
- E. J. Petersen, J. Akkanen, J. V. K. Kukkonen and W. J. Weber, Jr, Environ. Sci. Technol., 2009, 43, 2969–2975 CrossRef CAS.
- M. H. Li and C. P. Huang, Carbon, 2011, 49, 1672–1679 CrossRef CAS.
- L. Canesi, C. Ciacci, M. Betti, R. Fabbri, B. Canonico, A. Fantinati, A. Marcornini and G. Pojana, Environ. Int., 2008, 34, 1114–1119 CrossRef CAS.
- J. E. Ward and D. J. Kach, Mar. Environ. Res., 2009, 68, 137–142 CrossRef CAS.
- A. Baun, N. B. Hartmann, K. Grieger and K. O. Kusk, Ecotoxicology, 2008, 17, 387–395 CrossRef CAS.
- X. S. Zhu, J. Zhou and Z. H. Cai, Environ. Sci. Technol., 2011, 45, 3753–3758 CrossRef CAS.
- J. R. Burkett, L. M. Hight, P. Kenny and J. J. Wilker, J. Am. Chem. Soc., 2010, 132, 12531–12533 CrossRef CAS.
- M. Zuykov, E. Pelletier and S. Demers, Mar. Environ. Res., 2011, 71, 17–21 CrossRef CAS.
- B. P. Jackson, H. Pace, A. Lanzirotti, R. Smith and J. F. Ranville, Anal. Bioanal. Chem., 2009, 394, 911–917 CrossRef CAS.
- M. Heinlaan, A. Kahru, K. Kasemets, B. Arbeille, G. Prensier and H. C. Dubourguier, Water Res., 2011, 45, 179–190 CrossRef CAS.
- M. N. Moore, Environ. Int., 2006, 32, 967–976 CrossRef CAS.
- J. E. Ward and D. J. Kach, Mar. Environ. Res., 2009, 68, 137–142 CrossRef CAS.
- P. Rosenkranz, Q. Chaudhry, V. Stone and T. F. Fernandes, Environ. Toxicol. Chem., 2009, 28, 2142–2149 CrossRef CAS.
- S. B. Lovern, J. R. Strickler and R. Klaper, Environ. Sci. Technol., 2007, 41, 4465–4470 CrossRef CAS.
- M. Zuykov, E. Pelletier, C. Belzile and S. Demers, Chemosphere, 2011, 84, 701–706 CrossRef CAS.
- L. Canesi, C. Ciacci, R. Fabbri, A. Marcomini, G. Pojana and G. Gallo, Mar. Environ. Res., 2012, 76, 16–21 CrossRef CAS.
- R. J. Griffitt, N. J. Brown-Peterson, D. A. Savin, C. S. Manning, I. Boube, R. A. Ryan and M. Brouwer, Environ. Toxicol. Chem., 2012, 31, 160–167 CrossRef CAS.
- S. Kashiwada, M. E. Ariza, T. Kawaguchi, Y. Nakagame, B. S. Jayasinghe, K. Gartner, H. Nakamura, Y. Kagami, T. Sabo-Attwood, P. L. Ferguson and G. T. Chandler, Environ. Sci. Technol., 2012, 46, 6278–6287 CrossRef CAS.
- R. J. Griffitt, A. Feswick, R. Weil, K. Hyndman, P. Carpinone, K. Powers, N. D. Denslow and D. S. Barber, Environ. Toxicol., 2011, 26, 541–551 CrossRef CAS.
- T. M. Scown, R. M. Goodhead, B. D. Johnston, J. Moger, M. Baalousha, J. R. Lead, R. van Aerle, T. Iguchi and C. R. Tyler, Environ. Chem., 2010, 7, 36–49 CrossRef CAS.
- W. F. Vevers and A. N. Jha, Ecotoxicology, 2008, 17, 410–420 CrossRef CAS.
- T. H. Chen, C. Y. Lin and M. C. Tseng, Mar. Pollut. Bull., 2011, 63, 303–308 CrossRef CAS.
- C. S. Ramsden, T. J. Smith, B. J. Shaw and R. D. Handy, Ecotoxicology, 2009, 18, 939–951 CrossRef CAS.
- J. Zhao, Z. Y. Wang, X. Y. Liu, X. Y. Xie, K. Zhang and B. S. Xing, J. Hazard. Mater., 2011, 197, 304–310 CrossRef CAS.
- B. K. Gaiser, T. F. Fernandes, M. A. Jepson, J. R. Lead, C. R. Tyler, M. Baalousha, A. Biswas, G. J. Britton, P. A. Cole, B. D. Johnston, Y. Ju-Nam, P. Rosenkranz, T. M. Scown and V. Stone, Environ. Toxicol. Chem., 2012, 31, 144–154 CrossRef CAS.
- J. Ferreira, D. M. Barros, L. A. Geracitano, G. Fillmann, C. E. Fossa, E. A. de Almeida, M. D. Castro Prado, B. R. Almeida Neves, M. V. Brant Pinheiro and J. Monserrat, Environ. Toxicol. Chem., 2012, 31, 961–967 CrossRef CAS.
- C. J. Smith, B. J. Shaw and R. D. Handy, Aquat. Toxicol., 2007, 82, 94–109 CrossRef CAS.
- R. D. Handy, T. B. Henry, T. M. Scown, B. D. Johnston and C. R. Tyler, Ecotoxicology, 2008, 17, 396–409 CrossRef CAS.
- R. J. Griffitt, K. Hyndman, N. D. Denslow and D. S. Barber, Toxicol. Sci., 2009, 107, 404–415 CrossRef CAS.
- M. Yeo and M. Kang, Korean J. Chem. Eng., 2009, 26, 711–718 CrossRef CAS.
- B. J. Shaw and R. D. Handy, Environ. Int., 2011, 37, 1083–1097 CrossRef CAS.
- G. Laban, L. F. Nies, R. F. Turco, J. W. Bickham and M. S. Sepulveda, Ecotoxicology, 2010, 19, 185–195 CrossRef CAS.
- B. D. Johnston, T. M. Scown, J. Moger, S. A. Cumberland, M. Baalousha, K. Linge, R. van Aerle, K. Jarvis, J. R. Lead and C. R. Tyler, Environ. Sci. Technol., 2010, 44, 1144–1151 CrossRef CAS.
- B. Jovanovic and D. Palic, Aquat. Toxicol., 2012, 118, 141–151 CrossRef.
- P. Zhang, X. He, Y. H. Ma, K. Lu, Y. L. Zhao and Z. Y. Zhang, Chemosphere, 2012, 89, 530–535 CrossRef CAS.
- L. Canesi, C. Ciacci, D. Vallotto, G. Gallo, A. Marcomini and G. Pojana, Aquat. Toxicol., 2010, 96, 151–158 CrossRef CAS.
- B. Jovanovic, L. Anastasova, E. W. Rowe, Y. J. Zhang, A. R. Clapp and D. Palic, Ecotoxicol. Environ. Saf., 2011, 74, 675–683 CrossRef CAS.
- L. H. Hao, Z. Y. Wang and B. S. Xing, J. Environ. Sci., 2009, 21, 1459–1466 CrossRef CAS.
- W. Zhang, M. Kalive, D. G. Capco and Y. S. Chen, Nanotechnology, 2010, 21, 355103 CrossRef.
- D. M. Aruguete, J. S. Guest, W. W. Yu, N. G. Love and M. F. Hochella, Environ. Chem., 2010, 7, 28–35 CrossRef CAS.
- S. Kang, M. Herzberg, D. F. Rodrigues and M. Elimelech, Langmuir, 2008, 24, 6409–6413 CrossRef CAS.
- O. Choi and Z. Q. Hu, Environ. Sci. Technol., 2008, 42, 4583–4588 CrossRef CAS.
- S. B. Liu, L. Wei, L. Hao, N. Fang, M. W. Chang, R. Xu, Y. H. Yang and Y. Chen, ACS Nano, 2009, 3, 3891–3902 CrossRef CAS.
- S. George, S. J. Lin, Z. X. Jo, C. R. Thomas, L. J. Li, M. Mecklenburg, H. Meng, X. Wang, H. Y. Zhang, T. Xia, J. N. Hohman, S. Lin, J. I. Zink, P. S. Weiss and A. E. Nel, ACS Nano, 2012, 6, 3745–3759 CrossRef CAS.
- I. Romer, T. A. White, M. Baalousha, K. Chipman, M. R. Viant and J. R. Lead, J. Chromatogr., A, 2011, 1218, 4226–4233 CrossRef.
- K. Donaldson, X. Y. Li and W. MacNee, J. Aerosol Sci., 1998, 29, 553–560 CrossRef CAS.
- M. Hull, A. J. Kennedy, C. Detzel, P. Vikesland and M. A. Chappell, Environ. Sci. Technol., 2012 Search PubMed , in press.
- C. M. Goodman, C. D. McCusker, T. Yilmaz and V. M. Rotello, Bioconjugate Chem., 2004, 15, 897–900 CrossRef CAS.
- J. L. Bouldin, T. M. Ingle, A. Sengupta, R. Alexander, R. E. Hannigan and R. A. Buchanan, Environ. Toxicol. Chem., 2008, 27, 1958–1963 CrossRef CAS.
- D. H. Lin, N. Liu, K. Yang, L. Z. Zhu, Y. Xu and B. S. Xing, Carbon, 2009, 47, 2875–2882 CrossRef CAS.
- D. H. Lin and B. S. Xing, Environ. Sci. Technol., 2008, 42, 5917–5923 CrossRef CAS.
- D. H. Lin, N. Liu, K. Yang, B. S. Xing and F. C. Wu, Environ. Pollut., 2010, 158, 1270–1274 CrossRef CAS.
- D. Li, D. Y. Lyon, Q. Li and P. Alvarez, Environ. Toxicol. Chem., 2008, 27, 1888–1894 CrossRef CAS.
- T. P. Dasari and H. M. Hwang, Sci. Total Environ., 2010, 408, 5817–5823 CrossRef CAS.
- A. J. Bone, B. P. Colman, A. P. Gondikas, K. M. Newton, K. H. Harrold, R. M. Cory, J. M. Unrine, S. J. Klaine, C. W. Matson and R. T. Di Giulio, Environ. Sci. Technol., 2012, 46, 6925–6933 CrossRef CAS.
- J. W. Chen, Z. M. Xiu, G. V. Lowry and P. Alvarez, Water Res., 2011, 45, 1995–2001 CrossRef CAS.
- K. Van Hoecke, K. De Schamphelaere, P. Van der Meeren, G. Smagghe and C. R. Janssen, Environ. Pollut., 2011, 159, 970–976 CrossRef CAS.
- Z. Y. Wang, J. Li, J. Zhao and B. S. Xing, Environ. Sci. Technol., 2011, 45, 6032–6040 CrossRef CAS.
- M. R. Choi, K. J. Stanton-Maxey, J. K. Stanley, C. S. Levin, R. Bardhan, D. Akin, S. Badve, J. Sturgis, J. P. Robinson, R. Bashir, N. J. Halas and S. E. Clare, Nano Lett., 2007, 7, 3759–3765 CrossRef CAS.
- C. L. Azevedo Costa, I. S. Chaves, J. Ventura-Lima, J. L. Ribas Ferreira, L. Ferraz, L. M. de Carvalho and J. M. Monserrat, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2012, 155, 206–212 CrossRef CAS.
- X. Z. Zhang, H. W. Sun, Z. Y. Zhang, Q. Niu, Y. S. Chen and J. C. Crittenden, Chemosphere, 2007, 67, 160–166 CrossRef CAS.
- L. P. Fang, O. K. Borggaard, P. E. Holm, H. Hansen and N. Cedergreen, Environ. Toxicol. Chem., 2011, 30, 2553–2561 CrossRef CAS.
- A. Oukarroum, S. Polchtchikov, F. Perreault and R. Popovic, Environ. Sci. Pollut. Res., 2012, 19, 1755–1762 CrossRef CAS.
- A. Lapresta-Fernandez, A. Fernandez and J. Blasco, TrAC, Trends Anal. Chem., 2012, 32, 40–59 CrossRef CAS.
- R. J. Miller, S. Bennett, A. A. Keller, S. Pease and H. S. Lenihan, PLoS One, 2012, 7, e30321 CAS.
- Y. Z. Wang and F. M. Li, Sci. China, Ser. G: Phys., Mech. Astron., 2012, 55, 1210–1224 CrossRef.
- D. G. Wei, R. Dave and R. Pfeffer, J. Nanopart. Res., 2002, 4, 21–41 CrossRef CAS.
- A. N. Shipway, E. Katz and I. Willner, ChemPhysChem, 2000, 1, 18–52 CrossRef CAS.
- J. Moger, B. D. Johnston and C. R. Tyler, Opt. Express, 2008, 16, 3408–3419 CrossRef CAS.
- X. Y. Yang, R. E. Edelmann and J. T. Oris, Aquat. Toxicol., 2010, 100, 202–210 CrossRef CAS.
- S. C. Hayden, G. X. Zhao, K. Saha, R. L. Phillips, X. N. Li, O. R. Miranda, V. M. Rotello, M. A. El-Sayed, I. Schmidt-Krey and U. Bunz, J. Am. Chem. Soc., 2012, 134, 6920–6923 CrossRef CAS.
- X. S. Zhu, Y. Chang and Y. S. Chen, Chemosphere, 2010, 78, 209–215 CrossRef CAS.
- R. D. Holbrook, K. E. Murphy, J. B. Morrow and K. D. Cole, Nat. Nanotechnol., 2008, 3, 352–355 CrossRef CAS.
- K. Fent, C. J. Weisbrod, A. Wirth-Heller and U. Pieles, Aquat. Toxicol., 2010, 100, 218–228 CrossRef CAS.
- S. J. Lin, Y. Zhao, T. Xia, H. Meng, Z. X. Ji, R. Liu, S. George, S. J. Xiong, X. Wang, H. Y. Zhang, S. Pokhrel, L. Madler, R. Damoiseaux, S. Lin and A. E. Nel, ACS Nano, 2011, 5, 7284–7295 CrossRef CAS.
- M. Manabe, N. Tatarazako and M. Kinoshita, Aquat. Toxicol., 2011, 105, 576–581 CrossRef CAS.
- B. Gilbert, C. F. Sirine, T. Xia, S. Pokhrel, L. M. Dler and A. E. Nel, ACS Nano, 2012, 6, 4921–4930 CrossRef CAS.
- N. F. Reuel, A. Dupont, O. Thouvenin, D. C. Lamb and M. S. Strano, ACS Nano, 2012, 6, 5420–5428 CrossRef CAS.
- A. Kumar, A. K. Pandey, S. S. Singh, R. Shanker and A. Dhawan, Cytometry, Part A, 2011, 79, 707–712 Search PubMed.
- U. Dembereldorj, E. O. Ganbold, J. H. Seo, S. Y. Lee, S. I. Yang and S. W. Joo, Vib. Spectrosc., 2012, 59, 23–28 CrossRef CAS.
- A. A. Shemetov, I. Nabiev and A. Sukhanova, ACS Nano, 2012, 6, 4585–4602 CrossRef CAS.
- C. D. Walkey, J. B. Olsen, H. B. Guo, A. Emili and W. Chan, J. Am. Chem. Soc., 2012, 134, 2139–2147 CrossRef CAS.
- J. M. Unrine, S. E. Hunyadi, O. V. Tsyusko, W. Rao, W. A. Shoults-Wilson and P. M. Bertsch, Environ. Sci. Technol., 2010, 44, 8308–8313 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
