Biological accumulation of engineered nanomaterials: a review of current knowledge
Wen-Che
Hou
*ac,
Paul
Westerhoff
a and
Jonathan D.
Posner
*b
aArizona State University, School of Sustainable Engineering and the Built Environment, Tempe, AZ 85287, USA
bUniversity of Washington, Mechanical Engineering, Chemical Engineering, Seattle, WA 98195, USA. E-mail: jposner@uw.edu; Tel: +1 206 543-9834
cNational Research Council Associate, Ecosystems Research Division, National Exposure Research Laboratory, United States Environmental Protection Agency, Athens, GA 30605, USA. E-mail: hou.wen-che@epa.gov; Tel: +1 706 337-8129
First published on 17th December 2012
Abstract
Due to the widespread use of engineered nanomaterials (ENMs) in consumer and industrial products, concerns have been raised over their impacts once released into the ecosystems. While there has been a wealth of studies on the short-term acute toxic effects of ENMs over the past decade, work on the chronic endpoints, such as biological accumulation, has just begun to increase in last 2–3 years. Here, we comprehensively review over 65 papers on the biological accumulation of ENMs under a range of ecologically relevant exposure conditions in water, soil or sediment with the focus on quantitative comparison among these existing studies. We found that daphnid, fish, and earthworm are the most commonly studied ecological receptors. Current evidence suggests that ENM accumulation level is generally low in fish and earthworms with logarithmic bioconcentration concentration factor and biota-sediment accumulation factor ranging from 0.85–3.43 (L kg−1) and −2.21–0.4 (kg kg−1), respectively. ENMs accumulated in organisms at the lower trophic level can transfer to higher trophic level animals with the occurrence of biomagnification varying depending on the specific food chain studied. We conclude the review by identifying the challenges and knowledge gaps and propose paths forward.
 Wen-Che Hou | Wen-Che Hou holds a Ph.D. in Civil Engineering from Purdue University. He is now at the U. S. Environmental Protection Agency at Athens, Georgia as a National Research Council associate. His research interest is in the field of environmental nanomaterial chemistry. |
 Paul Westerhoff | Paul Westerhoff holds a Ph.D. (1995) in environmental engineering from the University of Colorado at Boulder. He is a Professor in the School of Sustainable Engineering and the Built Environment and Associate Dean for Research in the Ira A. Fulton Schools of Engineering at Arizona State University. His research focuses on emerging water quality issues and their treatment using physicochemical processes. He has more than 130 peer-reviewed publications. |
 Jonathan D. Posner | Jonathan D. Posner is an Associate Professor of Mechanical Engineering at University of Washington with courtesy appointments in Chemical Engineering at UW and the Consortium for Science Policy and Outcomes at Arizona State University. His research area is micro and nanoscale transport phenomena at the intersection of chemistry, biology, and medicine. |
Environmental impactThe widespread use of engineered nanomaterials (ENMs) in consumer and industrial products and their subsequent release into the ecosystems have raised concerns over their potential environmental impacts. While there has been a wealth of studies on the short-term acute toxicity of ENMs, work on the chronic endpoints, such as biological accumulation, has just begun to increase. Research in organic and metal pollutants has shown that measuring the pollutant concentrations accumulated in biota is more reflective of the pollutant's intrinsic toxicity. A review on the current research dealing with the ecological accumulation of emerging ENMs and identifying the knowledge gaps and path forward is needed. |
Introduction
Over the past five years there has been growing evidence on the ecotoxicological effects of engineered nanomaterials (ENMs).1–3 ENMs that are now in more than 1300 commercial products have found widespread applications as drug carriers, molecular probes, catalysts, cosmetics, antibiotics, microelectronic components, image contrast agents, opacifiers, and fillers for plastics and rubber.4–6 Nanomaterials have several potential routes to come into contact with organisms, such as (1) incidental release (e.g., emission from vehicles); (2) release from industrial products or processes as well as commercial products during intended uses (e.g., using soaps or toothpastes containing nanosilver, washing clothing containing nanomaterials),7–9 which enter sewers and flow to wastewater treatment plants and then discharge to surface waters; (3) application of biosolids from wastewater treatment plants (WWTPs) to agricultural lands or landfills; (4) pesticides applied on agricultural fields or residential yards; (5) accidental spill of materials or waste streams discharged during manufacturing; (6) contact during usage of consumer products, such as sun screen or cosmetics; and (7) direct infiltration or runoff of excretion (urine/feces) of ENMs from humans or livestock. While the last decade has seen a wealth of acute toxicity studies focusing on the short-term effects of ENMs (e.g., mortality, stress), the work on the chronic endpoints, such as biological and ecological accumulation, has just begun to increase in the last 2–3 years.Environmental risk assessment involves both hazard evaluation (i.e., toxicity) as well as the potential for exposure. Toxicity studies of ENMs to mammalian cells and a wide range of organisms from single cell organisms, such as bacteria and algae, to more complex organisms, including aquatic worms and crustaceans (e.g., water flea), earthworm, plants, and fish, have been growing rapidly in the past decade.1,10–15 The studies provide knowledge regarding the acute toxicity of major ENMs. While the acute toxicity can be determined using test organisms by dosing varied amounts of chemicals (e.g., lethal dose 50% LD50), these dosages may not necessarily reflect the realistic environmental concentrations and the corresponding toxicity that occur in the ecosystems.
Assessment of the potential exposure concentrations in the environment is controlled by ENM's collective environmental behaviors, such as removal from wastewater treatment systems,16,17 aggregation,18–20 deposition,18,19 and chemical transformation (i.e., persistence).21–29 Using nominal concentration (i.e., added concentration) can misrepresent the intrinsic toxic effect of a pollutant, especially in complex environmental systems.30 The bioavailable fraction of the added concentration can be significantly reduced due to aggregation, strong binding to environmental matrix such as soil or sediment organic carbon, or due to sorption to container walls during laboratory exposure.30 Not all the pollutant in the ambient environment will reach an organism, and it is usually the fraction retained by the organism (i.e., internal concentration) that exerts the greatest toxic effect.31 Research in organic pollutants has shown that measuring the pollutant concentration accumulated in organisms more accurately reflects the intrinsic toxicity of the pollutant.30 If a chemical substance tends to persist in the environment, it is more likely to undergo long range transport and to accumulate in biota. Metal pollutants are generally considered persistent (i.e., they never disappear), and this may also be applicable to some metal ENMs, such as TiO2. There is a general lack of knowledge regarding ENM persistence with only a handful of studies showing that ENMs can be degraded by biodegradation,32,33 by ligand reaction,23,24 or by phototransformation.26–29,34 This suggests that attention needs to be paid to the potential for ENMs to accumulate in ecosystems.
Typically, organic chemicals and some metals (e.g., methylmercury) accumulate in fatty tissues and organs in aquatic and terrestrial organisms. ENMs may follow similar paradigms and their accumulation in biota and transfer through a food chain are more likely to exert long term and wide ranging ecotoxicological impacts.
Biological accumulation potential is an important indicator besides persistence and toxicity, that has been used globally by many regulatory agencies for assessment of hazard potentials for existing or new chemical substances. Agencies such as the U. S. Environmental Protection Agency (USEPA) in its Toxic Substances Control Act (TSCA), as well as the Registration, Evaluation, and Authorization of Chemicals (REACH) of the European Commission, have quantified biological accumulation using the bioconcentration factor (BCF), which is the quotient of pollutant concentration in organisms to that in water. If the BCF value of a chemical substance is greater than 5000 (log BCF value = 3.7), the chemical is considered “very bioaccumulative” and may be subject to actions that reduce environmental risk, depending on other factors, including persistence, exposure, and hazards.
The present review aims to provide an overview of current knowledge of ENM accumulation in biota. While some articles have reviewed the ecotoxicity, fate, and transport of ENMs,1,6,10–12,15,35,36 to our knowledge there has been no review that focuses on the ecological accumulation issues. Here, we critically review existing ENM biological accumulation studies across a range of ENMs in aquatic as well as terrestrial animals. While cell uptake of ENMs in vitro or in growth media is useful in elucidation of cellular uptake mechanisms, we focus on studies of ENM accumulation in organisms using ecologically relevant exposure conditions (i.e., via water, soil, or sediment). We exclude uptake in plants, as this topic has been the concern of other reviews.10,13 We organize the review as follows. We first describe the terminology in order to provide a coherent discussion in the review. We then detail the literature related to ENM uptake in aquatic and terrestrial species with the focus on quantitative comparison across the results. We conclude the review by identifying the challenges and knowledge gaps associated with the current research on ENM biological uptake and propose paths forward.
1 Ecological accumulation of engineered nanomaterials
Organisms can experience several exposure routes to pollutants that lead to uptake. Here we briefly describe the exposure routes typical of molecular pollutants and the specific endpoints associated with them in an effort to guide the discussion of this review and to compare and contrast the uptake behavior with ENMs.37,38 The relevant endpoints are as follows.Bioconcentration
Bioconcentration is a process in which pollutants are absorbed by the organisms from the ambient environment (i.e., water or air) only via respiratory and/or dermal surfaces (i.e., passive uptake). This process can be quantitatively expressed by bioconcentration factor (BCF), which is the quotient of the chemical concentration in an organism to that in water. BCF has units of L kg−1 because the aqueous and organism concentrations are conventionally expressed as chemical mass per L and chemical mass per kg biomass, respectively. BCF is measured as it reaches a steady state and is a net result of uptake and elimination processes from the organism. The elimination processes may include metabolic transformation, fecal egestion, and gill elimination, and growth dilution.37 Note that bioconcentration does not include uptake via contaminated diets and thus can only be determined in controlled laboratory environment without dietary uptake of pollutants.Bioaccumulation
In situations where exposure through contaminated food in addition to that through ambient sources occurs, bioaccumulation is used and bioaccumulation factor (BAF) is expressed as chemical concentration in an organism (chemical mass per kg biomass) to that in water (chemical mass per L). The field measured bioaccumulation endpoint is generally referred to as BAF, as the dietary exposure is difficult to exclude in field conditions. If the exposure occurs in soil or a benthic (e.g., river or lake sediment) environment, the relevant bioaccumulation endpoint is usually represented by biota-sediment accumulation factor (BSAF), which is the ratio of chemical concentration in an organism to that in the sediment.Biomagnification
Biomagnification is a process where the pollutant concentration in the organism of higher trophic level exceeds that in the organism of lower level at a steady state within a food chain. The biomagnification end point can be quantitatively described by the biomagnification factor (BMF), which is the ratio of the pollutant concentration in the predator to that in its prey. A BMF larger than unity generally indicates that biomagnification occurs in a food web.Existing biological accumulation studies of ENMs using ecologically relevant exposure conditions (i.e., via water, soil, or sediment) are summarized in Table 1. In Table 1, the organisms used have included three major categories as aquatic invertebrates and vertebrates as well as terrestrial invertebrates. Daphnids, aquatic worms, fish, and earthworms are the 4 most commonly used organisms, which can be attributed to their recommended use as test organisms in standard test guidelines.39–43 We summarize the BCF or BAF values reported in or estimated from the existing studies in Tables 2 and 3 for aquatic organisms (daphnids and fish) and earthworms, respectively. For aquatic organism studies, this review is focused on those in freshwater ecosystems, because research on marine or estuary systems is limited.44–49 In the few marine organism studies, Ferry et al. found that gold nanorods preferentially accumulated to biofilms and clams in a simulated marine food web that also included seawater, sediment, microbes, fish, snail, shrimp, and plants.48 As expected, ENMs in marine ecosystems aggregate to a greater extent than in freshwater systems as a result of ENM surface charge screening in seawater (i.e., elevated concentration of salts), thereby reducing the bioavailability of ENMs.49 While the bioavailability is reduced, the existing studies suggest that ENMs are still bioavailable to organisms in marine systems.44–49
| Category | Organism | ENM & property | Exposure condition | Major findings | Ref. |
|---|---|---|---|---|---|
| Aquatic invertebrate or protozoa | Daphnid (Daphnia magna) | Degussa P25 TiO2 w/ a primary particle size = 21 nm, forming ∼3500 nm aggregates in exposure solution. | 24 h waterborne exposure to 0.1–1 mg L−1 TiO2 in reconstituted water at pH = 8 w/ or w/o algae (as diet), followed by 72 h depuration. | BCF (L kg−1 dry weight) = 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 563 and 118 563 and 118![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 063 for 0.1 and 1 mg L−1 TiO2. BCF (L kg−1 dry weight) = 1232 at 1 mg L−1 TiO2 with diet. Incomplete depuration was observed. 063 for 0.1 and 1 mg L−1 TiO2. BCF (L kg−1 dry weight) = 1232 at 1 mg L−1 TiO2 with diet. Incomplete depuration was observed. |
51 |
| Daphnid (Ceriodaphnia dubia) | Fe2O3 NP w/ a nominal size of 20–40 nm aggregated up to 700 nm in media. | 48 h waterborne exposure to 1–50 mg L−1 Fe2O3 NP followed by 72 h depuration in moderately hard water (MHW). | Uptake increased with increased NP concentrations. NPs mainly accumulated in the gut. Significant yet incomplete depuration was observed. | 65 | |
| Daphnid (Daphnia magna) | Radiolabeled AgNP coated w/ cysteine. Primary NP size = 20 nm, but aggregated to 40–50 nm in exposure solution. | Waterborne exposure to 2–500 μg L−1 AgNP in artificial freshwater or via dietary exposure to AgNP pre-exposed algae. | Ionic Ag uptake rates were higher than AgNP. Ingestion of AgNP associated diet accounted for 70% of total AgNP accumulation. AgNP depuration was incomplete in 4 days. | 57 | |
| Daphnid (Daphnia magna) | Citrate coated 20 nm Au NP | 24 h waterborne exposure to 0.5 mg L−1 Au NP in MHW. | Au NPs were observed to retain in the gut with no apparent absorption into tissues. | 66 | |
| Daphnid (Daphnia magna) | Carbonate coated AgNP w/ a size of 20 nm | 48 h waterborne exposure to 0.02–0.5 mg L−1 AgNP in artificial freshwater. | BCF = 4.6 × 104 (L kg−1 dry weight) for exposure to 0.5 mg L−1 AgNP. | 58 | |
| Daphnid (Daphnia magna) | C60 aggregates w/ a size of 10–200 nm | 48 h waterborne exposure in reconstituted hard water with diet. | Estimated BCF (L kg−1 dry weight) = 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for exposed nC60 concentration of 30 mg L−1. 000 for exposed nC60 concentration of 30 mg L−1. |
54 | |
| Daphnid (Daphnia magna) | C60 aggregates w/ a size of 235 nm | 24 h waterborne exposure in artificial freshwater at pH = 6.8 followed by depuration. | Estimated BCF (L kg−1 dry weight) = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 25 000 and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for 0.5 and 2 mg L−1 of nC60. 50% depuration in 2 days. 000 for 0.5 and 2 mg L−1 of nC60. 50% depuration in 2 days. |
52 | |
| Daphnid (Daphnia magna) | C60 aggregates w/ a size of 99 nm | 48 h waterborne exposure in MHW at pH = 7. | Estimated BCF (L kg−1 dry weight) = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 437 000 and 437![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 for mother and baby daphnia at 0.2 mg L−1 nC60. 500 for mother and baby daphnia at 0.2 mg L−1 nC60. |
56 | |
| Daphnid (Daphnia magna) | Phospholipid coated SWCNT | 96 h waterborne exposure to 2.5 ppm SWCNT in MHW at pH = 7.6. | Qualitatively showing that daphnids took up phospholipid coated SWNT. SWCNT may be biomodified by removing (digesting) the lipid coating. | 60 | |
| Daphnid (Ceriodaphnia dubia) | Hydroxylated and carboxylated MWCNT | 48 h waterborne exposure to 32 mg CNT/L in MHW w/ or w/o algae as food. | Qualitatively showing that MWCNTs were retained in the gut. Feeding during depuration facilitated MWCNT elimination. | 61 | |
| Daphnid (Daphnia magna) | Acid oxidized MWCNT | 48 h waterborne exposure to 0.04–0.4 mg L−1 CNT in artificial freshwater followed by depuration. | BCF (L kg−1 dry biomass) = 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–440 000–440![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Minimal depuration w/o feeding, 50–85% depuration in 2 days with feeding. 000. Minimal depuration w/o feeding, 50–85% depuration in 2 days with feeding. |
53 | |
| Daphnid (Daphnia magna) | Polyethyleneimine (PEI) coated MWNT w/ negative, positive, or neutral surface charge | 48 h waterborne exposure to 0.025–0.25 mg L−1 CNT in artificial freshwater followed by depuration. | Surface coating of MWNT has no apparent effect on bioaccumulation. | 55 | |
| Daphnid (Daphnia magna) | Fluorescent polystyrene (PS) NPs w/ sizes of 20 or 1000 nm. Particle sizes unchanged in exposure media. | 24 h waterborne exposure to 2 μg L−1 PS NPs in reconstituted hard water followed by 4 h depuration. | 20 nm PS NPs accumulated to a greater extent than 1000 nm NPs on surface area and number basis. 20 nm PS NPs retained stronger than 1000 nm ones during depuration. Absorption of NPs into gut's epithelium was observed. | 70 | |
| Crustacean (Thamnocephalus platyurus) | C60 aggregates w/ a size of 500 nm | 1 h waterborne exposure to 3–6 mg L−1 nC60 in MHW. | nC60 accumulated in the guts or excreted, forming micron scale aggregates. | 130 | |
| Daphnid (Ceriodaphnia dubia) | Carboxyl CdSe@ZnS QDs | 24 h waterborne exposure to 0.2–0.6 ppb QD in MHW at pH = 7.2 | Qualitatively showing that bioaccumulation of QD to C. dubia occurred. | 131 | |
| Daphnid (Ceriodaphnia dubia) w/algae as food. | Carboxyl CdSe@ZnS QDs | 24 h feeding C. dubia with QD pre-exposed algae (as diet) in MHW. | Qualitatively showing that trophic transfer occurs from algae to water flea. | 94 | |
| Daphnid (Daphnia magna) | Mercaptoundecanoic acid coated CdSe@ZnS QDs w/ a size of ∼5 nm. Aggregated to 25 nm in solution. | 36 h waterborne exposure to 15 nM QDs. | Qualitatively showing that QDs accumulated in the gut with no apparent absorption into tissue. | 62 | |
| Daphnid (Daphnia magna) | Poly(acrylic acid)-octylamine or poly (maleic anhydridealt-1-octadecene) coated CdSe@ZnS QD w/ PEG modification. Size = 14–43 nm. | Waterborne exposure to 7.7 nM QDs ([Cd] = 0.6 ppm) in MHW at pH = 7.2–7.6 | PEG modification leads to higher uptake. QDs were not completely excreted after 48 h depuration. | 63 | |
| Ciliated protozoa (Tetrahymena pyriformis) | Carboxylated or biotinylated CdSe@ZnS QDs w/ a size of 12 nm | 7 d waterborne exposure in hard water followed by depuration. | BCF = 1766 and 2474 (L kg−1 dry weight) for carboxylated and biotinylated QDs. Significant depuration in 8 days. | 93 | |
| Rotifer (Brachionus calyciflorus) | Carboxylated and biotinylated CdSe@ZnS QDs w/ a size of 12 nm | QD trophic transfer from ciliates to rotifer | BMF (dry weight) = 0.60 and 0.29 for carboxylated and biotinylated QDs. | 93 | |
| Ciliated protozoa (Tetrahymena thermophila) | Citrate coated CdSe QDs w/ a mean size of 5 nm | Trophic transfer from bacteria (pre-exposed to 75 mg L−1 (as Cd) QD) to ciliated protozoa | Trophic transfer occurred with a BMF (dry weight) = 5.4. | 92 | |
| Ciliated protozoa (Tetrahymena thermophila) | Acid oxidized SWCNT w/ size of 2–20 × 500 nm | 24 h exposure to 11.9 mg SWCNT L−1 in Osterhout's medium. | Qualitatively showing that SWCNT uptake occurs. Internalized SWCNT impeded ingestion and digestion of diet. | 132 | |
| Oligochaetes (Lumbriculus variegates) | Al2O3 w/ a size of 11 nm. Forming aggregates w/ size range of 120–500 nm in pure water. | 28 d exposure to 25 g Al2O3 per kg sediment. | Marginal uptake was observed. | 133 | |
| Oligochaetes (Lumbriculus variegates) | HCl-purified SWCNT and MWCNT | 28 d exposure to 0.03 and 0.37 mg CNT kg−1 dry sediment in river sediments followed by depuration. | BSAF (kg OC kg−1 lipid) = 0.28 and 0.40 for SWCNT and MWCNT. Nearly complete depuration in 1 day. No apparent CNT absorption into tissue. | 124 | |
| Oligochaetes (Lumbriculus variegates) | HCl purified or acid oxidized MWCNT | 28 d exposure to 0.37 mg CNT g−1 dry sediment. | BSAF (kg dry sediment per kg dry biomass) = 0.67. Oxidizing MWCNT has no observed effect on BSAF. | 110 | |
| Polychaets (Arenicola marina) | TiO2 with a primary particle = 23.2 nm | 10 d exposure to 1–3 g TiO2 per kg marine sediment. | BSAF = 0.156–0.196 (kg OC per kg lipid). No observed TiO2 across gut epithelium cells. | 44 | |
| Polychaete (Nereis diversicolor) | 30 nm AgNPs w/ citrate coating | 10 d exposure to 250 mg AgNP per kg dry estuary sediment. | Absorption of AgNPs into gut tissues was observed and endocytosis was likely the route for cellular uptake. | 45 | |
| Polychaete (Streblospio benedicti) | Carboxylated SWCNTs | 14 d exposure in marine sediments w/ 364 g CNT kg−1 dry sediment. | No measurable bioaccumulation after defecation. | 46 | |
| Polychaets (Arenicola marina) | SWCNT dispersed with surfactants | 10 d exposure to 0.003–0.03 g CNT per kg marine sediment. | Qualitatively showing bioaccumulation of SWCNT is minimal in sediment. | 44 | |
| Copepod (Amphiascus tenuiremis) | Carboxylated SWCNTs | 14 d exposure in marine sediments w/ 364 g CNT per kg dry sediment. | No measurable bioaccumulation after defecation. | 46 | |
| Amphipod (Leptocheirus plumulosus) | Carboxylated CdSe@ZnS QD w/ a size of 15–20 nm. | 96 h exposure to QD or OQ contaminated algae in artificial sea water. | Waterborne exposure led to greatest QD accumulation than dietary exposure or exposure to ionic Cd. | 47 | |
| Freshwater snail (Lymnaea stagnalis) | 10–20 nm AgNP w/ citrate or humic acid coating | Waterborne exposure to 0.04–9 ppb Ag (as AgNP or ionic Ag) or dietary exposure to Ag pre-exposed algae (as diet). | Ag uptake rates were greater for Ag ion than for AgNP in either route. Both Ag forms (as AgNP or Ag ion) lost extremely slowly once accumulated from diet. | 134 | |
| Freshwater snail (Lymnaea stagnalis) | 65Cu labeled CuO NP w/ spherical shape (7 nm) or as rod (7 × 40 nm) | 24 h waterborne exposure to 0.2 to 2000 μg Cu per L in MHW. | Label Cu NP was able to distinguish Cu body burden from background versus NP exposure. | 135 | |
| Freshwater snail (Lymnaea stagnalis) | 67Zn labeled ZnO NP w/ spherical shape (110 nm) or mixed shapes (spherical, trigonal, rod, 20–70 nm) | 4 h dietary exposure to 5–60 mg ZnO per g diet followed by 2–15 days of depuration. | Label ZnO NP was able to distinguish Zn accumulation from background versus NP exposure. | 136 | |
| Clam (Corbicula fluminea) | Al2O3 w/ a size of 11 nm. Forming aggregates w/ size range of 120–500 nm in pure water. | 28 d exposure to 25 g per kg sediment nano- or micron-sized Al2O3. | No difference was observed in Al body burden for nano- or micron-sized Al2O3. | 133 | |
| Clam (Corbicula fluminea) | Bovine serum albumin stabilized 7.8, 15, 46 nm AuNPs. AuNP size unchanged during exposure. | 180 h waterborne exposure to 2–8 mg L−1 AuNP in MHW. | AuNPs were largely retained in the digestive tract or excreted in feces. | 137 | |
| Aquatic vertebrate | Carp (Cyprinus carpio) | Degussa P25 TiO2 w/ a primary particle size = 21 nm | 25 d waterborne exposure to 10 mg L−1 TiO2 in tap water at pH = 7.8. | Elevated Ti was detected in muscle, gill, liver, stomach, and intestine with intestine showing the largest accumulation. BCF (L kg−1 whole body dry weight) = 495. | 73 |
| Carp (Cyprinus carpio) | Degussa P25 TiO2 w/ a primary particle size = 21 nm | 20 d waterborne exposure to 10 mg L−1 TiO2 in tap water at pH = 7.8. | Elevated Ti was detected in skin and scale, muscle, gill, and other organs and gut combined. BCF (L kg−1 whole body dry weight) = 325. | 74 | |
| Carp (Cyprinus carpio) | Degussa P25 TiO2 w/ a primary particle size = 21 nm | 20 d waterborne exposure to 10 mg L−1 TiO2 in tap water at pH = 7.8. | Elevated Ti was detected in skin and scale, muscle, gill, and other organs and gut combined. BCF (L kg−1 whole body dry weight) = 617. | 72 | |
| Carp (Cyprius carpio) | AgNP with a primary size of 35 nm. | 21 day waterborne exposure to 0.01–0.1 mg L−1 AgNP in dechlorinated tap water. | Ag was detected in liver, bile, intestine, and gills with intestine showing the greatest accumulation. Nanosized AgNPs show greater accumulation than micron-sized ones. | 78 | |
| Zebrafish | AgNP w/ a primary size of 26.6 nm. AgNP aggregated to 44.5–216 nm in media. | 48 h waterborne exposure to 1 mg L−1 AgNP in MHW. | Particulate Ag is a greater contributor to the body burden than the soluble portion of Ag. | 76 | |
| Zebrafish (Danio rerio) | Degussa P25 TiO2 w/ a primary particle size = 21 nm, forming ∼2500 nm aggregates in exposure media. | 14 d waterborne exposure to 0.1–1 mg L−1 TiO2 in reconstituted water at pH = 8, followed by 7 days depuration. | BCF (L kg−1 dry weight) = 25 and 181 for 0.1 and 1 mg L−1 TiO2. Significant, yet incomplete depuration was observed. | 96 | |
| Zebrafish (Danio rerio) | Degussa P25 TiO2 w/ a primary particle = 21 nm. | Trophic transfer from daphnids pre-exposed to 0.1–1 mg L−1 TiO2 to zebrafish in reconstituted water at pH = 8. | Trophic transfer of TiO2 occurred with BMF values (dry weight) < 0.024. Exposure via contaminated food results in larger body burden to fish than via waterborne TiO2. | 96 | |
| Zebrafish (Danio rerio) | ZnO w/ a primary particle size ≤ 100 nm | 14 d waterborne exposure to 0.5–5 mg ZnO per L in artificial freshwater. | ZnO aggregates fell out of solution. No significant uptake occurred. | 80 | |
| Zebrafish (Danio rerio) | CeO2 w/ a primary particle size ∼25 nm | 14 d waterborne exposure to 0.5–5 mg CeO2 per L in artificial freshwater. | CeO2 aggregates fell out of solution. No significant uptake occurred. | 80 | |
| Zebrafish (Danio rerio) | Poly(acrylic acid)-octylamine coated CdSe@ZnS QD w/ a size of 5.2 nm | Trophic transfer from QD contaminated daphnids to zebrafish. | Trophic transfer occurred with low BMF of 0.04 and 0.004 for adult and juvenile zebrafish. Lost >99% body burden in 7 days depuration. | 95 | |
| Zebrafish (Danio rerio) | 12 and 50 nm citrate coated Au NP | Dietary exposure to 0.1 μg Au per fish per day for 36 days or to 0.04 μg Au per fish per day for 60 days. | Au was detected in brain, liver, and muscles in the high exposure concentration, but not in low exposure level. Large (50 nm) Au NPs accumulated greater than small ones (12 nm). | 138 | |
| Rainbow trout (Oncorhynchus mykiss) | Degussa P25 TiO2 w/ a primary size of 21 nm | 14 d waterborne exposure to 0.1–1 mg L−1 TiO2 in tap water. | TiO2 NP uptake was low with no significant accumulation in gill, liver, muscles, or brain. | 139 | |
| Rainbow trout (Oncorhynchus mykiss) | Degussa P25 TiO2 w/ a primary size of 21 nm | Dietary exposure to 10 or 100 mg TiO2 per kg diet for 8 weeks followed by 2 weeks of depuration. | Elevated Ti was detected in gill, liver, intestine, brain, and spleen w/ accumulated Ti still remaining after depuration. | 75 | |
| Rainbow trout (Oncorhynchus mykiss) | TiO2 w/ a primary particle size ≤ 100 nm, aggregating to > 1000 nm in exposure medium. | 14 d waterborne exposure to 0.05–5 mg L−1 TiO2 in artificial freshwater. | TiO2 aggregates fell out of solution. No significant uptake occurred. Some TiO2 aggregates crossed gill membrane. | 80 | |
| Rainbow trout (Oncorhynchus mykiss) | 10 or 35 nm AgNP or micron-sized Ag particles. AgNP formed large aggregates (>600 nm) in solution. | 10 d waterborne exposure to 0.01–0.1 mg L−1 AgNP at pH = 7.6–8.5. | AgNP accumulation was limited, yet detectable in gill and liver. No apparent size effect was observed. | 77 | |
| Medaka (Oryzias latipes) | Cationic, neutral, and anionic 10 nm AuNPs w/ hydrophilic or hydrophobic coating. AuNP size unchanged during exposure. | 5 d waterborne exposure to 800 ppb AuNP in conditioned water. | Cationic AuNPs w/ hydrophobic coating exhibited greatest accumulation. Elevated Au was detected in brain, heart, liver, gill, and intestine w/ intestine showing the greatest accumulation. Complete depuration in 120 h was observed for hydrophilic AuNPs. | 79 | |
| Medaka (Oryzias latipes) | Fluorescent PS NP w/ a size of 39.4 nm | 7 d waterborne exposure to 10 mg L−1 PS NPs at pH = 7.2. | NPs accumulated in various organs including brain, gill, liver, kidney, intestine and blood with the greatest accumulation found in gills and intestines. | 91 | |
| Estuarine mesocosm | Mesocosms contained sea water, sediment, sea grass, microbes, biofilms, snails, clams, shrimp and fish | CTAB coated positively charged Au nanorods, 65 nm × 15 nm. | 12 d exposure in mesocosms simulating estuarine environment. | BCF (L kg−1 dry weight) = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 for biofilm, 8.21 for sea grass, 115 for shrimp, 474 for fish, 167 for snail, 22 300 for biofilm, 8.21 for sea grass, 115 for shrimp, 474 for fish, 167 for snail, 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 for clam 800 for clam |
48 |
| Terrestrial invertebrate | Earthworm (Eisenia fetida) | Al2O3 w/ a size of 11 nm. Forming aggregates w/ size range of 120–500 nm in pure water. | 28 d exposure to 0.1–13 g Al per kg soil. | Uptake of nano-sized Al2O3 is larger than micron-sized Al2O3. | 87 |
| Earthworm (Eisenia fetida) | TiO2 w/ a primary particle size of 10–20 nm | 7 d exposure to 0.1–5 g kg−1 dry soil in artificial soil | BSAF = 0.01–0.02 (kg dry soil per kg wet biomass) | 86 | |
| Earthworm (Eisenia fetida) | ZnO w/ a primary particle size = 10–20 nm | 7 d exposure to 0.1–5 g kg−1 dry soil in artificial soil. | BSAF = 0.01–0.37 (kg dry soil per kg wet biomass) | 86 | |
| Earthworm (Eisenia veneta) | ZnO w/ a primary size of 100 nm | 21 day exposure to 0.25–0.75 g kg−1 dry soil or 1 day aqueous exposure to 0.12 g L−1 ZnO. | Particulate ZnO shows uptake into tissues in aqueous exposure. | 140 | |
| Earthworm (Eisenia fetida) | Molecular C60 or C60 aggregates | 28 d exposure to 0.25 to 300 mg C60 per kg dry soils with 0.3–5% OC content followed by depuration. | BSAF (kg dry soil per kg dry biomass) = 0.22–0.79 and 0.065–0.13 for 0.25 and 100 mg C60 per kg dry soils. ∼70% depuration in 1 day. | 82 | |
| Earthworm (Eisenia fetida) | HCl-purified SWCNT and MWCNT | 28 d exposure to 0.03 to 0.3 mg CNT per kg dry soils having 1.7–5.5% OC content followed by depuration. | BSAF (kg dry soil per kg dry biomass) = 0.022–0.0061 and 0.014 to 0.023 for SWCNT and MWCNT. OC content has no effect on BSAF. | 83 | |
| Earthworm (Eisenia fetida) | HCl purified or acid oxidized MWCNT | 28 d exposure to 0.3 mg CNT per g dry soil | BSAF (kg dry soil per kg dry biomass) = 0.03 for earthworm. Oxidizing MWCNT has no observed effect on BSAF. | 110 | |
| Earthworm (Eisenia fetida) | Polyethyleneimine (PEI) coated MWNT w/ negative, positive, or neutral surface charge | 28 d exposure to 0.03 to 0.3 mg CNT per kg dry soils with 1.6–5.5% OC content followed by depuration. | Surface coating of MWNT has no apparent effect on bioaccumulation. Some depuration in 1 day. | 85 | |
| Earthworm (Eisenia fetida) | Cu NP w/ a size of 20–40 nm or <100 nm. | 28 d exposure to 5–50 mg per dry artificial soil of ionic or particulate Cu. | Body burden generally increased with increased exposure concentration w/ no clear trend on size effect. | 141 | |
| Earthworm (Eisenia fetida) | Citrate coated 20 and 55 nm AuNPs | 28 d exposure to 5, 20, 50 mg Au per kg dry artificial soil | Particulate AuNP can be absorbed into earthworm tissue. | 90 | |
| Earthworm (Eisenia fetida) | 30–50 nm AgNP w/ hydrophilic or amphiphilic coating. | 28 d exposure to 10–1000 mg per dry artificial soil of ionic or particulate Ag. | Earthworms accumulated Ag from ionic Ag more than from particulate Ag. No surface coating effect was observed. | 142 | |
| Isopods (Porcellio scaber) | ZnO w/ a primary particle size < 100 nm | 28 d exposure to ZnO contaminated leaves (as food) in Petri dish. | BAF (kg dry leaf per kg dry biomass) = 0.1 and 0.2 for exposure to 6.2 and 2.5 g ZnO per kg dry leaf. Exposure to other forms of Zn (i.e., microsized ZnO or ZnCl2) showed similar results. | 143 | |
| Isopods (Porcellio scaber) | TiO2 w/ a primary particle size < 25 nm | 14 d dietary exposure to 0.1–5 mg g−1 dry food in Petri dishes. | NP internalization into gut epithelial cells occurred at dose concentration at 5 mg g−1 food and may be attributed to cell membrane disruption. | 144 | |
| Tobacco hornworm (Manduca sexta) | Tannic acid-coated 5, 10, and 15 nm AuNPs, negatively charged. | Trophic transfer from plants pre-exposed to 100 mg L−1 AuNP before feeding to the worms. | AuNP trophic transferred into the tissue of worms. BMF (dry weight) = 6.2, 11.6, 9.2 for 5, 10, and 15 nm AuNP. | 97 |
| Organism | ENMs | n | Nominal exposure concentration (mg L−1) | Range of log (BCF) | Mean log (BCF) (95% C.I.) | Ref. |
|---|---|---|---|---|---|---|
| a Steady state BCF is not tested. b Some BCF values reported here are calculated based on reported or our estimated dry weight based body burden divided by nominal exposure concentrations, as the actual ENM concentration remained in the exposure medium is not measured or clearly indicated in the studies. The dry weight body burden is estimated by dividing the wet weight body burden by the dry-to-wet weight ratio of 0.08 for daphnid.52 c BCF values for gut, liver, gill, brain, or skin. d BCF based on wet biomass, as appropriate dry-to-wet weight ratio is not available. | ||||||
| Daphnid | TiO2 | 2 | 0.1–1 | 4.75–5.05 | 4.91 (0.31) | 51 |
| AgNP | 9 | 0.002–0.5 | 3.16–4.66a,b | 4.04 (0.39) | 57 and 58 | |
| nC60 | 3 | 0.2–2 | 4.18–4.98b | 4.52 (0.47) | 52 and 56 | |
| Carbon nanotube | 11 | 0.04–0.4 | 3.74–5.64 | 4.70 (0.38) | 53 and 55 | |
| Fish | TiO2 | 5 | 0.5–10 | 1.40–2.79b | 2.33 (0.49) | 72–74 |
| CeO2 | 2 | 0.5–5 | 2.31–3.43a,b,c | 2.87 (1.10) | 80 | |
| AgNP | 6 | 0.01–0.1 | 0.85–1.62a,b,c,d | 1.27 (0.23) | 77 | |
| AuNP | 1 | 0.0057 | 2.68c | 2.68 | 48 | |
| ENMs | n | Nominal exposure concentration (mg kg−1 dry soil) | Range of log (BSAF) | Mean log (BSAF) (95% C.I.) | Ref. |
|---|---|---|---|---|---|
| a Steady state BCF is not tested. b BSAF values reported here are calculated based on reported or our estimated dry weight body burden divided by nominal exposure concentrations, as the actual ENM concentration remained in the exposure medium is not measured or reported. The dry weight body burden is estimated by dividing the wet weight body burden by a factor of 0.15 for E. fetida.145 | |||||
| TiO2 | 2 | 1000–5000 | −1.14 to −0.86a,b | −1.00 (0.28) | 86 |
| ZnO | 6 | 100–5000 | −1.08 to 0.40a,b | −0.27 (0.39) | 140 |
| Au NP | 6 | 5–50 | −1.14 to −0.5a,b | −0.75 (0.20) | 90 |
| Ag NP | 6 | 5–50 | −1.18 to −0.85a,b | −0.99 (0.11) | 141 |
| Cu NP | 6 | 10–1000 | −0.33–0.27a,b | −0.05 (0.16) | 142 |
| C60 | 4 | 0.25–300 | −1.19 to −0.30 | −0.83 (0.37) | 82 |
| Carbon nanotube | 10 | 30–500 | −2.21 to −1.15 | −1.58 (0.22) | 83,85 and 110 |
Note that not all the reports we include in this review strictly use the above terminology or their quantitative measures. We have made an effort to translate whatever is reported to this common set of quantitative measures and terminology provided here. To facilitate comparison, we use the dry biomass or dry soil based BCF or BSAF values, as most current studies have reported the dry weight body burden and that also minimizes the variability associated with the water content of the organisms tested. BCFs and BSAFs for many hydrophobic organic contaminants (HOCs) are traditionally reported by normalizing by the lipid content of the test organisms because there is evidence that HOCs preferentially reside in the lipid phase of animals due to their similarity in nonpolar nature.50 However, since there is limited evidence showing that this is also true for ENMs, currently there is no consensus on reporting.
Accumulation in daphnid
Table 2 summarizes daphnid BCF values directly reported from BCF studies51–53 as well as our estimate from body burden based on dry biomass and exposure concentration.54–58 The estimate of BCF may introduce some uncertainties. For example, as the actual exposed concentrations in water are not reported or measured in some studies,54–58 we use the nominal exposure concentrations (i.e., initial concentrations) to approximate the aqueous ENM concentrations in BCF calculation. This approach likely underestimates the BCF values, which should be the quotient of the body burden to the ENM concentration remaining in water, because the ENM concentration can decrease in the aqueous phase due to sorption to container walls, aggregation and subsequent settling, and organism uptake.59 Additionally, whether ENM bioaccumulation reached a steady state value was not confirmed or clearly stated in some studies.57,58 This may also cause underestimation in our calculation of BCFs. Other studies also demonstrated that ENMs can accumulate in daphnids, but are not included in Table 2, because the results reported therein are either only qualitative or not easy to convert to BCF for comparison.60–63In Table 2, the mean log BCF values for daphnids across various ENMs are generally large, ranging from 3.16 to 5.64. This suggests that ENMs have a high possibility to accumulate in daphnids. The USEPA TSCA considers bioaccumulative substances as having log BCF values in the range 3–3.7 and very bioaccumulative substances with log BCF values ≥3.7. The compiled log BCF values for the well-known bioaccumulative p,p′-DDT fall in the range 3.07–5.06 for daphnids.64
Many hydrophobic and bioaccumulative organic pollutants, such as polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), insecticides, and some metals (e.g., methylmercury), tend to absorb into the fatty tissue of organisms. However, for ENMs, the majority of the existing studies has indicated that ENMs taken up by daphnids mainly remain in the guts with limited absorption into the gut tissue,51–53,60–63,65,66 suggesting that ingestion of ENMs is the major route for uptake. This is consistent with the feeding behavior of daphnids that are able to filter a large volume of water relative to their body size and retain fine particulate materials (as food) in the guts. Given that daphnids can filter particles with sizes ranging from 0.4–40 μm,67,68 most of the retained materials are likely aggregates of ENMs. While the experiments were designed for BCFs, the accumulation in the gut raises doubt about the suitability of using BCF to interpret the data, since BCF is used when exposure occurs via passive uptake (i.e., dermal or gill absorption). In this review, we tentatively use BCF as a general description for the phenomenon observed. Once ingested, the food particles will be digested and then the nutrients will transport across the gut epithelium to storage cells.69 However, this appears to contrast with most existing observations for ENMs that show limited translocations across gut walls. One exception is the work by Rosenkranz et al. who demonstrated that daphnids take up micron-sized (1000 nm) fluorescent carboxylated polystyrene (PS) particles more efficiently than nano-sized ones (20 nm), and some particles of both sizes absorb across gut walls, accumulating in the lipid droplets within storage cells as revealed by tracing the fluorescence of the particles distributed within daphnids and confirmed by electron microscopy.70 They suggested that the uptake of 10 nm PS nanoparticles is unintentional as opposed to 1000 nm PS nanoparticles that fall in the size range that can be actively retained by daphnids. Nano-sized particles can possibly be captured in the food groove via interception, inertial impaction, gravitational and diffusional deposition, yet in a less efficient way than active filtering.71 The observation of organic PS nanoparticle deposition in lipid droplets of daphnids is analogous to the partitioning of traditional lipophilic HOCs to the lipid phase. One recent study also shows that fullerene C60 accumulation to daphnids increases proportionally to the increase in daphnid's lipid content.56 However, such an observation is not unequivocally reported in other work with carbon nanotubes and C60.52,53,55 The apparent contradiction suggests that ENM absorption and distribution within organisms warrants further investigation as it has a profound impact on how ENMs reach target organs and exert toxic effects there.
Following exposure, daphnids are able to purge ENMs from their bodies. Tervonen et al. reported that daphnids eliminate 75% of accumulated nC60 in 48 h of depuration in nC60 free freshwater.52 However, the depuration is not complete and the 25% of nC60 mass remaining in daphnids is significant. Similar observations have also been reported for carbon nanotubes (CNTs),53,55 nano TiO2 (nTiO2),51 silver nanoparticles (AgNPs),57 quantum dots (QDs),63 iron oxide,65 and PS nanoparticles.70 Feeding daphnids during waterborne exposure and depuration impacts the accumulation behavior for ENMs. Zhu et al. has shown that feeding daphnids with green algae during waterborne exposure to nTiO2 greatly reduces body burden from 61.0 to 1.0 (g per kg biomass).51 Feeding also helps to eliminate ENMs accumulated in daphnids. For example, Petersen et al. indicated that feeding daphnids during depuration significantly decreases the CNT body burden from minimal elimination w/o food to 85% removal in 24 h.53,55 Nevertheless in all cases, the elimination is not complete, suggesting the potential for trophic transfer of ENMs.
Table 2 suggests that there is a lack of dependence of bioconcentration on the ENMs composition, aspect ratio, primary particle size, and surface coatings. Petersen et al. indicated that CNT surface modification with polymers of positive, neural, or negative charges has a minimal effect on CNT uptake by daphnids, although it was not clearly stated if the surface charge characteristics and the CNT size remains the same in the exposure solution (i.e., as opposed to in pure water) was not clearly stated.55 Several studies report that pristine nanoparticles with nanoscale primary sizes aggregate to submicron or micron scale agglomerates in the exposure solution (e.g., moderately hard water) due to the presence of salts.51,63 The physical and chemical alterations of ENMs, such as aggregation or stabilization by coating with organic matter in exposure media, can diminish the impact of the pristine ENM properties and highlights the importance of characterizing ENMs interactions in the exposure media.
Accumulation in fish
Table 2 summarizes the fish BCF values directly reported in or estimated from the information reported in the literature. In contrast to HOCs, which show relatively similar bioaccumulation in daphnid and fish, the ENM mean log BCF values in fish range from 1.27–2.87, which are 1–2 orders of magnitude smaller than those of daphnids. For example, the compiled log BCF values of p,p′-DDT are in the range 3.07–5.06 for daphnids and 2.48–5.56 for fish.64 This discrepancy in bioaccumulation behavior for HOCs and ENMs implies that the underlying bioaccumulation mechanism of ENM is unique from HOCs. While HOCs rely on partitioning into the lipid phase of organisms and thus the lipid content normalized BCF values stay relatively constant across species, the knowledge regarding the bioaccumulation mechanisms for ENMs in fish is limited. The log BCF values for Ag NP are one order of magnitude lower than those of the other ENMs, because they are based on wet biomass (dry biomass data not available nor an appropriate wet to dry biomass conversion ratio).Before absorption into fish tissues and organs, ENMs need to cross several barriers on the fish surface. Fish gills are generally identified as an important site for xenobiotic exposure, as it is the place where the chemical exchange (e.g., gases, ions, and small molecular nutrients) between fish and the environment (i.e., water) occurs.11,14 Other possible exposure routes may include gut exposure (i.e., ingestion) and dermal exposure. The greater ENM accumulation in daphnids may be partly attributed to their feeding behavior, as they are well-known filter feeders that capture a large amount of particulate materials in the guts. To elucidate the mechanism for the uptake and distribution of ENMs in fish, recent studies have begun to examine the biodistribution of ENM in fish organs and tissues. Kashiwada measured the distribution of fluorescent non-ionized PS nanoparticles with a size of 39.4 nm in transparent medaka upon waterborne exposure. He found that fluorescence was extensively detected in various organs with gill and intestine exhibiting the greatest increase in fluorescence. The increase in fluorescence was also observed in gallbladder, blood, testis, liver, kidney, and brain, although theses values are not statistically significant. The author suggests that PS nanoparticles are absorbed through gill and gut and enter the circulation system (i.e., blood stream) to various organs, although it is not clear if the fluorescent tag on the nanoparticles is leached out and reaches the organs during exposure. Similar observations were also reported for nTiO2 uptake in carp72–74 or trout,75 Ag NP uptake in zebrafish,76 trout,77 or carp,78 and Au NP uptake in medaka.79 Ferry et al. found that Au NP mass largely resided in combined organs and gut content samples of fish, but was not detectable in brain, gills, or skin and muscle tissues, indicating limited Au NP transport through the circulatory system or absorption through skin or gills.48 Similar results have also been reported for nTiO2, Ag NP, and CeO2 nanoparticles, although detectable mass of ENMs was also found in gills and livers.72–74,76–80 Taken together, current literature suggests that the bioaccumulation potential of ENMs to fish is relatively low and that the major route of ENMs uptake for fish may be oral (i.e., direct ingestion of ENMs) or food exposure (i.e., ingestion of ENM contaminated diets).
Accumulation in earthworm
The earthworm is an important and ecologically relevant receptor that plays a key role in the structure and function of the soil environment. It has been used as a test organism in several recent ENM bioaccumulation studies. Table 3 summarizes the BASF values for ENMs reported or estimated from the data reported in the literature. The mean log BSAF values range from −1.68 to −0.34 across various ENMs. Currently there is no regulatory criterion of BSAF for chemical substances, but the ENM BSAF values are generally low compared with typical HOCs. For example, the earthworm BSAF values for phenanthrene and pyrene have been reported in the range 0.51–0.9081,82 and 0.47–1.15 (kg dry soil per kg dry biomass),83,84 respectively. The ENM BSAF values are fairly insensitive to the organic carbon (OC) content of the soils in large contrast to HOCs. For example, Petersen et al. indicated that single-walled and multi-walled CNTs exhibit a similar bioaccumulation behavior to earthworm in soil with varied OC content from 1.6–5.7%, suggesting that the equilibrium partitioning theory for HOC bioaccumulation may not be applicable for CNTs.55,83Other reports suggest that ENM surface properties have minimal impact on biological accumulation behavior. CNTs with different surface functionalities (i.e., acid purified, acid oxidized, polyethyleneimine (PEI) functionalized, etc.) and charge characteristics exhibit similar bioaccumulation in earthworms.85 The results are consistent with the observation that the accumulation of CNTs in daphnids is independent of surface characteristics.55
While many studies have shown that metal oxide or metallic ENMs can bioaccumulate in earthworms,86,87 there has been no clear indication on the uptake route (i.e., via dissolved ions versus particulate NPs), as some of the metal and metal oxide ENMs are soluble.88,89 Unrine et al. studied the bioaccumulation behavior of Au NPs to earthworm and compared it to ionic Au.90 They found that ionic Au is ∼5 times more bioaccumulative than particulate Au. There is no apparent size dependency on bioaccumulation for Au NPs of 20 or 55 nm based on Au mass, although Au NPs of both primary sizes formed larger aggregates (∼100 nm) in the soil pore water. To further elucidate the uptake route, they used synchrotron micro-X-ray absorbance near-edge spectroscopy (μXANES) (i.e., to distinguish between zero valent versus ionic Au) combined with TEM imaging coupled with elemental analysis. They showed that zero valent Au in particulate form accumulates in earthworm tissue. In other words, the study provided some evidence that absorption of Au NPs into earthworm tissue occurs. The result is consistent with the other handful of studies that indicate occurrences of absorption and translocation of ENMs in other organisms.45,70,80,91
2 Biomagnification of engineered nanomaterials
The relatively high bioaccumulation and incomplete depuration of ENMs in some lower trophic level organisms (e.g., daphnid) points to the possibility for trophic transfer and biomagnification through the food chain. This has led to increased research in the food chain transfer behavior of ENMs. Table 4 summarizes the current reports on trophic transfer behavior for ENMs. The bacterial community is at the lower level of food chain. Werlin et al. demonstrated that trophic transfer occurs from CdSe QD-contaminated bacteria (Pseudomonas aeruginosa) to ciliated protozoa (Tetrahymena thermophila).92 They showed that the QD body burden in ciliates is ∼5 times as high as that in the bacteria they ingested (i.e., BMF = ∼5), indicating that biomagnification occurs. In another study, Holbrook et al. tested if CdSe–ZnS core–shell QD trophic transfer occurs in a simulated aquatic food web involving bacteria (Escherichia coli)-ciliates (Tetrahymena thermophila)-rotifers (Brachionus calyciflorus).93 In contrast, they did not observe trophic transfer from bacteria to ciliates due to the lack of QD internalization into bacterial cells. The discrepancy would suggest that bacterial strain and/or QD surface functionality play a role in the bacterial uptake of QDs. Skipping bacteria, they showed that ciliates can directly uptake QDs and trophic transfer to the predator, rotifers. However, the body burden in rotifers does not exceed that in ciliates (BMF = 0.29–0.62), indicating no biomagnification.| ENMs | n | Food chain involved | Range of BMFb | Mean BMF (95% C.I.) | Ref. |
|---|---|---|---|---|---|
| a n indicates number of observations. b Based on dry biomass unless noted. c Based on wet biomass. d Steady state BMF is not tested. | |||||
| TiO2 | 2 | Daphnid to zebrafish | 0.009–0.024 | 0.016 (0.015) | 96 |
| QDs | 2 | Daphnid to zebrafish | 0.004–0.04 | 0.022 (0.035) | 95 |
| QDs | 3 | Ciliated protozoa to rotifer | 0.29–0.62c | 0.50 (0.21) | 93 |
| QDs | 1 | Bacteria to ciliated protozoa | 5.37d | 5.37 | 92 |
| Au NP | 3 | Leaf to worm | 6.2–11.6d | 9.1 (3.1) | 97 |
Trophic transfer has also been reported for other high trophic level aquatic food chains, such as QDs transfer from algae to daphnid,94 and QDs or nTiO2 transfer from daphnid to fish.95,96 Biomagnification was not observed in these other studies with BMF ranging from 0.004–0.04. In a simulated terrestrial food chain, Judy et al. showed that Au concentrations in tobacco hornworms were larger than those in the Au NP pre-exposed tobacco leaves by a factor of 6.2–11.6 (i.e., BMF = 6.2–11.6) with no apparent dependency on Au NP sizes (5, 10, and 15 nm).97
Traditional biomagnifying contaminants, such as methylmercury, PCBs, and DDT, have great potential to accumulate up the food chains. For example, DDT has BMF values in the range of 1–45 in typical aquatic food chains.98 The pollutants can eventually reach human via consumption of contaminated foods (e.g., fish). Additional research is needed to further evaluate the impact of ENMs, considering that current studies used rather simplified food chains with exposure routes and ecological receptors at higher trophic levels (e.g., mammals) largely unknown.
3 Discussion
There is no current, systematic evaluation and correlation of the bioaccumulation data from existing studies. Here, we make a first effort to correlate and compare the aqueous or sediment phase concentrations versus the ENM concentrations accumulated in the biota from the existing studies across various ENMs. Fig. 1 shows the data for the distribution between aqueous organisms (i.e., daphnid and fish) and water. For both organisms, there is no clear dependence on the various ENMs reported in the literature. Therefore, we describe the data for each organism with a linear relationship of aqueous ENM concentration (Cw) versus body burden (Cbiomass). The slope for daphnid data (38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 313 L kg−1, n = 21, 95% confidence interval = 36
313 L kg−1, n = 21, 95% confidence interval = 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852–39
852–39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 775 L kg−1, R2 = 0.99) is ∼2 orders of magnitude higher than that of fish (430 L kg−1, n = 27, 95% confidence interval = 364–496 L kg−1, R2 = 0.85). The larger slope for daphnid data indicates that ENMs tend to accumulate more in daphnids, which may be attributable to their unique uptake behavior by filtering ENMs as discussed earlier. We note that the data presented in Fig. 1 carry similar uncertainties (e.g., nominal or measured Cw, wet or dry weight based Cbiomass, etc.) as those in daphnid and fish BCFs (Tables 2 and 3), as they are from the same data set. The large deviation from the fit line for fish data for Ag NP is due to the use of wet weight based Cbiomass in fish organs, for which an appropriate dry-to-wet biomass ratio is not available. Fig. 2 reports the earthworm-soil distribution of ENMs. The ENM exposure concentration (Cs) and body burden (Cbiomass) are both dry-weight based. The slope of Cbiomass to Cs is 0.095 (n = 40, 95% confidence interval = 0.065–0.126, R2 = 0.35).
775 L kg−1, R2 = 0.99) is ∼2 orders of magnitude higher than that of fish (430 L kg−1, n = 27, 95% confidence interval = 364–496 L kg−1, R2 = 0.85). The larger slope for daphnid data indicates that ENMs tend to accumulate more in daphnids, which may be attributable to their unique uptake behavior by filtering ENMs as discussed earlier. We note that the data presented in Fig. 1 carry similar uncertainties (e.g., nominal or measured Cw, wet or dry weight based Cbiomass, etc.) as those in daphnid and fish BCFs (Tables 2 and 3), as they are from the same data set. The large deviation from the fit line for fish data for Ag NP is due to the use of wet weight based Cbiomass in fish organs, for which an appropriate dry-to-wet biomass ratio is not available. Fig. 2 reports the earthworm-soil distribution of ENMs. The ENM exposure concentration (Cs) and body burden (Cbiomass) are both dry-weight based. The slope of Cbiomass to Cs is 0.095 (n = 40, 95% confidence interval = 0.065–0.126, R2 = 0.35).
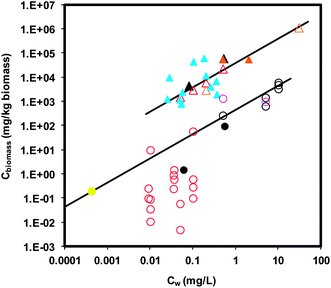 | ||
| Fig. 1 Aquatic organism–water distribution of ENMs. Cw indicates the aqueous concentration of ENMs. Cbiomass indicates body burden. Triangular and circular symbols represent daphnid (n = 21) and fish data (n = 27), respectively. Solid symbols represent measured data of Cw and dry weight Cbiomass. Open symbols represent data of nominal Cw (i.e., added concentration) and/or wet weight Cbiomass. Nanoparticles are represented by different colors: black for TiO2, pink for CeO2, red for Ag NP, yellow for Au NP, orange for C60, and blue for MWCNTs. Lines represent the linear relationship of Cwversus Cbiomass. The slopes for daphnid and fish are 38313 (95% confidence interval = 36852–39775; R2 = 0.99) and 430 (95% confidence interval = 364–496; R2 = 0.85) (L kg−1), respectively. | ||
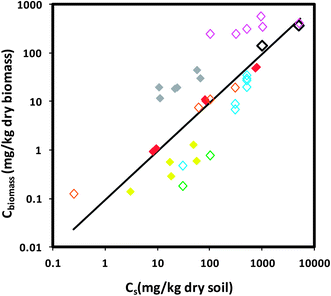 | ||
| Fig. 2 Earthworm–soil distribution of ENMs (n = 40). Cs indicates the exposure concentration of ENMs in soil based on dry soil mass. Cbiomass indicates body burden based on dry weight biomass. Solid symbols show the measured data of Cs and dry weight Cbiomass. Open symbols show the data of nominal Cs (i.e., added concentration) and/or estimated dry weight Cbiomass. We estimated dry weight Cbiomass by dividing the reported wet weight Cbiomass by the dry-to-wet weight ratio of 0.15 for earthworms.145 ENMs are indicated by symbols of different colors: black for TiO2, pink for ZnO, red for Ag NP, yellow for Au NP, grey for Cu NP, orange for C60, green for SWCNTs, and blue for MWCNTs. Lines represent linear relationship of Csversus Cbiomass. The slope is 0.095 (95% confidence interval = 0.065–0.126; R2 = 0.35). | ||
Existing literature suggests that there is a general lack of observation of ENM absorption into animal tissues. The lack of absorption in animal tissues is in contrast to the results in vitro using single cellular organisms. For example, several recent studies have shown that many cells are able to internalize ENMs across the cellular barriers.99–103 Kastarelos et al. reported that functionalized CNTs can be internalized by a range of eukaryotic (e.g., fish and mammalian cells) and prokaryotic cells (i.e., fungi, yeast, and bacteria).101 It is generally believed that receptor-mediated endocytosis is a major route leading to the internalization of ENMs.104 However, internalization has also been observed in prokaryotic cells that lack an endocytosis mechanism and in eukaryotic cells (e.g., red blood cells) under conditions inhibiting endocytosis.101 The discrepancy in the results from in vivo versus in vitro studies suggests that other biological barriers (e.g., epithelium) not tested in in vitro studies play a role and caution needs to be taken when extrapolating in vitro results to in vivo studies.
Most current studies were performed in relatively well-controlled conditions (i.e., in the laboratory or in mesocosms) with high exposure concentrations of non-labeled ENMs (i.e., >sub ppm) to demonstrate that ENM accumulation in organisms occurs. The measured environmental concentration of ENMs is essentially non-existent with a few modeling studies that suggest the potential environmental concentration of ENMs at sub microgram per liter level (i.e., ppb) in surface waters.105,106 It is challenging to detect biological accumulation at such low exposure levels of ENMs in complex environmental matrices of soil, natural waters and organism tissues. Another challenge associated with the detection of ENM ecological accumulation is related to the characterization of the chemical and physical forms of ENM in biota. Most current studies used methods relying on bulk analysis of elemental concentrations by using inductively coupled plasma (ICP) with mass spectroscopy (MS) or by detecting the radioactivity of radio-labeled nanomaterials (e.g., 14C-labeled carbon nanotubes) following sample digestion. The methods are useful in terms of tracking the distributions of elemental concentrations of ENMs between phases (i.e., biota, water, soil, and sediments), but they do not yield the quantitative information regarding the physical form of the accumulated elements (i.e., particulate versus ionic), especially for ENMs known to dissolve to ions (e.g., ZnO, Ag NP)107,108 and size and particle number distributions. It has been shown that imaging techniques, such as TEM coupled with energy dispersive X-ray spectroscopy (EDS) elemental mapping45,90,92 or coherent anti-Stokes Raman scattering spectroscopy (CARS),80 can reveal the physical form and spatial distribution (i.e., adsorption on versus absorption into tissues or cells) of ENMs in tissues, although the results appear to be qualitative and tedious sample preparation and imaging hinder routine analysis. For more comprehensive discussions, we refer to a recent article that reviews recent advances and highlights areas that require additional research in measurement of ENMs in complex environmental and biological samples.109
There is a potentially wide range of ENMs with varied physicochemical properties (e.g., size, shape, core composition, chirality, crystallinity, surface functionality) that require risk assessment. In an effort to address this issue, recently attempts have been made to examine the applicability of empirical methods to predict ENM accumulation in biota, such as determination of ENM octanol–water partitioning coefficient (Kow) which is commonly used as the fate, transport, and bioaccumulation descriptors for HOCs.110–116 Some ENM Kow values appear to be experiment dependent and may not have thermodynamic significance because the ENMs are not able to cross the octanol–water interface without vigorous mixing and tend to accumulate at the interface.110–112 Initially dispersing CNTs in water versus octanol before contact with the other phase also gives different results.110 Petersen et al. found that their measured Kow for multi-walled carbon nanotubes (MWNTs, log Kow = ∼3) does not correlate with their bioaccumulation behavior, as MWNTs do not bioaccumulate appreciably to earthworm (BSAF = ∼0.03) in contrast to HOCs having similar Kow values.110 Similar observations have also been reported for fullerene C60.82 It is suggested that the relatively large size of ENMs compared to HOCs and/or strong sorption to soil OC may hinder the absorption of ENMs to organism tissue, limiting ENM accumulation in biota.82 The limited bioavailability in soil systems may explain the discrepancy in toxicity results where the exposure in soils or activated sludge leads to no toxicity117,118 as opposed to exposure in vitro.119–121 This highlights the need to characterize the bioavailability and toxicity of ENMs in ecologically relevant conditions. In our previous work, we have also measured the distribution of ENM between water and simulated biomembranes (i.e., lipid bilayers) in an effort to use a more relevant organic phase and overcome some challenges associated with the poorly defined octanol–water interface. The results indicate that the lipid bilayer-water distributions of both C60 and fullerol nano-aggregates are pH-dependent with accumulation in lipid bilayers increasing systematically as pH decreased. The lipid bilayer-water distribution coefficient (Klipw) for C60 was larger than that of fullerol (i.e., polyhydroxylated C60) at a given pH, indicating greater propensity for C60 aggregates to interact with lipid bilayers.122 In that work, we provided a preliminary comparison between the measured lipid-water distribution and bioaccumulation studies in daphnia, which suggests that the surrogate measurement may be useful in predicting biological accumulation. In another work, we explored the dosimetric selection on the distribution of gold nanoparticles (Au NPs) to lipid bilayers.123 Using tannic acid functionalized Au NP from 5–100 nm, we found that smaller sized Au NPs accumulate more rapidly to lipid bilayers than larger ones. Large Au NPs distribute to the lipid bilayer to a greater extent on the basis of Au mass accumulated than small Au NPs, but the trend reverses if we compared by the number of accumulated Au NPs. The faster accumulation to lipid bilayers for small nanoparticles can be qualitatively rationalized by the Smoluchowski collision rate equations and the classical colloid theory (i.e., Derjaguin–Landau–Verwey–Overbeek (DLVO) theory). Our analysis suggests that number concentration, along with Au NP diameter, may be the more appropriate dosimetric parameter for mechanistically describing the nano–bio interaction. Across the various Au NP sizes, we measure the lipid bilayer–water distribution coefficient (Klipw = Clip/Cw) as 450 L per kg lipid, which is independent of dosimetric units. Overall, our studies suggest that lipid bilayer–water distribution may be a promising predictor for biological accumulation in aquatic organisms.
4 Summary and outlook
Currently, there is no standardized ecological accumulation test protocol specifically designed for ENMs. Some current work has adopted the protocols developed for molecular pollutants (e.g., Kow and sorption to soil and lipid). Concerns exist that the current protocols may not be applicable to ENMs, because they were established assuming that the distribution of molecular pollutants between biota and the environmental matrices (i.e., soil, water) follows equilibrium partitioning theory.35 In our previous studies on ENM distribution between simulated biota (i.e., lipid bilayer biomembrane) and water, we observed that a pseudo-steady state was established generally within 1 day of mixing.122,123 While trying to extend the mixing time to weeks as used in traditional soil sorption studies of organic pollutants to reach equilibrium, we encountered the problem of lipid bilayer disintegration, limiting our ability to observe long-term results. Similar steady state distribution between organisms and environmental matrices has also been reported previously.51–53,82,83,110,124 On the other hand, a few studies that aimed to test if ENM distribution follows partitioning behavior indicated that carbonaceous ENM accumulation in organisms is insensitive to organic carbon content of exposure media (i.e., sediment or soil). This contrasts with the bioaccumulation behavior of traditional hydrophobic pollutants, suggesting that partitioning may be irrelevant to the tested ENMs.82,110 However, there is an exception showing that hydrophobic fullerene C60 accumulation in daphnids is proportional to daphnid lipid content where traditional hydrophobic organic pollutants preferentially accumulate.56 Nano–bio interface interaction is an emerging field in which colloidal forces are generally invoked to describe the interaction dynamics.125 It is of general belief that equilibrium thermodynamics is irrelevant in colloidal interactions. However, given the current inconclusive evidence showing that this is also the case for biological accumulation of ENMs, further research is needed to address the issue, especially as the particle size is reduced.There is a need to develop global descriptors and modeling frameworks in prediction of the ecological accumulation of ENMs. Such framework may be similar to the quantitative structure–activity relationship (QSAR) modeling that correlates single or multiple descriptors that capture the physicochemical properties of the chemical substances to their chemical or biological activity.126 While the physicochemical descriptors can be derived from measurements or theoretical calculations, their corresponding activity is usually obtained from experimental assays. Currently, there are very few nano-QSAR studies with none focusing on biological accumulation of ENMs.127,128 Selecting the physicochemical property descriptors is of critical importance in establishing QSARs. The representative physicochemical properties should be reflective of the mechanisms responsible for the specific activity. Traditionally, Kow has been shown to be a robust predictor of biological accumulation for a wide range of HOCs,37 due to the similarity in both processes that rely on partitioning. However, the use of Kow for ENMs has been under scrutiny. Novel nano-descriptors that capture the interaction dynamics at the nano–bio interfaces need to be developed for biological accumulation of ENMs. The potential descriptors could include the lipid bilayer–water distribution coefficient as suggested in our earlier work or attachment/deposition efficiency (α) that recently began to be used to describe ENM-(bio)interface interactions.122,123,129
The lack of standardized test protocols for ecological accumulation of ENMs may contribute to the inconsistency in data reporting. In our survey of the literature, we found problems that can cause difficulty in quantitative comparison among studies. For example, the wet weight biomass based body burden measured as whole body and/or in specific organs was used in the literature without reporting the dry weight (or weight ratio) or vice versa. The dry weight body burden can be orders of magnitude larger than wet weight body burden (for example, daphnid), which can cause errors in inter-study comparison without considering weight ratio. While the dry-to-wet weight ratio for some test animals may be found in the literature (assuming small variability between individuals or species), others are not readily available especially for organs and tissues. We recommend that future research provide the information (i.e., dry-to-wet weight ratio) to facilitate comparisons. ENM concentration can decrease over time due to aggregation and settling out of solution, sorption to the walls of experimental containers, and uptake by organisms. For this reason, we recommend that future research measure and monitor the steady state ENM concentrations in the exposure media throughout experiments and report them rather than only reporting nominal (i.e., added) concentration, as it may not accurately reflect the actual exposure level. We believe that these recommendations will aid in the creation of a consistent data set from future work that can ultimately be used to develop nano-QSARs in prediction of biological accumulation of ENMs.
Acknowledgements
Financial support was provided by the United States Department of Energy under Award no. DE-FG02-08ER64613 with Daniel Drell as program manager, National Science Foundation grant number CBET-0932885, NIH Grand Opportunities (RC2) program through NANO-GO NIEHS grant DE- FG02-08ER64613, and Semiconductor Research Corporation task number 425.025.References
- S. J. Klaine, P. J. J. Alvarez, G. E. Batley, T. F. Fernandes, R. D. Handy, D. Y. Lyon, S. Mahendra, M. J. McLaughlin and J. R. Lead, Nanomaterials in the environment: behavior, fate, bioavailability, and effects, Environ. Toxicol. Chem., 2008, 27, 1825–1851 CrossRef CAS.
- M. R. Wiesner, G. V. Lowry, P. Alvarez, D. Dionysiou and P. Biswas, Assessing the risks of manufactured nanomaterials, Environ. Sci. Technol., 2006, 40, 4336–4345 CrossRef CAS.
- A. Nel, T. Xia, L. Mädler and N. Li, Toxic potential of materials at the nanolevel, Science, 2006, 311, 622–627 CrossRef CAS.
- An inventory of nanotechnology-based consumer products currently on the market of the Project of Emerging Nanotechnology, http://www.nanotechproject.org/inventories/consumer/analysis_draft/, accessed 1 March 2011.
- B. Nowack, J. F. Ranville, S. Diamond, J. A. Gallego-Urrea, C. Metcalfe, J. Rose, N. Horne, A. A. Koelmans and S. J. Klaine, Potential scenarios for nanomaterial release and subsequent alteration in the environment, potential scenarios for nanomaterial release and subsequent alteration in the environment, Environ. Toxicol. Chem., 2012, 31, 50–59 CrossRef CAS.
- E. J. Petersen, L. Zhang, N. T. Mattison, D. M. O'Carroll, A. J. Whelton, N. Uddin, T. Nguyen, Q. Huang, T. B. Henry, R. D. Holbrook and K. L. Chen, Potential release pathways, environmental fate, and ecological risks of carbon nanotubes, Environ. Sci. Technol., 2011, 45, 9837–9856 CrossRef CAS.
- T. M. Benn and P. Westerhoff, Nanoparticle silver released into water from commercially available sock fabrics, Environ. Sci. Technol., 2008, 42, 4133–4139 CrossRef CAS.
- T. Benn, B. Cavanagh, K. Hristovski, J. D. Posner and P. Westerhoff, The release of nanosilver from consumer products used in the home, J. Environ. Qual., 2010, 39, 1875 CrossRef CAS.
- R. Kaegi, A. Ulrich, B. Sinnet, R. Vonbank, A. Wichser, S. Zuleeg, H. Simmler, S. Brunner, H. Vonmont, M. Burkhardt and M. Boller, Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment, Environ. Pollut., 2008, 156, 233–239 CrossRef CAS.
- E. Navarro, A. Baun, R. Behra, N. B. Hartmann, J. Filser, A.-J. Miao, A. Quigg, P. H. Santschi and L. Sigg, Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi, Ecotoxicology, 2008, 17, 372–386 CrossRef CAS.
- R. Handy, T. B. Henry, T. M. Scown, B. D. Johnston and C. R. Tyler, Manufactured nanoparticles: their uptake and effects on fish—a mechanistic analysis, Ecotoxicology, 2008, 17, 396–409 CrossRef CAS.
- J. Fabrega, S. N. Luoma, C. R. Tyler, T. S. Galloway and J. R. Lead, Silver nanoparticles: behaviour and effects in the aquatic environment, Environ. Int., 2011, 37, 517–531 CrossRef CAS.
- C. M. Rico, S. Majumdar, M. Duarte-Gardea, J. R. Peralta-Videa and J. L. Gardea-Torresdey, Interaction of nanoparticles with edible plants and their possible implications in the food chain, J. Agric. Food Chem., 2011, 59, 3485–3498 CrossRef CAS.
- R. D. Handy, G. Al-Bairuty, A. Al-Jubory, C. S. Ramsden, D. Boyle, B. J. Shaw and T. B. Henry, Effects of manufactured nanomaterials on fishes: a target organ and body systems physiology approach, J. Fish Biol., 2011, 79, 821–853 CrossRef CAS.
- A. Baun, N. B. Hartmann, K. Grieger and K. O. Kusk, Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing, Ecotoxicology, 2008, 17, 387–395 CrossRef CAS.
- M. A. Kiser, P. Westerhoff, T. Benn, Y. Wang, J. Pérez-Rivera and K. Hristovski, Titanium nanomaterial removal and release from wastewater treatment plants, Environ. Sci. Technol., 2009, 43, 6757–6763 CrossRef CAS.
- L. K. Limbach, R. Bereiter, E. Müller, R. Krebs, R. Gälli and W. J. Stark, Removal of oxide nanoparticles in a model wastewater treatment plant: influence of agglomeration and surfactants on clearing efficiency, Environ. Sci. Technol., 2008, 42, 5828–5833 CrossRef CAS.
- A. R. Petosa, D. P. Jaisi, I. R. Quevedo, M. Elimelech and N. Tufenkji, Aggregation and deposition of engineered nanomaterials in aquatic environments: role of physicochemical interactions, Environ. Sci. Technol., 2010, 44, 6532–6549 CrossRef CAS.
- K. L. Chen, B. A. Smith, W. P. Ball and D. H. Fairbrother, Assessing the colloidal properties of engineered nanoparticles in water: case studies from fullerene C60 nanoparticles and carbon nanotubes, Environ. Chem., 2010, 7, 10–27 CrossRef CAS.
- E. M. Hotze, T. Phenrat and G. V. Lowry, Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment, J. Environ. Qual., 2010, 39, 1909 CrossRef CAS.
- G. V. Lowry, K. B. Gregory, S. C. Apte and J. R. Lead, Transformations of nanomaterials in the environment, Environ. Sci. Technol., 2012, 46, 6893–6899 CrossRef CAS.
- G. V. Lowry, B. P. Espinasse, A. R. Badireddy, C. J. Richardson, B. C. Reinsch, L. D. Bryant, A. J. Bone, A. Deonarine, S. Chae, M. Therezien, B. P. Colman, H. Hsu-Kim, E. S. Bernhardt, C. W. Matson and M. R. Wiesner, Long-term transformation and fate of manufactured Ag nanoparticles in a simulated large scale freshwater emergent wetland, Environ. Sci. Technol., 2012, 46, 7027–7036 CrossRef CAS.
- C. Levard, E. M. Hotze, G. V. Lowry and G. E. Brown, Environmental transformations of silver nanoparticles: impact on stability and toxicity, Environ. Sci. Technol., 2012, 46, 6900–6914 CrossRef CAS.
- A. P. Gondikas, A. Morris, B. C. Reinsch, S. M. Marinakos, G. V. Lowry and H. Hsu-Kim, Cysteine-induced modifications of zero-valent silver nanomaterials: implications for particle surface chemistry, aggregation, dissolution, and silver speciation, Environ. Sci. Technol., 2012, 46, 7037–7045 CrossRef CAS.
- J. D. Fortner, D.-I. Kim, A. M. Boyd, J. C. Falkner, S. Moran, V. L. Colvin, J. B. Hughes and J.-H. Kim, Reaction of water-stable C60 aggregates with ozone, Environ. Sci. Technol., 2007, 41, 7497–7502 CrossRef CAS.
- W.-C. Hou and C. T. Jafvert, Photochemical transformation of aqueous C60 clusters in sunlight, Environ. Sci. Technol., 2009, 43, 362–367 CrossRef CAS.
- W.-C. Hou and C. T. Jafvert, Photochemistry of aqueous C60 clusters: evidence of 1O2 formation and its role in mediating C60 phototransformation, Environ. Sci. Technol., 2009, 43, 5257–5262 CrossRef CAS.
- W.-C. Hou, L. Kong, K. A. Wepasnick, R. G. Zepp, D. H. Fairbrother and C. T. Jafvert, Photochemistry of aqueous C60 clusters: wavelength dependency and product characterization, Environ. Sci. Technol., 2010, 44, 8121–8127 CrossRef CAS.
- L. Kong, O. Tedrow, Y. F. Chan and R. G. Zepp, Light-initiated transformations of fullerenol in aqueous media, Environ. Sci. Technol., 2009, 43, 9155–9160 CrossRef CAS.
- B. I. Escher and J. L. M. Hermens, Peer reviewed: internal exposure: linking bioavailability to effects, Environ. Sci. Technol., 2004, 38, 455A–462A CrossRef CAS.
- S. N. Luoma and P. S. Rainbow, Metal Contamination in Aquatic Environments: Science and Lateral Management, Cambridge University Press, 1st edn, 2008 Search PubMed.
- K. M. Schreiner, T. R. Filley, R. A. Blanchette, B. B. Bowen, R. D. Bolskar, W. C. Hockaday, C. A. Masiello and J. W. Raebiger, White-rot basidiomycete-mediated decomposition of C60 fullerol, Environ. Sci. Technol., 2009, 43, 3162–3168 CrossRef CAS.
- B. L. Allen, P. D. Kichambare, P. Gou, I. I. Vlasova, A. A. Kapralov, N. Konduru, V. E. Kagan and A. Star, Biodegradation of single-walled carbon nanotubes through enzymatic catalysis, Nano Lett., 2008, 8, 3899–3903 CrossRef CAS.
- C.-Y. Chen and C. T. Jafvert, Photoreactivity of carboxylated single-walled carbon nanotubes in sunlight: reactive oxygen species production in water, Environ. Sci. Technol., 2010, 44, 6674–6679 CrossRef CAS.
- R. D. Handy, G. Cornelis, T. Fernandes, O. Tsyusko, A. Decho, T. Sabo-Attwood, C. Metcalfe, J. A. Steevens, S. J. Klaine, A. A. Koelmans and N. Horne, Ecotoxicity test methods for engineered nanomaterials: practical experiences and recommendations from the bench, Environ. Toxicol. Chem., 2012, 31, 15–31 CrossRef CAS.
- E. J. Petersen and T. B. Henry, Methodological considerations for testing the ecotoxicity of carbon nanotubes and fullerenes: review, Environ. Toxicol. Chem., 2012, 31, 60–72 CrossRef CAS.
- J. A. Arnot and F. A. P. Gobas, A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms, Environ. Rev., 2006, 14, 257–297 CrossRef CAS.
- R. P. Schwarzenbach, P. M. Gschwend and D. M. Imboden, Environmental Organic Chemistry, Wiley-Interscience, 2nd edn, 2002 Search PubMed.
- OECD guidelines for the testing chemicals No. 305E. Bioconcentration: Flow-through fish test, 1996.
- OECD guidelines for the testing chemicals No. 305E. Daphnia sp., Acute Immobilization Test, 2004.
- OECD guidelines for the testing chemicals No. 315. Bioaccumulation in Sediment-dwelling Benthic Oligochaetes, 2008.
- OECD guidelines for the testing chemicals No. 317. Bioaccumulation in Terrestrial Oligochaetes, 2010.
- Ecological effects test guidelines, OPPTS 850.1730, Fish BCF.
- T. Galloway, C. Lewis, I. Dolciotti, B. D. Johnston, J. Moger and F. Regoli, Sublethal toxicity of nano-titanium dioxide and carbon nanotubes in a sediment dwelling marine polychaete, Environ. Pollut., 2010, 158, 1748–1755 CrossRef CAS.
- J. García-Alonso, F. R. Khan, S. K. Misra, M. Turmaine, B. D. Smith, P. S. Rainbow, S. N. Luoma and E. Valsami-Jones, Cellular internalization of silver nanoparticles in gut epithelia of the estuarine polychaete Nereis diversicolor, Environ. Sci. Technol., 2011, 45, 4630–4636 CrossRef.
- P. L. Ferguson, G. T. Chandler, R. C. Templeton, A. DeMarco, W. A. Scrivens and B. A. Englehart, Influence of sediment–amendment with single-walled carbon nanotubes and diesel soot on bioaccumulation of hydrophobic organic contaminants by benthic invertebrates, Environ. Sci. Technol., 2008, 42, 3879–3885 CrossRef CAS.
- B. P. Jackson, D. Bugge, J. F. Ranville and C. Y. Chen, Bioavailability, toxicity, and bioaccumulation of quantum dot nanoparticles to the amphipod Leptocheirus plumulosus, Environ. Sci. Technol., 2012, 46, 5550–5556 CrossRef CAS.
- J. L. Ferry, P. Craig, C. Hexel, P. Sisco, R. Frey, P. L. Pennington, M. H. Fulton, I. G. Scott, A. W. Decho, S. Kashiwada, C. J. Murphy and T. J. Shaw, Transfer of gold nanoparticles from the water column to the estuarine food web, Nat. Nanotechnol., 2009, 4, 441–444 CrossRef CAS.
- F. R. Khan, S. K. Misra, J. García-Alonso, B. D. Smith, S. Strekopytov, P. S. Rainbow, S. N. Luoma and E. Valsami-Jones, Bioaccumulation dynamics and modeling in an estuarine invertebrate following aqueous exposure to nanosized and dissolved silver, Environ. Sci. Technol., 2012, 46, 7621–7628 CrossRef CAS.
- D. Mackay, Correlation of bioconcentration factors, Environ. Sci. Technol., 1982, 16, 274–278 CrossRef CAS.
- X. Zhu, Y. Chang and Y. Chang, Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna, Chemosphere, 2010, 78, 209–215 CrossRef CAS.
- K. Tervonen, G. Waissi, E. J. Petersen, J. Akkanen and J. V. K. Kukkonen, Analysis of fullerene-C60 and kinetic measurements for its accumulation and depuration in Daphnia magna, Environ. Toxicol. Chem., 2010, 1072–1078 CAS.
- E. J. Petersen, J. Akkanen, J. V. K. Kukkonen and W. J. Weber, Biological uptake and depuration of carbon nanotubes by Daphnia magna, Environ. Sci. Technol., 2009, 43, 2969–2975 CrossRef CAS.
- E. Oberdörster, S. Zhu, T. M. Blickley, P. McClellan-Green and M. L. Haasch, Ecotoxicology of carbon-based engineered nanoparticles: effects of fullerene (C60) on aquatic organisms, Carbon, 2006, 44, 1112–1120 CrossRef.
- E. J. Petersen, R. A. Pinto, D. J. Mai, P. F. Landrum and W. J. J. Weber, Influence of polyethyleneimine graftings of multi-walled carbon nanotubes on their accumulation and elimination by and toxicity to Daphnia magna, Environ. Sci. Technol., 2011, 45, 1133–1138 CrossRef CAS.
- X. Tao, J. D. Fortner, B. Zhang, Y. He, Y. Chen and J. B. Hughes, Effects of aqueous stable fullerene nanocrystals (nC60) on Daphnia magna: evaluation of sub-lethal reproductive responses and accumulation, Chemosphere, 2009, 77, 1482–1487 CrossRef CAS.
- C.-M. Zhao and W.-X. Wang, Biokinetic uptake and efflux of silver nanoparticles in Daphnia magna, Environ. Sci. Technol., 2010, 44, 7699–7704 CrossRef CAS.
- C.-M. Zhao and W.-X. Wang, Comparison of acute and chronic toxicity of silver nanoparticles and silver nitrate to Daphnia magna, Environ. Toxicol. Chem., 2011, 30, 885–892 CrossRef CAS.
- C. W. Isaacson, C. Y. Usenko, R. L. Tanguay and J. A. Field, Quantification of fullerenes by LC/ESI-MS and its application to in vivo toxicity assays, Anal. Chem., 2007, 79, 9091–9097 CrossRef CAS.
- A. P. Roberts, A. S. Mount, B. Seda, J. Souther, R. Qiao, S. Lin, P. C. Ke, A. M. Rao and S. J. Klaine, In vivo biomodification of lipid-coated carbon nanotubes by Daphnia magna, Environ. Sci. Technol., 2007, 41, 3025–3029 CrossRef CAS.
- A. J. Kennedy, M. S. Hull, J. A. Steevens, K. M. Dontsova, M. A. Chappell, J. C. Gunter and C. A. J. Weiss, Factors influencing the partitioning and toxicity of nanotubes in the aquatic environment, Environ. Toxicol. Chem., 2008, 27, 1932–1941 CrossRef CAS.
- B. P. Jackson, H. E. Pace, A. Lanzirotti, R. Smith and J. F. Ranville, Synchrotron X-ray 2D and 3D elemental imaging of CdSe/ZnS quantum dot nanoparticles in Daphnia magna, Anal. Bioanal. Chem., 2009, 394, 911–917 CrossRef CAS.
- N. A. Lewinski, H. Zhu, H.-J. Jo, D. Pham, R. R. Kamath, C. R. Ouyang, C. D. Vulpe, V. L. Colvin and R. A. Drezek, Quantification of water solubilized CdSe/ZnS quantum dots in Daphnia magna, Environ. Sci. Technol., 2010, 44, 1841–1846 CrossRef CAS.
- USEPA ECOTOX Database Release 4.0, http://cfpub.epa.gov/ecotox/ecotox_home.cfm, accessed 11 May 2011.
- J. Hu, D. Wang, J. Wang and J. Wang, Bioaccumulation of Fe2O3 (magnetic) nanoparticles in Ceriodaphnia dubia, Environ. Pollut., 2012, 162, 216–222 CrossRef CAS.
- S. B. Lovern, H. A. Owen and R. Klaper, Electron microscopy of gold nanoparticle intake in the gut of Daphnia magna, Nanotoxicology, 2008, 2, 43–48 CrossRef CAS.
- W. Geller and H. Müller, The filtration apparatus of Cladocera: filter mesh-sizes and their implications on food selectivity, Oecologia, 1981, 49, 316–321 CrossRef.
- M. Gophen and W. Geller, Filter mesh size and food particle uptake by Daphnia, Oecologia, 1984, 64, 408–412 CrossRef.
- C. W. M. Bodar, E. G. van Donselaar and H. J. Herwig, Cytopathological investigations of digestive tract and storage cells in Daphnia magna exposed to cadmium and tributyltin, Aquat. Toxicol., 1990, 17, 325–337 CrossRef CAS.
- P. Rosenkranz, Q. Chaudhry, V. Stone and T. F. Fernandes, A comparison of nanoparticle and fine particle uptake by Daphnia magna, Environ. Toxicol. Chem., 2009, 28, 2142–2149 CrossRef CAS.
- J. Gerritsen, K. G. Porter and J. R. Strickler, Not by sieving alone: observations of suspension feeding in Daphnia, Bull. Mar. Sci., 1988, 43, 366–376 Search PubMed.
- H. Sun, X. Zhang, Z. Zhang, Y. Chen and J. C. Crittenden, Influence of titanium dioxide nanoparticles on speciation and bioavailability of arsenite, Environ. Pollut., 2009, 157, 1165–1170 CrossRef CAS.
- H. Sun, X. Zhang, Q. Niu, Y. Chen and J. C. Crittenden, Enhanced accumulation of arsenate in carp in the presence of titanium dioxide nanoparticles, Water, Air, Soil Pollut., 2006, 178, 245–254 CrossRef.
- X. Zhang, H. Sun, Z. Zhang, Q. Niu, Y. Chen and J. C. Crittenden, Enhanced bioaccumulation of cadmium in carp in the presence of titanium dioxide nanoparticles, Chemosphere, 2007, 67, 160–166 CrossRef CAS.
- C. S. Ramsden, T. J. Smith, B. J. Shaw and R. D. Handy, Dietary exposure to titanium dioxide nanoparticles in rainbow trout, (Oncorhynchus mykiss): no effect on growth, but subtle biochemical disturbances in the brain, Ecotoxicology, 2009, 18, 939–951 CrossRef CAS.
- R. J. Griffitt, K. Hyndman, N. D. Denslow and D. S. Barber, Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles, Toxicol. Sci., 2009, 107, 404–415 CrossRef CAS.
- T. M. Scown, E. M. Santos, B. D. Johnston, B. Gaiser, M. Baalousha, S. Mitov, J. R. Lead, V. Stone, T. F. Fernandes, M. Jepson, R. van Aerle and C. R. Tyler, Effects of aqueous exposure to silver nanoparticles of different sizes in rainbow trout, Toxicol. Sci., 2010, 115, 521–534 CrossRef CAS.
- B. K. Gaiser, T. F. Fernandes, M. A. Jepson, J. R. Lead, C. R. Tyler, M. Baalousha, A. Biswas, G. J. Britton, P. A. Cole, B. D. Johnston, Y. Ju-Nam, P. Rosenkranz, T. M. Scown and V. Stone, Interspecies comparisons on the uptake and toxicity of silver and cerium dioxide nanoparticles, Environ. Toxicol. Chem., 2011, 31, 144–154 CrossRef.
- Z.-J. Zhu, R. Carboni, M. J. Quercio Jr, B. Yan, O. R. Miranda, D. L. Anderton, K. F. Arcaro, V. M. Rotello and R. W. Vachet, Surface properties dictate uptake, distribution, excretion, and toxicity of nanoparticles in fish, Small, 2010, 6, 2261–2265 CrossRef CAS.
- B. D. Johnston, T. M. Scown, J. Moger, S. A. Cumberland, M. Baalousha, K. Linge, R. van Aerle, K. Jarvis, J. R. Lead and C. R. Tyler, Bioavailability of nanoscale metal oxides TiO2, CeO2, and ZnO to fish, Environ. Sci. Technol., 2010, 44, 1144–1151 CrossRef CAS.
- W. C. Ma, J. Immerzeel and J. Bodt, Earthworm and food interactions on bioaccumulation and disappearance in soil of polycyclic aromatic hydrocarbons: studies on phenanthrene and fluoranthene, Ecotoxicol. Environ. Saf., 1995, 32, 226–232 CrossRef CAS.
- D. Li, J. D. Fortner, D. R. Johnson, C. Chen, Q. Li and P. J. J. Alvarez, Bioaccumulation of 14C60 by the earthworm Eisenia fetida, Environ. Sci. Technol., 2010, 44, 9170–9175 CrossRef CAS.
- E. J. Petersen, Q. Huang and J. J. Weber, Bioaccumulation of radio-labeled carbon nanotubes by Eisenia foetida, Environ. Sci. Technol., 2008, 42, 3090–3095 CrossRef CAS.
- E. J. Petersen, R. A. Pinto, P. F. Landrum and W. J. Weber Jr, Influence of carbon nanotubes on pyrene bioaccumulation from contaminated soils by earthworms, Environ. Sci. Technol., 2009, 43, 4181–4187 CrossRef CAS.
- E. J. Petersen, R. A. Pinto, L. Zhang, Q. Huang, P. F. Landrum and W. J. J. Weber, Effects of polyethyleneimine-mediated functionalization of multi-walled carbon nanotubes on earthworm bioaccumulation and sorption by soils, Environ. Sci. Technol., 2011, 45, 3718–3724 CrossRef CAS.
- C. W. Hu, M. Li, Y. B. Cui, D. S. Li, J. Chen and L. Y. Yang, Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida, Soil Biol. Biochem., 2010, 42, 586–591 CrossRef CAS.
- J. G. Coleman, D. R. Johnson, J. K. Stanley, A. J. Bednar, C. A. Weiss, R. E. Boyd and J. A. Steevens, Assessing the fate and effects of nano aluminum oxide in the terrestrial earthworm, Eisenia fetida, Environ. Toxicol. Chem., 2010, 29, 1575–1580 CrossRef CAS.
- T. Xia, M. Kovochich, M. Liong, L. Mädler, B. Gilbert, H. Shi, J. I. Yeh, J. I. Zink and A. E. Nel, Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties, ACS Nano, 2008, 2, 2121–2134 CrossRef CAS.
- T. J. Brunner, P. Wick, P. Manser, P. Spohn, R. N. Grass, L. K. Limbach, A. Bruinink and W. J. Stark,
In vitro cytotoxicity of oxide nanoparticles:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) comparison to asbestos, silica, and the effect of particle solubility, Environ. Sci. Technol., 2006, 40, 4374–4381 CrossRef CAS.
comparison to asbestos, silica, and the effect of particle solubility, Environ. Sci. Technol., 2006, 40, 4374–4381 CrossRef CAS. - J. M. Unrine, S. E. Hunyadi, O. V. Tsyusko, W. Rao, W. A. Shoults-Wilson and P. M. Bertsch, Evidence for bioavailability of Au nanoparticles from soil and biodistribution within earthworms (Eisenia fetida), Environ. Sci. Technol., 2010, 44, 8308–8313 CrossRef CAS.
- S. Kashiwada, Distribution of nanoparticles in the see-through medaka (Oryzias latipes), Environ. Health Perspect., 2006, 114, 1697–1702 CAS.
- R. Werlin, J. H. Priester, R. E. Mielke, S. Kramer, S. Jackson, P. K. Stoimenov, G. D. Stucky, G. N. Cherr, E. Orias and P. A. Holden, Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain, Nat. Nanotechnol., 2011, 6, 65–71 CrossRef CAS.
- R. D. Holbrook, K. E. Murphy, J. B. Morrow and K. D. Cole, Trophic transfer of nanoparticles in a simplified invertebrate food web, Nat. Nanotechnol., 2008, 3, 352–355 CrossRef CAS.
- J. L. Bouldin, T. M. Ingle, A. Sengupta, R. Alexander, R. E. Hannigan and R. A. Buchanan, Aqueous toxicity and food chain transfer of quantum dots™ in freshwater algae and Ceriodaphnia dubia, Environ. Toxicol. Chem., 2008, 27, 1958–1963 CrossRef CAS.
- N. A. Lewinski, H. Zhu, C. R. Ouyang, G. P. Conner, D. S. Wagner, V. L. Colvin and R. A. Drezek, Trophic transfer of amphiphilic polymer coated CdSe/ZnS quantum dots to Danio rerio, Nanoscale, 2011, 3, 3080–3083 RSC.
- X. Zhu, J. Wang, X. Zhang, Y. Chang and Y. Chen, Trophic transfer of TiO2 nanoparticles from daphnia to zebrafish in a simplified freshwater food chain, Chemosphere, 2010, 79, 928–933 CrossRef CAS.
- J. D. Judy, J. M. Unrine and P. M. Bertsch, Evidence for biomagnification of gold nanoparticles within a terrestrial food chain, Environ. Sci. Technol., 2011, 45, 776–781 CrossRef CAS.
- X. Wang and W.-X. Wang, Uptake, absorption efficiency and elimination of DDT in marine phytoplankton, copepods and fish, Environ. Pollut., 2005, 136, 453–464 CrossRef CAS.
- J. A. Kloepfer, R. E. Mielke and J. L. Nadeau, Uptake of CdSe and CdSe/ZnS quantum dots into bacteria via purine-dependent mechanisms, Appl. Environ. Microbiol., 2005, 71, 2548–2557 CrossRef CAS.
- E. E. Connor, J. Mwamuka, A. Gole, C. J. Murphy and M. D. Wyatt, Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity, Small, 2005, 1, 325–327 CrossRef CAS.
- K. Kostarelos, L. Lacerda, G. Pastorin, W. Wu, S. WieckowskiSebastien, J. Luangsivilay, S. Godefroy, D. Pantarotto, J.-P. Briand, S. Muller, M. Prato and A. Bianco, Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type, Nat. Nanotechnol., 2007, 2, 108–113 CrossRef CAS.
- L. K. Limbach, Y. Li, R. N. Grass, T. J. Brunner, M. A. Hintermann, M. Muller, D. Gunther and W. J. Stark, Oxide nanoparticle uptake in human lung fibroblasts: effects of particle size, agglomeration, and diffusion at low concentrations, Environ. Sci. Technol., 2005, 39, 9370–9376 CrossRef CAS.
- L. Au, Q. Zhang, C. M. Cobley, M. Gidding, A. G. Schwartz, J. Chen and Y. Xia, Quantifying the cellular uptake of antibody-conjugated Au nanocages by two-photon microscopy and inductively coupled plasma mass spectrometry, ACS Nano, 2010, 4, 35–42 CrossRef CAS.
- M. N. Moore, Do nanoparticles present ecotoxicological risks for the health of the aquatic environment?, Environ. Int., 2006, 32, 967–976 CrossRef CAS.
- N. C. Mueller and B. Nowack, Exposure modeling of engineered nanoparticles in the environment, Environ. Sci. Technol., 2008, 42, 4447–4453 CrossRef CAS.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS.
- J. Liu and R. H. Hurt, Ion release kinetics and particle persistence in aqueous nano-silver colloids, Environ. Sci. Technol., 2010, 44, 2169–2175 CrossRef CAS.
- R. B. Reed, D. A. Ladner, C. P. Higgins, P. Westerhoff and J. F. Ranville, Solubility of nano-zinc oxide in environmentally and biologically important matrices, Environ. Toxicol. Chem., 2012, 31, 93–99 CrossRef CAS.
- F. von der Kammer, P. L. Ferguson, P. A. Holden, A. Masion, K. R. Rogers, S. J. Klaine, A. A. Koelmans, N. Horne and J. M. Unrine, Analysis of engineered nanomaterials in complex matrices (environment and biota): general considerations and conceptual case studies, Environ. Toxicol. Chem., 2012, 31, 32–49 CrossRef CAS.
- E. J. Petersen, Q. Huang and W. J. Weber Jr, Relevance of octanol–water distribution measurements to the potential ecological uptake of multi-walled carbon nanotubes, Environ. Toxicol. Chem., 2010, 29, 1106–1112 CAS.
- J. Giri, M. S. Diallo, W. A. Goddard III, N. F. Dalleska, X. Fang and Y. Tang, Partitioning of poly(amidoamine) dendrimers between n-octanol and water, Environ. Sci. Technol., 2009, 43, 5123–5129 CrossRef CAS.
- K. D. Hristovski, P. K. Westerhoff and J. D. Posner, Octanol–water distribution of engineered nanomaterials, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2011, 46, 636–647 CrossRef CAS.
- C. T. Jafvert and P. P. Kulkarni, Buckminsterfullerene's (C60) octanol–water partition coefficient (Kow) and aqueous solubility, Environ. Sci. Technol., 2008, 42, 5945–5950 CrossRef CAS.
- X. Wang, T. Xia, S. A. Ntim, Z. Ji, S. George, H. Meng, H. Zhang, V. Castranova, S. Mitra and A. E. Nel, Quantitative techniques for assessing and controlling the dispersion and biological effects of multiwalled carbon nanotubes in mammalian tissue culture cells, ACS Nano, 2010, 4, 7241–7252 CrossRef CAS.
- P. Wang, Q. Shi, H. Liang, D. W. Steuerman, G. D. Stucky and A. A. Keller, Enhanced environmental mobility of carbon nanotubes in the presence of humic acid and their removal from aqueous solution, Small, 2008, 4, 2166–2170 CrossRef CAS.
- M. J. Eckelman, M. S. Mauter, J. A. Isaacs and M. Elimelech, New perspectives on nanomaterial aquatic ecotoxicity: production impacts exceed direct exposure impacts for carbon nanotubes, Environ. Sci. Technol., 2012, 46, 2902–2910 CrossRef CAS.
- Z. Tong, M. Bischoff, L. Nies, B. Applegate and R. F. Turco, Impact of fullerene (C60) on a soil microbial community, Environ. Sci. Technol., 2007, 41, 2985–2991 CrossRef CAS.
- L. Nyberg, R. F. Turco and L. Nies, Assessing the impact of nanomaterials on anaerobic microbial communities, Environ. Sci. Technol., 2008, 42, 1938–1943 CrossRef CAS.
- D. Y. Lyon, L. K. Adams, J. C. Falkner and P. J. J. Alvarez, Antibacterial activity of fullerene water suspensions: effects of preparation method and particle size, Environ. Sci. Technol., 2006, 40, 4360–4366 CrossRef CAS.
- J. D. Fortner, D. Y. Lyon, C. M. Sayes, A. M. Boyd, J. C. Falkner, E. M. Hotze, L. B. Alemany, Y. J. Tao, W. Guo, K. D. Ausman, V. L. Colvin and J. B. Hughes, C60 in water: nanocrystal formation and microbial response, Environ. Sci. Technol., 2005, 39, 4307–4316 CrossRef CAS.
- C. M. Sayes, J. D. Fortner, W. Guo, D. Lyon, A. M. Boyd, K. D. Ausman, Y. J. Tao, B. Sitharaman, L. J. Wilson, J. B. Hughes, J. L. West and V. L. Colvin, The differential cytotoxicity of water-soluble fullerenes, Nano Lett., 2004, 4, 1881–1887 CrossRef CAS.
- W.-C. Hou, B. Y. Moghadam, P. Westerhoff and J. D. Posner, Distribution of fullerene nanomaterials between water and model biological membranes, Langmuir, 2011, 27, 11899–11905 CrossRef CAS.
- W.-C. Hou, B. Y. Moghadam, C. Corredor, P. Westerhoff and J. D. Posner, Distribution of functionalized gold nanoparticles between water and lipid bilayers as model cell membranes, Environ. Sci. Technol., 2012, 46, 1869–1876 CrossRef CAS.
- E. J. Petersen, Q. Huang and W. J. J. Weber, Ecological uptake and depuration of carbon nanotubes by Lumbriculus variegatus, Environ. Health Perspect., 2008, 116, 496–500 CAS.
- A. E. Nel, L. Mädler, D. Velegol, T. Xia, E. M. V. Hoek, P. Somasundaran, F. Klaessig, V. Castranova and M. Thompson, Understanding biophysicochemical interactions at the nano-bio interface, Nat. Mater., 2009, 8, 543–557 CrossRef CAS.
- T. Puzyn, D. Leszczynska and J. Leszczynski, Toward the development of “nano-QSARs”: advances and challenges, Small, 2009, 5, 2494–2509 CrossRef CAS.
- E. Burello and A. P. Worth, QSAR modeling of nanomaterials, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2011, 3, 298–306 CrossRef CAS.
- T. Puzyn, B. Rasulev, A. Gajewicz, X. Hu, T. P. Dasari, A. Michalkova, H.-M. Hwang, A. Toropov, D. Leszczynska and J. Leszczynski, Using nano-QSAR to predict the cytotoxicity of metal oxide nanoparticles, Nat. Nanotechnol., 2011, 6, 175–178 CrossRef CAS.
- P. Westerhoff and B. Nowack, Searching for global descriptors of engineered nanomaterial fate and transport in the environment, Acc. Chem. Res., 2012 DOI:10.1021/ar300030n.
- M. Patra, X. Ma, C. Isaacson, D. Bouchard, H. Poynton, J. M. Lazorchak and K. R. Rogers, Changes in agglomeration of fullerenes during ingestion and excretion in Thamnocephalus platyurus, Environ. Toxicol. Chem., 2011, 30, 828–835 CrossRef CAS.
- T. M. Ingle, R. Alexander, J. Bouldin and R. A. Buchanan, Absorption of semiconductor nanocrystals by the aquatic invertebrate Ceriodaphnia dubia, Bull. Environ. Contam. Toxicol., 2008, 81, 249–252 CrossRef CAS.
- P. Ghafari, C. H. St-Denis, M. E. Power, X. Jin, V. Tsou, H. S. Mandal, N. C. Bols and X. Tang, Impact of carbon nanotubes on the ingestion and digestion of bacteria by ciliated protozoa, Nat. Nanotechnol., 2008, 3, 347–351 CrossRef CAS.
- J. K. Stanley, J. G. Coleman, C. A. Weiss and J. A. Steevens, Sediment toxicity and bioaccumulation of nano and micron-sized aluminum oxide, Environ. Toxicol. Chem., 2010, 29, 422–429 CrossRef CAS.
- M.-N. Croteau, S. K. Misra, S. N. Luoma and E. Valsami-Jones, Silver bioaccumulation dynamics in a freshwater invertebrate after aqueous and dietary exposures to nanosized and ionic Ag, Environ. Sci. Technol., 2011, 45, 6600–6607 CrossRef CAS.
- S. K. Misra, A. Dybowska, D. Berhanu, M. N. Croteau, S. N. Luoma, A. R. Boccaccini and E. Valsami-Jones, Isotopically modified nanoparticles for enhanced detection in bioaccumulation studies, Environ. Sci. Technol., 2011, 46, 1216–1222 CrossRef.
- M.-N. Croteau, A. D. Dybowska, S. N. Luoma and E. Valsami-Jones, A novel approach reveals that zinc oxide nanoparticles are bioavailable and toxic after dietary exposures, Nanotoxicology, 2011, 5, 79–90 CrossRef CAS.
- M. S. Hull, P. Chaurand, J. Rose, M. Auffan, J.-Y. Bottero, J. C. Jones, I. R. Schultz and P. J. Vikesland, Filter-feeding bivalves store and biodeposit colloidally stable gold nanoparticles, Environ. Sci. Technol., 2011, 45, 6592–6599 CrossRef CAS.
- B. Geffroy, C. Ladhar, S. Cambier, M. Treguer-Delapierre, D. Brèthes and J.-P. Bourdineaud, Impact of dietary gold nanoparticles in zebrafish at very low contamination pressure: the role of size, concentration and exposure time, Nanotoxicology, 2012, 6, 144–160 CrossRef CAS.
- G. Federici, B. J. Shaw and R. D. Handy, Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects, Aquat. Toxicol., 2007, 84, 415–430 CrossRef CAS.
- H. L. Hooper, K. Jurkschat, A. J. Morgan, J. Bailey, A. J. Lawlor, D. J. Spurgeon and C. Svendsen, Comparative chronic toxicity of nanoparticulate and ionic zinc to the earthworm Eisenia veneta in a soil matrix, Environ. Int., 2011, 37, 1111–1117 CrossRef CAS.
- J. M. Unrine, O. V. Tsyusko, S. E. Hunyadi, J. D. Judy and P. M. Bertsch, Effects of particle size on chemical speciation and bioavailability of copper to earthworms (Eisenia fetida) exposed to copper nanoparticles, J. Environ. Qual., 2010, 39, 1942 CrossRef CAS.
- W. A. Shoults-Wilson, B. C. Reinsch, O. V. Tsyusko, P. M. Bertsch, G. V. Lowry and J. M. Unrine, Effect of silver nanoparticle surface coating on bioaccumulation and reproductive toxicity in earthworms (Eisenia fetida), Nanotoxicology, 2011, 5, 432–444 CrossRef CAS.
- Z. Pipan-Tkalec, D. Drobne, A. Jemec, T. Romih, P. Zidar and M. Bele, Zinc bioaccumulation in a terrestrial invertebrate fed a diet treated with particulate ZnO or ZnCl2 solution, Toxicology, 2010, 269, 198–203 CrossRef CAS.
- S. Novak, D. Drobne, J. Valant, Ž. Pipan-Tkalec, P. Pelicon, P. Vavpetič, N. Grlj, I. Falnoga, D. Mazej and M. Remškar, Cell membrane integrity and internalization of ingested TiO2 nanoparticles by digestive gland cells of a terrestrial isopod, Environ. Toxicol. Chem., 2012, 31, 1083–1090 CrossRef CAS.
- C. A. M. van Gestel, P. L. Kool and M. D. Ortiz, Metal-based nanoparticles in soil: new research themes should not ignore old rules and theories. Comments on the paper by Hu et al. (2010) “Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida.” Soil Biology & Biochemistry 42, 586–591, Soil Biol. Biochem., 2010, 42, 1892–1893 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
