Gas storage in renewable bioclathrates†
Weixing
Wang
*ab,
Chao
Ma
a,
Pinzhen
Lin
b,
Luyi
Sun
c and
Andrew I.
Cooper
*a
aDepartment of Chemistry, University of Liverpool, Crown Street, Liverpool L69 7ZD, UK
bMOE Key Laboratory of Enhanced Heat Transfer & Energy Conservation, South China University of Technology, Guangzhou, 510640, China
cDepartment of Chemistry and Biochemistry & Materials Science and Engineering Program, Texas State University–San Marcos, San Marcos, TX 78666, USA
First published on 1st November 2012
Abstract
Methane and carbon dioxide can be stored in ‘bioclathrate’ form—that is, as a clathrate supported in a biological structure—by using plants or fungi to greatly accelerate clathrate formation kinetics, thus avoiding the use of energy-intensive mixing technologies or petrochemically derived materials.
Broader contextGases such as methane and carbon dioxide are important in future energy schemes. The main strategies for storing and transporting these gases are compression, liquefaction, and physical adsorption in synthetic porous materials. All of these technologies introduce additional energy and materials requirements: for example, liquefaction may be energy-intensive for permanent gases with low boiling points, while synthetic porous sorbents can be expensive to scale up. Moreover, the applications of greatest topical interest, such as CO2 sequestration and natural gas/methane storage, would all need to operate on a very large scale with relatively low purity gas streams. Here, we explore a new solution to the storage and transportation of such gases that involves renewable biological materials such as plants and fungi. |
The main strategies for storing and transporting permanent gases are compression, liquefaction, and physical adsorption in porous materials.1–4 In this paper, we show that gases such as methane and carbon dioxide can be stored in clathrate form using naturally occurring plants and fungi as supports. Gas clathrates, also known as gas hydrates, are non-stoichiometric, crystalline inclusion compounds composed of a hydrogen-bonded water lattice that traps the gas molecule within polyhedral cavities.5,6 The best known compound in this class, methane gas hydrate, occurs in large volumes in the natural environment and has been considered as a material for transportation of natural gas from ‘stranded’, uneconomical gas reserves. From the point of view of cost, clathrates offer a potentially attractive answer to gas transportation since they can be composed of water and gas alone. Most gases, however, form clathrates with water at elevated pressures or at cryogenic temperatures:5–8 hence, as for compression or liquefaction strategies, the overall energy balance for clathrate formation would need to be considered. A further technical challenge lies in achieving the maximum potential gas storage capacity for a given clathrate system. For example, the maximum capacity of methane gas hydrate (MGH) is around 180 v/v STP,5 but this methane storage capacity cannot be achieved in practice without appropriate mixing or gas–liquid/gas–solid contacting technologies, which may be difficult to scale up. In general, gas clathrates form very slowly in unmixed, bulk water because the formation rate is inversely proportional to the thickness of the formation zone. Formation rates are accelerated by increasing contact between the gas and the liquid interface. Methods for this include grinding to produce small ice particles,9 the addition of surfactants,10 or the use of fine water droplets in the form of ‘dry water’.11,12 However, these gas–liquid contacting methods introduce additional energy requirements and, in some cases, petrochemical-derived materials, such as surfactants.
An overarching theme, therefore, in gas storage research is control over the gas–solid or gas–liquid interface, whether this is controlling pore size in synthetic porous sorbents1–4 or controlling ice crystallite9 or water droplet size11,12 to accelerate clathrate formation kinetics. Nature has also tackled gas transport through the evolution of biological structures such as alveoli in lungs, gills in fish, and stoma in the leaves of plants. As such, there is potential to discover natural, pre-structured materials that might act as gas storage substrates. Indeed, natural materials have been explored as ‘biosorbents’ for heavy metals13 and have also inspired synthetic materials chemists.14 We therefore decided to investigate biological, pre-structured materials to accelerate gas clathrate formation kinetics – that is, to form ‘bioclathrates’.
Fig. 1a shows the methane and carbon dioxide uptake kinetics at 273.2 K for gas clathrate formation in samples of mushroom (Agaricus bisporus), eggplant (Solanum melongena), and tomato (Solanum lycopersicum), compared with the kinetics for bulk, unstirred water. The mushroom and eggplant samples exhibit much faster methane absorption kinetics than bulk, unmixed water, which does not absorb appreciable quantities of gas under these conditions over a period of 500 minutes, most likely because of the formation of a clathrate ‘skin’ at the gas–water/ice interface. By contrast, the mushroom and eggplant materials, both of which have intrinsically high water contents, form methane clathrates with greatly accelerated kinetics. For the mushroom sample, the methane uptake reached a plateau of around 123 v/v CH4 (24.1 wt%) after 500 min. This storage capacity is lower than the US Department of Energy target for vehicular CH4 storage (180 v/v), for example, but these data demonstrate that plants, fungi, and potentially other lower-value, non-food biomass have promise for accelerating gas hydrate formation rates to store significant volumes of methane in the absence of any mixing technologies.
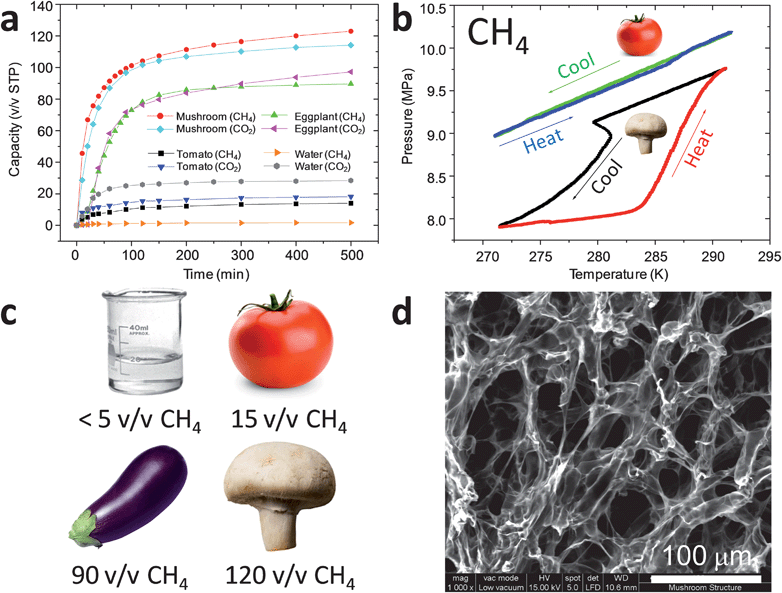 | ||
| Fig. 1 (a) CH4 and CO2 sorption kinetics for bulk water, tomato, eggplant (aubergine), and mushroom samples; (b) (P, T) plot illustrating formation of methane clathrate in mushroom but not tomato sample; (c) comparison of CH4 storage capacities after 500 min gas contact time for different natural materials, and for bulk, unstirred water; (d) scanning electron micrograph showing fine, porous structure of the mushroom sample which allows rapid gas transport to occur. Scale bar = 100 μm. | ||
The methane storage is reversible, and all of the methane can be released upon warming the material back to 290 K (Fig. 1b). Similar trends are observed with CO2, although the saturation uptake capacities for this gas, as expressed in units of v/v STP, are somewhat lower than for methane (Fig. 1a). While perhaps of less practical interest, krypton can be stored in bioclathrates in a similar fashion (Fig. S7†).
The gas storage capacities for mushroom and eggplant bioclathrates can be rationalized by the high water content in the materials (>90 wt%, as measured by thermogravimetric analysis). The formation of gas clathrates is exothermic, resulting in a characteristic pressure–temperature (P, T) plot where the temperature rises at the onset of clathrate formation at around 279 K for experiments conducted at this methane pressure (Fig. 1b). By comparison with other systems involving neat water,11,12 the onset temperature for methane clathrate formation does not seem to be greatly affected by the fact that the water in these materials is contained within a biological matrix.
Not all biological materials are equally effective in promoting gas clathrate formation (Fig. 1c). For example, the tomato sample, despite being 95 wt% water, absorbs very little CH4 or CO2 over this timescale. The much more rapid formation of the mushroom bioclathrates can be attributed its relatively large surface-to-volume ratio and its micro-channel structure, as revealed by electron microscopy (Fig. 1d). This shows that the material morphology, as well as the water content, is very important in determining the gas uptake kinetics in bioclathrates. It is possible that capillary effects play a role in enhancing gas transport kinetics and clathrate formation, although a detailed mechanistic study for these complex biological substrates is outside the scope of this first report.
In addition to being potentially renewable, plant and fungal materials should be insensitive to minor components in natural gas, such as sulfur-containing compounds, that might accumulate in or degrade synthetic porous supports. However, gas storage capacities and kinetics in bioclathrates were found to decrease substantially after the first storage cycle, at least for the materials tested here. We ascribe this to destabilization of the biological structures and loss of morphology as a result of the freeze–thaw process. Of course, it is unlikely that high-value food products such as eggplant would be used for gas transportation. However, even a single-use methane storage cycle could in principle be deployed as part of a combined operation involving waste agricultural biomass, seaweed, marine algae, or other inexpensive natural materials. For example, we tentatively propose a scheme where biomass is transported carrying a ‘cargo’ of methane hydrate from stranded natural gas reserves, prior to processing the biomass itself to produce additional biogas or other renewable fuels or feedstocks.
Conclusions
The natural, porous structure of certain plants and fungi, such as mushrooms, gives rise to greatly enhanced gas clathrate formation kinetics with respect to bulk, unmixed water. These natural materials can store up to around 120 v/v STP methane gas under the conditions investigated here. While the economics of using waste biomass for gas transportation are currently unclear, the use of pre-structured biological ‘sorbents’ represents a new approach to gas storage that has the advantage of using only natural, renewable materials.Notes and references
- R. E. Morris and P. S. Wheatley, Angew. Chem., Int. Ed., 2008, 47, 4966 CrossRef CAS.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469 CrossRef CAS.
- R. Dawson, E. Stöckel, J. R. Holst, D. J. Adams and A. I. Cooper, Energy Environ. Sci., 2011, 4, 4239–4245 CAS.
- T. M. McDonald, W. R. Lee, J. A. Mason, B. M. Wiers, C. S. Hong and J. R. Long, J. Am. Chem. Soc., 2012, 134, 7056–7065 CrossRef CAS.
- E. D. Sloan, Nature, 2003, 426, 353–359 CrossRef CAS.
- E. D. Sloan and C. A. Koh, Clathrate Hydrates of Natural Gases, CRC Press, Boca Raton, 2007 Search PubMed.
- Y. Jin, K. Matsumoto, J. Nagao and W. Shimada, J. Chem. Eng. Data, 2011, 56, 58–61 CrossRef CAS.
- G. Rehder, R. Eckl, M. Elfgen, A. Falenty, R. Hamann, N. Kahler, W. F. Kuhs, H. Osterkamp and C. Windmeier, Energies, 2012, 5, 2499–2523 CrossRef CAS.
- S. Takeya, W. Shimada, Y. Kamata, T. Ebinuma, T. Uchida, J. Nagao and H. Narita, J. Phys. Chem. A, 2001, 105, 9756–9759 CrossRef CAS.
- K. Okutani, Y. Kuwabara and Y. H. Mori, Chem. Eng. Sci., 2008, 63, 183–194 CrossRef CAS.
- W. X. Wang, C. L. Bray, D. J. Adams and A. I. Cooper, J. Am. Chem. Soc., 2008, 130, 11608–11609 CrossRef CAS.
- B. O. Carter, W. X. Wang, D. J. Adams and A. I. Cooper, Langmuir, 2010, 26, 3186–3193 CrossRef CAS.
- J. L. Wang and C. Chen, Biotechnol. Adv., 2009, 27, 195–226 CrossRef CAS.
- E. Dujardin and S. Mann, Adv. Mater., 2002, 14, 775–788 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ee23565j |
| This journal is © The Royal Society of Chemistry 2013 |
