High surface and magnetically recoverable mPANI/pFe3O4 nanocomposites for C–S bond formation in water†
D.
Damodara
a,
R.
Arundhathi
ab and
Pravin R.
Likhar
*a
aInorganic and Physical Chemistry Division, Indian Institute of Chemical Technology, Hyderabad-500007, India. E-mail: plikhar@iict.res.in; Fax: +91-40-2716-0921; Tel: +91-40-2719-3510
bDepartment of Materials Engineering Science, Graduate School of Engineering Science, Osaka University, 1-3 Machikaneyama, Toyonaka, Osaka 560-8531, Japan
First published on 12th November 2012
Abstract
A high surface area mPANI/pFe3O4 nanocomposite from mesoporous polyaniline and porous magnetic Fe3O4 was used as a catalyst in the S-arylation of thiophenol with aryl chlorides and in the C–S bond formation between aryl iodides and thiourea in water. The mesoporosity of the polyaniline enhances the efficiency and stability of the porous magnetic Fe3O4 nanoparticles in both coupling reactions. The mPANI/pFe3O4 nanocomposite can be recovered with an external magnet and reused several times due to the superparamagnetic nature of the porous Fe3O4 nanoparticles.
1. Introduction
Catalytic methods for C–S bond formation have received considerable attention due to their wide range of applications in pharmaceuticals and polymer material science.1 Diaryl sulfides in particular are found in numerous drugs, with a broad spectrum of therapeutic activities for diverse clinical applications in the treatment of cancer, HIV, Alzheimer's and Parkinson's diseases.2 Various transition metals were proven to be highly effective catalysts in C–C, C–O and C–N cross-coupling reactions.3,4 However, their catalytic activity in S-arylation is comparatively less explored5 due to the tendency of thiols to undergo an oxidative homocoupling S–S reaction and/or poisoning of the metal by sulphur containing compounds. The development of an environmentally friendly catalytic system composed of inexpensive metal-based heterogeneous catalysts and water as a reaction medium has drawn much attention in both academia and industry in recent times due to the easy separation of heterogeneous catalysts and water, as a green solvent, being non toxic, low cost and easily available. Iron-based heterogeneous catalysts have gained new significant attention in various cross-coupling reactions because of their low cost, non-toxicity and interesting catalytic activities. Despite these interesting facts, only limited literature reports are available on iron-based heterogeneous catalysts. Recently, Bolm and co-workers have explored the FeCl3/DMEDA catalytic system for the S-arylation of thiol.6 However, the recovery and reusability of the catalyst remains a major problem with homogenous catalysts.Nano-sized metals and metal oxides have been extensively used as catalysts for many organic transformations because of their high surface area and facile separation. Commensurate with the aforementioned requirements, we envisage that nano-sized magnetically recoverable and reusable iron oxide could be an appropriate heterogeneous catalyst for the S-arylation reaction. In this context, the applications of Fe3O4 nanoparticle-immobilized or supported catalysts have been successfully demonstrated by means of various strategies.7–10 However, the direct use of Fe3O4 nanoparticles without modification as a magnetically recoverable catalyst for organic reactions is very rare.11
Initially, Fe3O4 nanoparticles were prepared using a solvo-thermal process and employed in the C–S bond formation reaction of chlorobenzene and thiophenol in various solvents at different temperatures. However, the yield of the S-arylated product could not improved to 42% in water under reflux conditions after 8 h. The anticipated reason for the low yields of the S-arylated product could be the size and aggregation of the Fe3O4 particles during the reaction (verified by TEM analysis). The catalytic efficiency of the nanoparticles mainly depends on the thermal and chemical stability and they have a great tendency to deform and aggregate during the course of the chemical reaction. Therefore, the surface modification12,13 of metal nanoparticles with the use of an appropriate capping agent, such as polymers or surfactants, is essential in order to prevent aggregation. Immobilization of metal nanoparticles at the pore surface of mesoporous materials is of considerable technological importance in order to improve the accessibility, lifetime and the reusability of the catalyst in the field of molecular catalysis.14 In order to achieve nano-scale stable Fe3O4 particles, Fe3O4 was first functionalized with 3-aminopropyltrimethoxysilane (APTMS) and then the pFe3O4 nanoparticles were encaged in mPANI microspheres, in presence of polyvinylpyrrolidone (PVP) and sodium dodecyl benzene sulfonate (SDBS), by in situ polymerization to give high surface mesoporous polyaniline/porous magnetic Fe3O4, the mPANI/pFe3O4 nanocomposite.15 The porous nature of the magnetic Fe3O4 nanoparticles of the mPANI/pFe3O4 nanocomposite provides direct access to the Fe3O4 nanoparticles and the mesoporous polyaniline enhances both the thermal and chemical stability of the magnetic Fe3O4 nanoparticles. The catalytic application of mPANI/pFe3O4 was then explored in the S-arylation of thiophenol with aryl chlorides and in the C–S bond formation between aryl iodides and thiourea.
2. Experimental section
Preparation of the mPANI/pFe3O4 nanocomposite
Magnetic Fe3O4 particles were prepared using FeCl3.6H2O and sodium acetate in ethylene glycol through a solvo-thermal process. The homogeneous solution was transferred to a teflon-lined sealed-steel autoclave and heated at 200 °C. After 8 h, the autoclave was cooled to room temperature and the particles were washed with ethanol and dried in a vacuum at 60 °C for 12 h. Black magnetite particles were obtained. Then, the porous Fe3O4 particles were chemically modified using APTMS in anhydrous ethanol and amine functionalised porous Fe3O4 (NH2–Fe3O4) magnetite particles were obtained. mPANI coated pFe3O4 microspheres were prepared by an in situ surface polymerization method in the presence of PVP and SDBS. In a typical procedure, PVP and SDBS were dissolved in deionized water and the NH2–Fe3O4 particles were then added. The mixture was ultrasonically dispersed and a solution of aniline in HCl was added into the mixture with vigorous stirring. The mixture was mechanically stirred for 30 min at 20 °C and then an aqueous solution of APS was instantly added to the above mixture to start the oxidative polymerization. The reaction was performed under mechanical stirring for 5 h. The resulting precipitates were washed with deionized water and ethanol several times. Finally, the product was dried in a vacuum at 60 °C for 24 h in order to obtain the desired mPANI/pFe3O4 composite as a dark powder.Synthesis of unsymmetrical diaryl sulfides
The catalyst was used for the S-arylation of thiolphenol with deactivated aryl chlorides and also examined for the synthesis of symmetrical diaryl sulfides from the reaction of aryl iodides with thiourea under mild reaction conditions using water as a green solvent. The reaction vessel was charged with aryl chloride (1.0 mmol), thiophenol (1.0 mmol), KOH (1.5 mmol) and the catalyst (mPANI/pFe3O4) (25 mg, 5 mol%) in water (3 mL). The reaction mixture was then stirred for 8 h at the reflux temperature. The progress of the reaction was monitored by TLC. At the end of the reaction, the reaction mixture was allowed to cool to room temperature. The reaction mixture was then treated with an excess of cold water and the organic phase was extracted by adding ethyl acetate and then dried over anhydrous Na2SO4. The crude mixture was purified by chromatography on silica gel to afford the coupled product.Synthesis of symmetrical diaryl sulfides
A mixture of aryl iodide (1.0 mmol), thiourea (0.75 mmol), mPANI/pFe3O4 (25 mg, 6.6 mol%) and KOH (1.5 equiv.) was stirred at the reflux temperature for 24 h in water (3.0 mL). The progress of the reaction was monitored by TLC. When the reaction was complete, the reaction mixture was allowed to cool, a mixture of ethyl acetate and water (20 mL) was added and the catalyst was separated with the use of an external magnet. The organic solution was washed with brine water and dried with Na2SO4. The solvent and volatiles were completely removed under vacuum to give the crude product, which was purified by column chromatography on silica gel to obtain the desired symmetrical diaryl sulfide.3. Results and discussion
mPANI/pFe3O4 composites were prepared in three steps: (1) porous magnetite Fe3O4 nanoparticles were synthesized by a solvo-thermal approach in the presence of sodium acetate and ethylene glycol in a sealed teflon-lined stainless-steel autoclave at 200 °C; (2) chemical modification/functionalization of the Fe3O4 nanoparticles was achieved by APTMS. APTMS acts as an organic spacer, which may induce a steric repulsion with Fe3O4 through its –NH2 end group, and also restricts the crystal growth of magnetic nanoparticles (cluster formation). (3) mPANI/pFe3O4 nanocomposites were prepared by an in situ surface polymerization method in the presence of PVP and SDBS. Similarly, the thickness of the mesoporous PANI layers for the mPANI/pFe3O4 microsphere core/shell structure was tuned by changing the relative concentration of the Fe3O4@PVP + SDBS particles and the aniline/HCl concentration in the solution. The parameters, such as reaction time (stirring time) and stirring speed (rpm), play an important role in maintaining the thickness of the mesostructures of the PANI microspheres. The synthesized mPANI/pFe3O4 was well characterized by various analytical tools such as XRD, FTIR, TEM (see the ESI†), magnetic analysis, XPS, SEM and surface area analysis.Characterization studies
In order to study the magnetic behaviour of the mPANI/pFe3O4 nanocomposite, magnetization measurements were performed and superparamagnetic behaviour was seen from the obtained magnetic hysteresis loop. Almost immeasurable coercivity was shown for the porous Fe3O4 nanoparticles with and without a polyaniline coating. This gives evidence for the superparamagnetic nature of the samples. The saturation magnetization for the nanoparticles without the coating was detected to be 64.13 emu g−1 and that for the mesoporous polyanilne-coated nanoparticles was found to be 54.13 emu g−1. All the results were compared to our earlier reports.15To examine the physico-chemical change of the catalyst in the S-arylation reaction, we studied the X-ray photoelectron spectra (XPS) and scanning electron microscope (SEM) analysis of fresh mPANI/pFe3O4 nanocomposites and the used mPANI/pFe3O4 nanocomposites.
The XPS spectra of the fresh mPANI/pFe3O4 nanocomposites and used mPANI/pFe3O4 nanocomposites are shown in Fig. 1(A) and (B). The XPS of the C1s, O2p, N1s and Fe2p level give proof for the approximate chemical structure of the mPANI coated pFe3O4 nanocomposites. Peaks at 284, 398, 538 eV were ascribed to the carbon, nitrogen and oxygen in polyaniline. The binding energies at 712 and 725 eV are the characteristic peaks of Fe2p3/2 and Fe2p1/2 core level electrons. The binding energies for Fe3O4 of the fresh mPANI/pFe3O4 nanocomposites in Fig. 1(A) and of the used mPANI/pFe3O4 nanocomposites in Fig. 1(B) are in good agreement with the values reported for Fe3O4 in the literature, which indicates that there is no chemical change occurring in the Fe2p3/2 and Fe2p1/2 core level.
![XPS spectra of the fresh mPANI/pFe3O4 nanocomposites [A] and the used mPANI/pFe3O4 nanocomposites [B].](/image/article/2013/CY/c2cy20624b/c2cy20624b-f1.gif) | ||
| Fig. 1 XPS spectra of the fresh mPANI/pFe3O4 nanocomposites [A] and the used mPANI/pFe3O4 nanocomposites [B]. | ||
Scanning electron microscopy (SEM) images of the freshly prepared mPANI/pFe3O4 nanocomposite and the nanocomposite recovered after its 5th cycle suggest that there is no change in the morphology of the catalyst. The BET surface area and pore volume of the mesoporous PANI (mPANI) are calculated as 190 m2 g−1 and 0.78 cm3 g−1, respectively. The mean value for the narrow pore size of the Fe3O4 nanoparticles, calculated from the adsorption branch of the isotherms, is 2.1 nm. The BET surface area and total pore volume of the mPANI/pFe3O4 nanocomposite are calculated as 445 m2 g−1 and 0.29 cm3 g−1, respectively. Thus, the increase in surface area and decrease in pore volume suggest that the active sites are reasonably increased due to the porosity of the magnetic Fe3O4 nanoparticles and the thickness of the mesoporous PANI layers decreased. TEM images of the Fe3O4 particles reveal that the average diameter of the porous Fe3O4 particles is around 6 nm and the presence of white patches on the surface of magnetic Fe3O4 is due to the porous nature of the materials, which was confirmed by BET analysis (see the ESI†). The formed magnetite Fe3O4 nanoparticles are porous in nature and this was confirmed by the Barrett–Joyner–Halenda (BJH) method. The hystersis of the mPANI/pFe3O4 nanocomposite was found to be of type-IV and clearly shows two peaks for the pore size centred at about 2.5 nm and 5.5 nm respectively for two types of mesopores Fig. 2.
 | ||
| Fig. 2 (a) SEM images of the freshly prepared mPANI/pFe3O4 nanocomposite, (b) the mPANI/pFe3O4 nanocomposite recovered after the 5th cycle. | ||
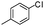
Initially, we tested the catalytic activity of mPANI/pFe3O4 in the S-arylation of thiophenol with the more challenging 4-methyl-chlorobenzene and found that the mPANI/pFe3O4 catalyst could give the S-arylated product in the presence of various bases and solvents (Table 1). Subsequently in the optimization, various reaction parameters such as catalysts, solvents, bases and temperature were studied. We have also compared the activity and efficiency of mPANI/pFe3O4 with other iron-based catalysts and found that the mPANI/pFe3O4 catalyst afforded a high yield due to its nano-size coupled with high surface porous Fe3O4. The effect of solvents was also studied and it was observed that the reaction was highly effective in polar solvents, such as DMF, water and DMSO, whereas the yield of the desired product was low in toluene, THF and dioxane. Although the yield afforded in DMF and water was 85% and 83% respectively, we preferred to use water as a reaction medium in the subsequent S-arylation reactions16 as water has several advantages over the other organic solvents. Among the various bases studied (e.g., K2CO3, K3PO4, Cs2CO3, NaOH and KOH), KOH proved to be a suitable base in combination with water. Thus, the optimized reaction conditions for S-arylation of 4-methyl-chlorobenzene (1.0 mmol), thiophenol (1.0 mmol) mPANI/pFe3O4 (25 mg, 5 mol%) and KOH (1.5 equiv.) in water (3.0 mL) at the reflux temperature afforded the desired product in an excellent yield (83%).
| Entry | Catalyst/wt. | Solvent | Base | Time (h)/Temp. °C | Yielda (%) |
|---|---|---|---|---|---|
| Reaction conditions: aryl chloride (1.0 mmol), thiophenol (1.0 mmol), catalyst (5 mol%, Fe 2.79 mg), base (1.5 mmol; 1.5 equiv.), solvent (3.0 mL).a Isolated yield of product, n.r. = no reaction, r.t = room temperature. | |||||
| 1 | nanoFe3O4/3.86 mg | Water | KOH | 8/reflux | 15 |
| 2 | nanoFe2O3/3.99 mg | Water | KOH | 8/reflux | 12 |
| 3 | Fe/C/56.81 mg | Water | KOH | 8/reflux | 21 |
| 4 | mPANI/pFe3O4/25 mg | DMF | K2CO3 | 8/120 | 56 |
| 5 | mPANI/pFe3O4/25 mg | DMF | K3PO4 | 10/120 | 58 |
| 6 | mPANI/pFe3O4/25 mg | DMF | Cs2CO3 | 8/120 | 67 |
| 7 | mPANI/pFe3O4/25 mg | DMF | NaOH | 9/120 | 68 |
| 8 | mPANI/pFe3O4/25 mg | DMF | KOH | 8/120 | 85 |
| 9 | mPANI/pFe3O4/25 mg | DMF | KOH | 24/rt | n.r |
| 10 | mPANI/pFe3O4/25 mg | Water | KOH | 8/reflux | 83 |
| 11 | mPANI/pFe3O4/25 mg | THF | KOH | 10/120 | 52 |
| 12 | mPANI/pFe3O4/25 mg | Dioxane | KOH | 10/120 | 47 |
| 13 | mPANI/pFe3O4/25 mg | DMSO | KOH | 8/120 | 71 |
| 14 | mPANI/pFe3O4/25 mg | PhMe | KOH | 10/120 | 44 |
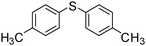
To explore the scope of the reaction and efficiency of mPANI/pFe3O4, S-arylation was studied with various functionalized aryl chlorides. The present mPANI/pFe3O4 catalyst could efficiently catalyze the coupling of thiophenol with electron-rich and electron-deficient aryl chlorides and unsymmetrical diaryl sulfides were obtained in moderate to high yields (Table 2).
| Entry | Substrate | Product | Isolated yielda (%) |
|---|---|---|---|
| Reaction conditions: substrate (1.0 mmol), thiophenol (1.0 mmol), catalyst (25 mg, 5 mol%, Fe 2.79 mg), KOH (1.5 mmol; 1.5 equiv.), water (3.0 mL), reflux temperature for 8 h.a Isolated yield. | |||
| 1 |

|

|
85 |
| 87 | |||
| 92 | |||
| 2 |
|

|
81 |
| 3 |

|

|
79 |
| 4 |

|
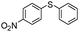
|
88 |
| 5 |

|

|
86 |
| 6 |
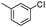
|

|
82 |
| 7 |

|

|
77 |
| 8 |
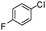
|

|
83 |
| 9 |

|
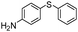
|
71 |
| 10 |

|

|
84 |
| 11 |

|

|
68 |
| 12 |

|
|
61 |
| 13 |
|

|
69 |
| 14 | C4H9Cl |
|
67 |
| 15 | C5H11Cl |

|
65 |
| 16 | C6H13Cl |

|
64 |
From the Table 2, it was observed that there is a slight decrease in the yields of the diaryl sulfides with electron-donating substituents on the aryl chlorides whereas the yields of the desired products increase in the presence of electron-withdrawing group containing aryl chlorides. The couplings of the aliphatic and heterocyclic chlorides with thiophenol also successfully afforded the corresponding product in moderate yields (Table 2, entries 13–16). We have successfully studied the application of porous Fe3O4 stabilized with mesoporous PANI in the synthesis of various diaryl sulfides.
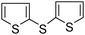
In the next part, we examined the catalytic activity of mPANI/pFe3O4 in the synthesis of symmetric diaryl sulphides from aryl iodides and thiourea. Despite the enormous applications of these symmetrical diary sulfides in various therapeutics, very few methodologies have been developed.17 To our knowledge, this is the first report on heterogeneous iron catalyzed C–S bond formation using thiourea and aryl halides in water. The optimized reaction conditions studied for the mPANI/pFe3O4 catalyzed C–S bond formation were iodobenzene, thiourea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75), mPANI/pFe3O4 (25 mg, 6.6 mol%) and KOH (1.5 equiv.) in water (3.0 mL) at the reflux temperature for 24 h to afford the desired product in a good yield (88%) (Table 3, entry 3). We observed that aryl iodides are more reactive with thiourea than aryl chlorides and bromides under the optimized reaction conditions. A variety of aryl iodides, including electron donating- and electron-withdrawing, and heterocyclic aryl iodides were used for the transformation into their corresponding symmetrical diaryl sulfides (Table 3, entries 2–8).
0.75), mPANI/pFe3O4 (25 mg, 6.6 mol%) and KOH (1.5 equiv.) in water (3.0 mL) at the reflux temperature for 24 h to afford the desired product in a good yield (88%) (Table 3, entry 3). We observed that aryl iodides are more reactive with thiourea than aryl chlorides and bromides under the optimized reaction conditions. A variety of aryl iodides, including electron donating- and electron-withdrawing, and heterocyclic aryl iodides were used for the transformation into their corresponding symmetrical diaryl sulfides (Table 3, entries 2–8).
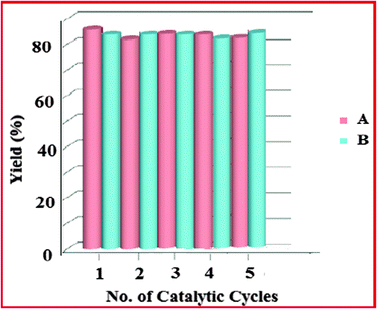
The separation of the mPANI/pFe3O4 nanocomposite catalyst using an external magnetic field from the reaction mixture is a very convenient and efficient process. Magnetic separation of the catalyst using an external magnet is an attractive alternative to filtration or centrifugation as it prevents loss of the catalyst and increases the reusability of the catalyst. The magnetite Fe3O4 particles are known for their paramagnetic property, which makes them amenable to magnetic separation. To investigate the consistency in terms of activity and efficiency of the catalysts using these impressive properties, a recoverability and reusability study was performed in the S-arylation reaction using 4-nitro-chlorobenzene with thiophenol and in the synthesis of symmetric diaryl sulfides from thiourea and 4-methoxy-iodobenzene (see the Recyclability study section). No significant loss of catalytic activity was observed for up to five cycles.
The possible reaction mechanism for the S-arylation of thiol is shown in Scheme 1. In the first step, the high surface porous Fe3O4 nanoparticles may undergo a reaction with aryl chloride to give intermediate [A]. The mesoporosity of the polyaniline in the mPANI/pFe3O4 composite provides direct access to the Fe3O4 nanoparticles. In the next step, the approach of the nucleophile (thiol) in the presence of a base may result in the formation of intermediate [B]. The catalytic cycle can be completed by a reductive elimination step via the generation of the cross-coupled product along with efficient separation of the catalyst.
 | ||
| Scheme 1 Possible reaction mechanism for the S-arylation of thiol. | ||
To confirm that the catalytic activity originated from the porous Fe3O4 and not from temporarily leached Fe3O4, a control experiment was performed by carrying out a reaction between 4-nitro-chlorobenzene and thiophenol which was terminated after 20% conversion (80 min). The catalyst was separated using an external magnet under hot conditions and the reaction was continued with the filtrate for 12 hours. No change in the conversion of 4-nitro-chlorobenzene to the desired product was observed. This result confirms the heterogeneous nature of the catalysis by the magnetic Fe3O4 nanoparticles.
Recyclability study
A: Reaction conditions: 4-nitro-chlorobenzene (1.0 mmol), thiophenol (1.0 mmol), catalyst (25 mg, 5 mol%), KOH (1.5 mmol; 1.5 equiv.), water (3.0 mL), reflux temperature for 8 h.
B: Reaction conditions: 4-methoxy-iodobenzene (1.0 mmol), thiourea (0.75 mmol), catalyst (25 mg, 6.6 mol%), KOH (1.5 mmol; 1.5 equiv.) water (3.0 mL), reflux temperature for 24 h.
4. Conclusions
We have developed a magnetically separable and efficient porous Fe3O4 catalyst stabilized with mesoporous PANI for the S-arylation of various aryl, alkyl and heterocyclic halides with thiophenol to obtain unsymmetrical diaryl sulfides in moderate to high yields. The application of the catalyst was also extended to the S-arylation of various aryl iodides with thiourea to obtain symmetrical diaryl sulfides selectively. The most attractive features of this protocol are the easy preparation of the catalyst from readily accessible reagents and that the catalyst can be used in a green solvent, water. In addition to this, the catalyst can be easily recovered under an external magnetic field and reused in the next consecutive S-arylation reaction.Acknowledgements
D.D. thanks UGC and R.A. thanks DST (GAP-0152), India for their fellowships. We also thank Dr. S. V. Manorama, Nanomaterial Science, IPC Division, CSIR-IICT for TEM analysis.Notes and references
- (a) G. Liu, J. R. Huth, E. T. Olejniczak, R. Mendoza, P. DeVries, S. Leitza, E. B. Reilly, G. F. Okasinski, S. W. Fesik and T. W. von Geldern, J. Med. Chem., 2001, 44, 1202 CrossRef CAS; (b) G. De Martino, M. C. Edler, G. La Regina, A. Coluccia, M. C. Barbera, D. Barrow, R. I. Nicholson, G. Chiosis, A. Brancale, E. Hamel, M. Artico and R. Silvestri, J. Med. Chem., 2006, 49, 947 CrossRef CAS; (c) A. Gangjee, Y. Zeng, T. Talreja, J. J. McGuire, R. L. Kisliuk and S. F. Queener, J. Med. Chem., 2007, 50, 3046 CrossRef CAS.
- (a) S. F. Nielsen, E. Ø. Nielsen, G. M. Olsen, T. Liljefors and D. Peters, J. Med. Chem., 2000, 43, 2217 CrossRef CAS.
- (a) J. Tsuji, Transition Metal Reagents and Catalysts: Innovations in Organic Synthesis, John Wiley & Sons, New York, 2000 Search PubMed; (b) M. Beller and C. Bolm, Transition Metals for Organic Synthesis, Wiley-VCH, Weinheim, Germany, 2nd edn, 2004 Search PubMed; (c) A. de Meijere and F. Diederich, Metal-Catalyzed Cross-Coupling Reactions, Wiley-VCH, Weinheim, Germany, 2nd edn, 2004 Search PubMed; (d) J. Montgomery, Angew. Chem., Int. Ed., 2004, 43, 3890 CrossRef CAS; (e) T. Kurita, M. Abe, T. Maegawa, Y. Monguchi and H. Sajiki, Synlett, 2007, 2521 CAS.
- (a) Z. Li, Y. Fu, L. Liu and Q.-X. Guo, Chin. J. Org. Chem., 2005, 25, 1508 CAS; (b) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS; (c) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Chem. Rev., 2007, 107, 5318 CrossRef CAS; (d) J. Tsuji, Palladium Reagents and Catalysts, John Wiley & Sons, New York, 2004 Search PubMed; (e) D. Prim, J.-M. Campagne, D. Joseph and B. Andrioletti, Tetrahedron, 2002, 58, 2041 CrossRef CAS.
- For a review, see: (a) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954–6971 CrossRef CAS; (b) T. Kondo and T.-A. Mitsudo, Chem. Rev., 2000, 100, 3205–3220 CrossRef CAS.
- (a) A. Correa, M. Carril and C. Bolm, Angew. Chem., Int. Ed., 2008, 47, 2880 CrossRef CAS; (b) S. L. Buchwald and C. Bolm, Angew. Chem., Int. Ed., 2009, 48, 5586 CrossRef CAS; (c) J.-R. Wu, C.-H. Lin and C.-F. Lee, Chem. Commun., 2009, 4450 RSC.
- (a) S. Sun and H. Zeng, J. Am. Chem. Soc., 2002, 124, 8204 CrossRef CAS; (b) A. H. Latham and M. E. Williams, Acc. Chem. Res., 2008, 41, 411 CrossRef CAS.
- (a) S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064 CrossRef CAS; (b) V. Polshettiwar, B. Baruwati and R. S. Varma, Chem. Commun., 2009, 1837 RSC; (c) S. Luo, X. Zheng, H. Xu, X. Mi, L. Zhang and J.-P. Cheng, Adv. Synth. Catal., 2007, 349, 2431 CrossRef CAS.
- (a) V. Polshettiwar, B. Baruwati and R. S. Varma, Green Chem., 2009, 11, 127 RSC; (b) M. Kotani, T. Koike, K. Yamaguchi and N. Mizuno, Green Chem., 2006, 8, 735 RSC; (c) R. S. Schwab, D. Singh, E. E. Alberto, P. Piquini, O. E. D. Rodrigues and A. L. Braga, Catal. Sci. Technol., 2011, 1, 569 RSC.
- (a) D.-H. Zhang, G.-D. Li, J.-X. Lia and J.-S. Chen, Chem. Commun., 2008, 3414 RSC; (b) M. Kawamura and K. Sato, Chem. Commun., 2006, 4714 Search PubMed; (c) M. Kawamura and K. Sato, Chem. Commun., 2007, 3404 RSC; (d) A. Hu, G. T. Yee and W. Lin, J. Am. Chem. Soc., 2005, 127, 12486 CrossRef CAS; (e) G. Chouhan, D. Wang and H. Alper, Chem. Commun., 2007, 4809 RSC.
- For using superparamagnetic iron oxide as a catalyst for the synthesis of α-aminonitriles, see: M. M. Mojtahedi, M. S. Abaee and T. Alishiri, Tetrahedron Lett., 2009, 50, 2322 CrossRef CAS.
- (a) D. Bourissou, O. Guerret, F. P. Gabbai and G. Bertrand, Chem. Rev., 2000, 100, 39 CrossRef CAS; (b) S. K. Yen, L. L. Koh, F. E. Hahn, H. V. Huynh and T. S. A. Hor, Organometallics, 2006, 25, 5105 CrossRef CAS; (c) J. Ye, W. Chen and D. Wang, Dalton Trans., 2008, 4015 RSC; (d) N. Marion and S. P. Nolan, Acc. Chem. Res., 2008, 41, 1440 CrossRef CAS.
- (a) X. Zhang, Z. Xi, A. Liu and W. Chen, Organometallics, 2008, 27, 4401 CrossRef CAS; (b) D. Meyer, M. A. Taige, A. Zeller, K. Hohlfeld, S. Ahrens and T. Strassner, Organometallics, 2009, 28, 2142 CrossRef CAS.
- (a) G. A. Somorjai and R. M. Rioux, Catal. Today, 2005, 100, 201 CrossRef CAS; (b) C. J. Zhong and M. M. Maye, Adv. Mater., 2001, 13, 1507 CrossRef CAS; (c) W. Schartl, Adv. Mater., 2000, 12, 1899 CrossRef CAS.
- R. Arundhathi, D. Damodara, P. R. Likhar, M. L. Kantam, P. Saravanan, T. Magdaleno and S. H. Kwon, Adv. Synth. Catal., 2011, 353, 1591 CrossRef CAS.
- (a) M. Oba, K. Tanaka, K. Nishiyama and W. Ando, J. Org. Chem., 2011, 76, 4173 CrossRef CAS; (b) F. Ke, Y. Qu, Z. Jiang, Z. Li, D. Wu and X. Zhou, Org. Lett., 2011, 13, 454 CrossRef CAS; (c) D. J. C. Prasad and G. Sekar, Org. Lett., 2011, 13, 1008 CrossRef CAS; (d) I. W. J. Still and F. D. Toste, J. Org. Chem., 1996, 61, 7677 CrossRef CAS.
- (a) M. Carril, R. SanMartin, E. Dominguez and I. Tellitu, Chem.–Eur. J., 2007, 13, 5100 CrossRef CAS; (b) H.-J. Xu, Y.-F. Liang, X.-F. Zhou and Y.-S. Feng, Org. Biomol. Chem., 2012, 10, 2562 RSC; (c) L. Rout, P. Saha, S. Jammi and T. Punniyamurthy, Eur. J. Org. Chem., 2008, 640 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional characterization studies supporting the results which include: TEM, XPS and TPR measurements. See DOI: 10.1039/c2cy20624b |
| This journal is © The Royal Society of Chemistry 2013 |