Photocatalytic performances and activities in Ag-doped ZnAl2O4 nanorods studied by FTIR spectroscopy
Zhengru
Zhu
ab,
Qidong
Zhao
a,
Xinyong
Li
*ad,
Hong
Li
ac,
Moses
Tade
d and
Shaomin
Liu
*d
aState Key Laboratory of Fine Chemical, Key Laboratory of Industrial Ecology and Environmental Engineering (MOE), School of Environmental Science and Technology, Dalian University of Technology, Dalian, 116024, China. E-mail: xyli@dlut.edu.cn; Fax: +86-411-8470-7733; Tel: +86-411-8470-7733
bResearch Center of Hydrology and Water Source, Academy of City and Environmental Science, Liaoning Normal University, Dalian, 116029, China
cDepartment of Basic, Dalian Naval Academy, Dalian, 116018, China
dDepartment of Chemical Engineering, Curtin University, Perth, WA 6845, Australia
First published on 12th November 2012
Abstract
Porous and rod-shaped nanostructures of ZnAl2O4 were successfully synthesized by a simple hydrothermal method. The Ag-doped ZnAl2O4 catalyst, based on porous ZnAl2O4, was prepared by the incipient wetness impregnation strategy and showed an excellent photoelectric property and catalytic activity. The structural properties of the samples were systematically investigated by X-ray powder diffraction (XRD), Brunauer–Emmet–Teller (BET), transmission electron microscopy (TEM), Electron spin resonance (ESR), X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FT-IR) techniques. The photocatalytic degradation of toluene by the Ag-doped ZnAl2O4, ZnAl2O4 and P25 TiO2 samples was comparatively studied under UV lamp irradiation. The results indicate that the Ag-doped ZnAl2O4 sample synthesized at pH = 6 exhibited a higher capacity for the degradation of toluene. No obvious deactivation of the Ag-doped ZnAl2O4 nanorods was observed during the prolonged operation of 6 h. The Ag-doped ZnAl2O4 nanorods could be potentially applied in environmental purification in the near future.
1. Introduction
VOCs are defined as toxic organic compounds commonly emitted into the environment due to their ubiquitous presence in fuel and petroleum products.1 Many VOCs can also cause headaches, eye, nose and throat irritation and dizziness. Therefore, they are directly harmful to human beings. Toluene is used as an indicator of VOCs and this is due to the fact that it belongs to a group of VOCs, has lower toxicity than benzene and is safer for use in a laboratory environment.Photocatalytic oxidation techniques are promising technologies for air purification because the pollutants can be oxidized to H2O and CO2. Photodegradation usually occurs at room temperature and pressure for air purification and may be more cost-effective than other conventional techniques, such as activated carbon adsorption and chemical scrubbers, because semiconductor catalysts are inexpensive and capable of mineralizing most organic compounds effectively. Photocatalytic oxidation using titanium dioxide (TiO2) is cost-effective and can be carried out at room temperature and atmospheric pressure. Obee and Brown first reported an investigation into the photocatalytic oxidation of VOCs using TiO2 for indoor air. In the investigation, they used Degussa P25 TiO2 as a catalyst and focused the study on the effects of humidity on the oxidation rates of toluene.2 This report is based on the FTIR method, which is widely used for various industrial and environmental applications.3–5 The main advantages of the FTIR technique are its capability for on-line and in situ monitoring of volatile organic compounds and gases from various samples. Concerning the gas–solid regime, Ibusuki and Takeuchi6 carried out the complete photo-oxidation of toluene on TiO2 at room temperature. The nanoscale TiO2 samples were found to be highly active for the photodegradation of toluene. However, rapid deactivation of the TiO2 catalysts at room temperature is due to the chemisorption of intermediates, such as benzaldehyde and benzoic acid.7
Zinc aluminate (ZnAl2O4) has a chemical formula of AB2O4 with the normal spinel structure. ZnAl2O4 has attracted broad interest in the application of catalytic reactions, such as cracking, dehydration, hydrogenation and dehydrogenation.8–11 Zinc aluminate offers many advantages, such as high thermal and chemical stability, hydrophobic behavior, high mechanical resistance, low sintering temperature and high quantum yields.12–15 ZnAl2O4 is widely used as an electronic, ceramic and catalytic material and is emerging as one of the best wide band gap compound semiconductors (Eg = 3.8 eV) for various optoelectronic applications.16 At present, the synthesis methods of ZnAl2O4 include solid-state methods,17 sol–gel routes,18 combustion methods19 and solution-phase routes.20 The hydrothermal method is simple, reliable, without post-treatment, allows for easy mass production and preparation of different shaped nanometer materials and is also widely used.
In recent years, nanocrystalline materials like ZnAl2O4 spinels have gained considerable interest in the field of catalysis since they may be used as supports for noble metals as substitutes for more traditional materials.21–23 In particular, nanocrystalline zinc aluminate ZnAl2O4 with a high specific surface area, prepared by methods like mechanochemical, solvothermal and modified citrate techniques and a wet impregnation strategy, may be employed as an excellent support for Pt and Pd catalysts. Porous and nanocrystalline zinc aluminum with a spinel structure as a catalyst support may provide a higher catalytic activity, selectivity, reproducibility and higher energy transfer ratio. Moreover, loading noble metals onto these porous semiconductors with nanostructures further enhances the activities of the catalyst. Zawadzki et al. prepared a Pd/ZnAl2O4 system using a microwave-solvothermal method, whose catalytic properties were tested in hydrocarbon combustion.24 Wrzyszcz et al. have reported ZnAl2O4 with a spinel structure as a carrier for loading the noble metal Pt.25 A promoting effect from low amounts of Sn added to the Pt/ZnAl2O4 catalyst was also found by Pakhomov et al.26 but when higher amounts of Sn was added to Pt/ZnAl2O4, it decreases the catalytic activity. Ag species significantly improve the activity, selectivity, recycling and reproducibility of Ag catalyst systems, so loading the noble metal Ag can be a feasible way to enhance the catalytic activities of semiconductors but the amounts of the loading metal should be controlled in a suitable range. Excessive amounts of noble metals possibly become a recombination center for the electron and hole, meaning the activities of the catalysts decrease.
In this work, porous and rod-like ZnAl2O4 nanoparticles were prepared using a simple hydrothermal method at pH = 6 followed by silver loading on the zinc aluminum by a traditional impregnation method, which formed Ag-doped ZnAl2O4 nanorods. After systematic characterization of the nanorods and surface structures, the photocatalytic performances for degradation of gaseous toluene over the ZnAl2O4, Ag-doped ZnAl2O4 and commercial P25 TiO2 photocatalysts were comparatively studied by in situ FT-IR spectroscopy.
2. Experimental
2.1. Preparation of catalysts
All the materials were reagent grade and used without further purification. Deionized water was used as a solvent. The “rod-like” ZnAl2O4 nanocrystals were synthesized by a hydrothermal method. 0.1 M (50 mL) of an aqueous solution of Zn(NO3)2·6H2O and 0.2 M (50 mL) Al(NO3)3·9H2O were mixed into 100 mL of deionized water. The desired amounts of NH3·H2O were prepared and added to the salt solution to adjust the pH value. When the pH value reached 6, the solution was transferred to a Teflon vessel and kept at 200 °C for 20 h. The precipitate was then washed twice with deionized water and with ethanol, respectively. After that, the precipitate was dried overnight at 80 °C and the residue water was further removed by heating at 750 °C for 5 h. The ZnAl2O4 sample was then obtained.The Ag-doped ZnAl2O4 catalyst was synthesized by a conventional impregnation strategy.27 A AgNO3 solution was used as the precursor. The silver loading was 1 wt%. After evaporation, the catalyst was dried at 100 °C for 12 h and calcined at 400 °C in air for 6 h and allowed to cool slowly. Then the Ag-doped ZnAl2O4 sample was obtained.
2.2. Characterizations
Powder X-ray diffraction (XRD) patterns of the ZnAl2O4 and Ag-doped ZnAl2O4 samples were carried out on a Japan Rigaku D/Max-rA diffractometer using a Cu-target tube (λ = 0.15418 nm) and a graphite monochromator. The samples were step-scanned in steps of 0.016° (2θ) using a count time of 0.1 s step−1.Transmission electron microscopy (TEM) images of the samples were recorded with an FEI Tecnai G220 transmission electron microscope. For the sample preparation, the powder was ultrasonicated in ethanol and then a droplet of the suspension was deposited and dried on the sample support. Moreover, high resolution transmission electron microscopy (HRTEM, JEOL-2010 system at 200 kV) and selected area electron diffraction (SAED, Tecnai G220) were used to characterize the structure of the nanocrystals.
Diffuse reflectance UV-visible (DR-UV-Vis) spectra of the samples were recorded on a diffuse reflectance spectrophotometer (JASCO, UV-550).
Nitrogen adsorption–desorption isotherms of the samples were obtained on a Micromeritics ASAP-2000 surface area and pore size analyzer. The samples were degassed at 400 °C prior to the measurement. The specific surface area was calculated from the desorption isotherms by the BET equation, using the data in a P/P0 range between 0.05 and 1.0.
The chemical compositions and structures of these samples were determined by a Fourier transform infrared spectrophotometer (BRUKER VERTEX 70 Optics) using KBr pellets.
The separation and transfer behaviors of the photogenerated charge carriers in the samples were investigated using a lock-in-based surface photovoltage (SPV) measurement system, which consists of a monochromator (model Omni-λ 3005) and a lock-in amplifier (model SR830-DSP) with an optical chopper (model SR540) running at a frequency of 20 Hz.
X-ray photoelectron spectra (XPS) were measured using a Perkin-Elmer PHI 5600 X-ray photoelectron spectrometer using acrochromatic Al–Kα radiation (1486.6 eV) with Ar+ sputtering to remove the surface layer of the samples. Fractional atomic concentrations of the elements were calculated using empirically derived atomic sensitivity factors.
Electron spin resonance (ESR; EOL/JES-FA200) measurements were used to investigate the properties of trapping electrons and holes from different samples.
The photocatalytic activity (PCA) was measured through the concentration change of toluene using in situ FT-IR spectroscopy (BRUKER VERTEX 70 Optics). Degussa P25 TiO2 powder (75% anatase and 25% rutile) with a Brunauer–Emmett–Teller (BET) surface area of 50 m2 g−1, a commercial UV photocatalyst, was used for comparison with the ZnAl2O4 and Ag-doped ZnAl2O4 samples.
2.3. Photocatalytic test
The photocatalytic experiments were carried out in a self-made in situ IR quartz photoreaction cell,28 the dimensions of which were a tubular diameter of 4 cm and a path length 10 cm. Both ends were “capped” by IR-transparent NaCl crystal windows. Approximately 30 mg of the samples was pressed into a self-supported disk of 13 mm in diameter. The disk was mounted inside the sample holder (its diameter was about 13 mm) which was located at the center of the cell and allowed for UV-illumination. The sample holder was tilted by an angle of 30 degrees with respect to the IR path. The distance between the UV lamp and sample was about 15 cm. The light intensity at the sample holder, measured using a radiometer UVX Digital, was 80 mW cm−2. Two wafers were prepared in parallel.The catalysts used were prepared ZnAl2O4 and Ag/ZnAl2O4 and for comparative experiments, commercial P25 TiO2. The compressed air used in the reaction was metered (150 mL min−1) and pretreated to remove adventitious water or oxygen through three drying columns packed with silica gel, CaCl2 and molecular sieves, respectively. The reaction cell was purged with dry air for 1 h. After 1 h, the flux of the dry air was set at 20 mL min−1. Spectra of the clean catalyst surface were collected after this process and utilized as the background. The temperature of the reactor was always 293 K. Subsequently, the toluene substrate was fed by a syringe pump to a mixing tee, where toluene was vaporized and mixed with dry air. The gas flow rate was 2 μL h−1 for ca. 30 min.
Before starting the irradiation, the gas mixture was fed for 1 h in order to achieve the equilibration of the catalyst with the reagents. After 1 h, the inlet and outlet ports were shut off and the lamps were turned on. The infrared spectra were continuously collected during the reaction. The reactant concentration was measured by gas chromatography (Aligent 7890A, USA). During the reaction process, samples of approximately 1 μL were collected at the outlet every 0.5 h and the change in the concentration of toluene was analyzed by GC equipped with a FID detector (HP-5 capillary column (30 m × 320 μm × 0.25 m)) and a Porapak Q column (2 m × 20 mm). When the characteristic peaks of toluene did not obviously change, the lamps were turned off and the cell was purged with nitrogen.
3. Results and discussion
3.1. Characterization of the ZnAl2O4 and Ag-doped ZnAl2O4 samples
To identify the crystalline phase and compare the particle size, X-ray powder diffraction (XRD) analysis was performed on the samples. Fig. 1 shows that the final product was ZnAl2O4 with the expected inverse spinel structure and all the peaks can be indexed as the spinel ZnAl2O4 phase (JCPDS 05-0669). The characteristic peaks at 2θ of 31.2°, 36.8°, 44.7°, 55.6°, 59.3° and 65.2° correspond to the (220), (311), (400), (422), (511) and (440) crystallographic nucleation planes of the ZnAl2O4 spinel phase, respectively. The average sizes of the ZnAl2O4 and Ag-doped ZnAl2O4 samples determined by X-ray peak broadening analysis of the (311) reflection are about 6 nm and 5.8 nm, respectively. The particle size of the Ag-doped ZnAl2O4 sample is slightly smaller than that of the ZnAl2O4 sample. No peaks corresponding to Ag species could be observed in the X-ray patterns. This is due to the low content of Ag in the sample. | ||
| Fig. 1 XRD patterns of the ZnAl2O4 (a) and Ag-doped ZnAl2O4 samples (b). | ||
Fig. 2a and b show the TEM images of the ZnAl2O4 sample. The sample is formed through the agglomeration of numerous rod-like nanoparticles. Fig. 2c shows the TEM images of the Ag-doped ZnAl2O4 sample. The particles present partial condensation, possibly because the amount of Ag doping causes a slight change. The TEM images (Fig. 2a–c) of the ZnAl2O4 and Ag-doped ZnAl2O4 samples show that the diameter and length of these rods is ca. 4 nm and 40 nm, respectively, which is in agreement with the results obtained by the XRD analysis. Furthermore, for the HRTEM image (Fig. 2d) of the ZnAl2O4 sample, the crystal lattice fringe spacing is 0.29 nm, which is consistent with the cubic phase ZnAl2O4 (220) plane spacing.29 The pattern in the selected area electron diffraction (SAED) (inset of Fig. 2c) indicates that the ZnAl2O4 nanorods are polycrystalline in nature and the most distinct diffraction spots could be assigned sequentially to the ZnAl2O4 (220), ZnAl2O4 (311), ZnAl2O4 (400) and ZnAl2O4 (440) planes from the center, which can also be indexed by the diffraction peaks in the XRD pattern.
 | ||
| Fig. 2 TEM images (a, b) and a HRTEM image of the ZnAl2O4 sample (d), a TEM image of the Ag-doped ZnAl2O4 sample (c). Inset of (c): SAED image of the Ag-doped ZnAl2O4 sample. | ||
FTIR measurements were used to identify and characterize the resulting spinel nanocrystals. The FT-IR spectra of the ZnAl2O4 and Ag-doped ZnAl2O4 samples were recorded using KBr pellets and are shown in Fig. 3a. According to Dhak and Pramanik,30 the spinels display stretching bands in the range of 500–900 cm−1, associated with the vibrations of the metal–oxygen, aluminum–oxygen and metal–oxygen–aluminum bonds.31,32 Additionally, the bands at 3350 cm−1 can be assigned to the vibrational mode of chemically bonded hydroxyl groups.33 The bands at 1100 cm−1 can be assigned to the C–O–C stretching vibration mode.34 The signal at 2360 cm−1 is attributed to CO2. No other intermediate phases were detected by the FTIR spectra, which is in agreement with the results obtained by the XRD analysis.
 | ||
| Fig. 3 FTIR spectra of the ZnAl2O4 and Ag-doped ZnAl2O4 samples (a) and UV-Vis absorption spectra of the ZnAl2O4 and Ag-doped ZnAl2O4 samples (b). | ||
Fig. 3b shows the UV-Vis absorption spectra of the ZnAl2O4 and Ag-doped ZnAl2O4 samples. The absorptive intensity of the Ag-doped ZnAl2O4 sample is stronger than the ZnAl2O4 sample in the whole absorption spectra. This shows that Ag loading means the induction of the Ag-doped ZnAl2O4 sample for UV light becomes more intense.
The specific surface area and porosity of two samples have been determined (Fig. 4). The specific surface area of the ZnAl2O4 sample and Ag-doped ZnAl2O4 sample is 161.195 and 152.247 m2 g−1, respectively. The specific surface area decreases a little due to Ag loading. The curves are typical for type IV (according to the IUPAC classification), which is characteristic of inorganic porous oxides.35 Three hysteresis loops with three sloping adsorption branches and three relatively sharp steep desorption branches are observed at relatively high pressure (P = 0.6–1.0P0). It is well known that a distribution of cavities of various sizes but with the same diameter could give this type of hysteresis loop. Analysis of the porosity was carried out following the BJH method.36 These two samples display a monomodal pore size distribution with most frequent pore radii of about 3.9 nm and 6.8 nm, respectively (Fig. 4a). The prepared samples are mesoporous materials according to the distribution of pore sizes. The total pore volumes (at P/P0 = 0.99) for the Ag-doped ZnAl2O4 and ZnAl2O4 samples are 0.3723 and 0.2689 cm3 g−1 (Fig. 4b), respectively. The Ag-doped ZnAl2O4 sample prepared by a hydrothermal method has the highest surface area and monomodal pore size distribution. These features are important for the accessibility of reactant molecules to the catalysts.
 | ||
| Fig. 4 BJH pore size distributions (a) and N2 absorption–desorption isotherms (b) of the ZnAl2O4 and Ag-doped ZnAl2O4 samples. | ||
We determined the survey XPS spectra of the ZnAl2O4 and Ag-doped ZnAl2O4 samples (Fig. 5). The spectra indicate that no other elements were detected except for the original components and contaminated carbon. Fig. 5b shows the high resolution XPS spectra of the Zn peak with Zn 2p3/2 and Zn 2p1/2 at 1022.3 eV and 1045.9 eV, respectively (Fig. 5b). The peaks at about 1023 and 1022 eV can be assigned to octahedral and tetrahedral Zn2+ ions, respectively.37 In addition, Al peaks with Al 2p and Al 2s were found at 76.8 eV and 121.7 eV (Fig. 5c–d). The main peak at the higher binding energy can be ascribed to Al3+ ions placed at the octahedral sites and the Al 2p signal at the lower binding energy can be ascribed to Al3+ ions located at the tetrahedral sites.38 Furthermore, an O peak with O 1s at 533.6 eV (Fig. 5e) indicates that the existing form of the sample is ZnAl2O4. Fig. 5f shows the high resolution XPS spectra of the Ag peak with Ag 3d5/2 and Ag 3d3/2 at 367.6 eV and 372.1 eV, respectively. It is shown that the silver exists in the form of Ag0. Table 1 gives the results of the measurements from the XPS analysis, indicating that the atomic ratio of elements is Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O = 1
O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.16
2.16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.32 of the composition of the Ag-doped sample and the atom ratio of silver is 0.61%. These results are in good agreement with the XRD analysis.
4.32 of the composition of the Ag-doped sample and the atom ratio of silver is 0.61%. These results are in good agreement with the XRD analysis.
 | ||
| Fig. 5 The whole XPS spectra (a), Zn 2p XPS spectra (b), Al 2p XPS spectra (c), Al 2s XPS spectra (d), O 1s XPS spectra (e) of the ZnAl2O4 and Ag-doped ZnAl2O4 samples and Ag 3d XPS spectra (f) of the Ag-doped ZnAl2O4 sample. | ||
| Element | Electronic energy (eV) | Atom ratio (%) |
|---|---|---|
| Zn 2p3/2 | 1022.3 | 12.67 |
| Zn 2p1/2 | 1045.9 | |
| Al 2p | 76.8 | 27.35 |
| Al 2s | 121.7 | |
| O 1s | 533.6 | 54.78 |
| C 1s | 286 | 4.59 |
| Ag 3d5/2 | 367.6 | 0.61 |
| Ag 3d3/2 | 372.1 |
SPV can detect the illumination-induced change in the surface potential due to drift, accumulation and recombination of the photogenerated carriers in nanostructured materials.39Fig. 6a shows the SPV spectra of the ZnAl2O4 and Ag-doped ZnAl2O4 samples. The Ag-doped ZnAl2O4 sample exhibits a distinguished SPV response and its performance is evidently higher than that of the ZnAl2O4 sample. The SPV spectra are sensitive to the electron transition related process and subsequent charge separation. A higher SPV signal may suggest a higher separation rate of the photo-generated charge carriers.40 Due to the quantum confinement effect, the electronic band gap of the dispersed nanocrystals in the “rod-like” aggregation of Ag-doped ZnAl2O4 is widened with respect to ZnAl2O4, which would improve the redox activity of its photo-generated electrons and holes correspondingly so that a better photocatalytic activity could be expected for the Ag-doped ZnAl2O4 catalyst.
 | ||
| Fig. 6 The SPV spectra (a) and ESR spectra (b) of the ZnAl2O4 and Ag-doped ZnAl2O4 samples. | ||
Fig. 6b shows the ESR spectra at room temperature of the ZnAl2O4 and Ag-doped ZnAl2O4 samples. Not many defect centres are expected to be formed in a system like ZnAl2O4. The observed spectrum of Ag-doped ZnAl2O4 appears to be a superposition of at least two distinct centres. The ESR line of Ag-doped ZnAl2O4, labeled as II in Fig. 5b, is due to a centre characterized by a single ESR line with an isotropic g-value equal to 2.0046 and a 7 gauss linewidth. The most probable centre which can be observed is centre II, which is assigned to a F+ centre (an electron trapped at an anion vacancy). It is known that the cation disorder of aluminates like Ag-doped ZnAl2O4 provides a large number of lattice defects which may serve as trapping centres. It has been speculated41 that in oxides, the charges must be trapped near doubly (or more) charged defects in order for the charge to be delocalized, thus allowing it to interact with surrounding nuclei. Centre I is tentatively assigned to a V− centre (hole trapping at aluminum and zinc vacancies). It should be mentioned that a similar centre in neutron irradiated MgAl2O4 has also been ascribed to a V− centre.42
3.2. Comparison of the photocatalytic activities of the ZnAl2O4, Ag/ZnAl2O4 and P25 TiO2 catalysts
In previous work (not published), we have synthesized ZnAl2O4 nanoparticles via a hydrothermal method at different pH values (pH = 4, 5, 6, 8, 10, respectively). Moreover, the photocatalytic performances and activities for toluene over these catalysts have been studied by FTIR spectroscopy. The previous results have shown that the degradation rates of toluene in the photocatalytic process were up to 53.3%, 70.3%, 73.4%, 67.6% and 58.8% on the samples prepared at pH = 4, 5, 6, 8 and 10, respectively. The photocatalytic capacity of the ZnAl2O4 catalyst prepared under pH = 6 was higher than the other samples. Therefore, pH 6 was chosen for the sample preparation in this work.In order to obtain insights on the surface phenomena involved in the reaction process, toluene oxidation was studied by FT-IR spectroscopy. In the presence of the catalyst and irradiation, toluene consumption occurred. The results obtained for a representative run are reported in Fig. 7. At higher frequencies, peaks due to adsorbed toluene appear in the 3100–2800 cm−1 range, which have been assigned to asymmetric and symmetric νC–H of the methyl group (2997 and 2920 cm−1, respectively) (Fig. 7).43 A set of infrared transmission spectra obtained during the photocatalytic oxidation of toluene over the Ag-doped ZnAl2O4 sample are shown in Fig. 7a. Fig. 7b displays the photodegradation spectra for toluene over the ZnAl2O4, Ag-doped ZnAl2O4 and commercial P25 TiO2 samples for 6 h UV illumination. The length of the UV light is 254 nm. Prior to UV-lamp illumination (t = 0), the spectrum displays characteristic toluene bands at 2997 and 2920 cm−1. Upon irradiation, the intensity of these bands begins to decrease slowly. The responses corresponding to CO2 (2360 cm−1 and 2340 cm−1) increase when the reaction proceeds (Fig. 7). No other peaks over the Ag-doped ZnAl2O4 sample are observed (Fig. 7a). These results show that toluene was mineralized into carbon dioxide and water as the major species. From Fig. 7, no other products or intermediates exist. These results demonstrate that Ag-doped ZnAl2O4 has a better photocatalytic activity than the ZnAl2O4 and P25 TiO2 samples.
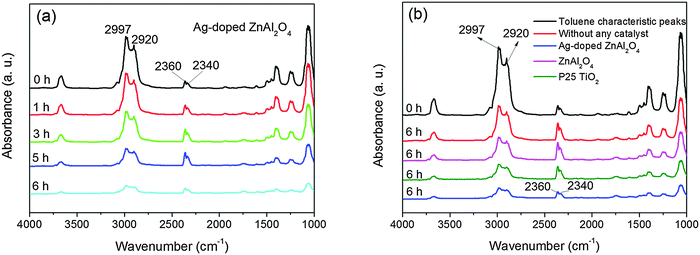 | ||
| Fig. 7 The FTIR spectra of the photodegradation of toluene over the Ag-doped ZnAl2O4 sample for 6 h UV illumination (a) and the ZnAl2O4, Ag-doped ZnAl2O4 and commercial P25 TiO2 samples under 6 h UV illumination (b). | ||
Meanwhile, we observed a concentration change in toluene by gas chromatography during UV illumination. The initial concentration of toluene is 750 mg m−3. Fig. 8a shows a comparison of the concentration change of toluene and UV illumination time without any catalyst and with the ZnAl2O4, Ag-doped ZnAl2O4 and P25 TiO2 samples for 6 h. After 6 h prolonged irradiation, the degradation percentages of toluene over the ZnAl2O4, Ag-doped ZnAl2O4 and P25 TiO2 catalysts reaches 71%, 82% and 75%, respectively. This shows that the Ag-doped ZnAl2O4 sample decreased gently. The Ag-doped ZnAl2O4 photocatalyst has a better photocatalytic activity for toluene.
 | ||
| Fig. 8 Photocatalytic degradation of toluene over the ZnAl2O4, Ag-doped ZnAl2O4 and commercial P25 TiO2 samples (a) and the reaction kinetics curves of the degradation of toluene using the ZnAl2O4, Ag-doped ZnAl2O4 and commercial P25 TiO2 samples (b) under UV illumination. | ||
The photocatalytic reaction for toluene meets with first-order reaction kinetics. The fitting curves (Fig. 8b) were obtained with the linefit method by using the experimental date in Fig. 8a. Table 2 shows that the kinetics constants and regression coefficients of the photocatalytic degradation of toluene without any catalyst and with the ZnAl2O4, Ag-doped ZnAl2O4 and P25 TiO2 samples during different processes, respectively.
| Experimental process | First-order kinetics constant (K, h−1) | Relative coefficient R2 |
|---|---|---|
| Photodegradation without catalyst | 0.0826 | 0.9862 |
| Photodegradation with ZnAl2O4 | 0.2013 | 0.9782 |
| Photodegradation with Ag-doped ZnAl2O4 | 0.3278 | 0.9833 |
| Photodegradation with P25 TiO2 | 0.2326 | 0.9741 |
The results show the kinetics constant for the photocatalytic degradation of toluene over the Ag-doped ZnAl2O4 sample is the biggest and that the kinetics constant for the photocatalytic degradation of toluene over the Ag-doped ZnAl2O4 sample is 1.6 and 1.4 times more than those of the ZnAl2O4 sample and P25 TiO2. Moreover, the results meet with the comparison of the photocatalytic activities for the Ag-doped ZnAl2O4 catalyst.
As obtained from the in situ FT-IR study, the Ag-doped ZnAl2O4 sample has more hydrogen-bonded surface water molecules and hydroxyl groups adsorbed at its surface due to its smaller crystallite size and higher specific surface area. The surface hydroxyl group would greatly affect the formation of hydroxyl radicals (˙OH) since these active species are generated via hole capture by the surface hydroxyl.44,45 According to the DRS analysis of these samples, Ag loading means the induction of the Ag-doped ZnAl2O4 sample for UV light becomes more intense. The XRD study indicated that the grain sizes of the Ag-doped ZnAl2O4 sample are smaller than that of the ZnAl2O4 sample. This shows that the hydrothermal method may result in defect sites on the surface of the ZnAl2O4 sample, which effectively reduces the recombination of the electron–hole pairs. From the ESR spectra, irradiation leads to trapping of an electron at an anionic vacancy and such trapping is the basis for the formation of F+ defect centres. Holes have also been trapped at aluminum and zinc vacancies, which form V− defect centres. The F+ defect centres on the surface of the Ag-doped ZnAl2O4 crystalline grains could accept more hydroxyl groups bonding to the metallic cations. We studied the mechanism of the roles of Ag by SPV and a literature survey qualitatively. The Ag nanoparticles could accept the photogenerated electrons from the conduction band of the ZnAl2O4 nanorods to activate molecular oxygen and further enforce the toluene oxidation. Ag could also be excited by the proper light to produce plasmatic charges. The excited Ag nanoparticles could contribute to the enhancement of the photocatalytic performance of the ZnAl2O4 nanorods, which also behave as good carriers for the Ag nanoparticles.46–50
Upon UV irradiation, Ag nanoparticles are excited and photo-induced electron–hole pairs are produced. The photo-induced electrons enter the conduction band of ZnAl2O4.51 The photo-induced holes could be easily captured by chemisorbed surface hydroxyl groups to produce hydroxyl radical groups, i.e., ˙OH, while the electrons could be trapped by adsorbed O2− to produce superoxide radicals, i.e., ˙O2−.
Both kinds of radical groups have been shown to be capable of contributing to the oxidation process of organic substances. These active species degrade toluene and mineralize it into H2O and CO2.52 These results demonstrate that the porous and rod-like nanoparticles of the Ag-doped ZnAl2O4 catalyst exhibit higher photocatalytic activity.
4. Conclusions
In summary, the synthesis of ZnAl2O4 nanoparticles was studied via a simple hydrothermal method at pH = 6. The Ag-doped ZnAl2O4 catalysts were prepared by a conventional impregnation strategy. The Ag-doped ZnAl2O4 sample with 1 wt% Ag has an attractive photovoltage response in the UV region and remarkable photocatalytic activities in the degradation of toluene. The porous and higher specific surface areas of the ZnAl2O4 catalyst with rod-like morphology leads to the good dispersion of the Ag species on the support, which ensures the access of the adsorbates towards the active sites and higher photo-induced reactivity. Toluene is degraded and mineralized into H2O and CO2 over the ZnAl2O4 and Ag-doped ZnAl2O4 catalysts. Moreover, no deactivation of the photocatalyst is observed during the prolonged experiment of 6 h. Rod-like Ag-doped ZnAl2O4 particles with an optimized performance in the photocatalytic degradation of environmental pollutants could be obtained for the sample with the proper preparation method, which could be potentially applied in environmental purification in the near future.Acknowledgements
This work was supported financially by the National Nature Science Foundation of China (NSFC-RGC 21061160495), the National High Technology Research and Development Program of China (863 Program) (No. 2010AA064902), the Major State Basic Research Development Program of China (973 Program) (No. 2011CB936002), the Excellent Talents Program of Liaoning Provincial University (LR2010090) and the Key Laboratory of Industrial Ecology and Environmental Engineering, China Ministry of Education.References
- C. Kennes and F. Thalasso, J. Chem. Technol. Biotechnol., 1998, 72, 303–319 CrossRef CAS.
- T. N. Obee and R. T. Brown, Environ. Sci. Technol., 1995, 29, 1223–1231 CrossRef CAS.
- R. A. Ketola, T. Kotiaho, M. E. Cisper and T. M. Allen, J. Mass Spectrom., 2002, 37, 457–459 CrossRef CAS.
- J. Bak and S. Clausen, Meas. Sci. Technol., 2002, 13, 150–156 CrossRef CAS.
- M. F. A. M. van Hest, A. de Graaf, M. C. M. van de Sanden and D. C. Schram, Plasma Sources Sci. Technol., 2000, 9, 615–624 CrossRef CAS.
- T. Ibusuki and K. Takeuchi, Atmos. Environ., 1986, 20, 1711–1715 CrossRef CAS.
- L. X. Cao, Z. Gao, S. L. Suib, T. N. Obee, S. O. Hay and J. D. Freihaut, J. Catal., 2000, 196, 253–261 CrossRef CAS.
- L. F. Peltier, P. Chaumette and J. Saussey, J. Mol. Catal. A: Chem., 1998, 132, 91–100 CrossRef.
- R. Roesky, J. Weiguny and H. Bestgen, Appl. Catal., A, 1999, 176, 213–220 CrossRef CAS.
- H. Grabowska, W. Mista and J. Trawczyski, Appl. Catal., A, 2001, 220, 207–213 CrossRef CAS.
- T. E. Nabarawy, A. A. Attia and M. N. Alaya, Mater. Lett., 1995, 24, 319–325 CrossRef.
- R. Pandey, J. D. Gale, S. K. Sampath and J. M. Recio, J. Am. Ceram. Soc., 1999, 12, 3337–3341 Search PubMed.
- J. Wrzyszcz, M. Zawadzki, J. Trawczynski, H. Grabowska and W. Mista, Appl. Catal., A, 2001, 210, 263–269 CrossRef CAS.
- S. Mathur, M. Veith, M. Haas, A. Shen, N. Lecerf, V. Huch, S. Hufner, R. Haberkorn, H. P. Beck and M. Jilavi, J. Am. Ceram. Soc., 2001, 84, 1921–1928 CrossRef CAS.
- Y. Yang, X. W. Sun, B. K. Tay, J. X. Wang, Z. L. Dong and H. M. Fan, Adv. Mater., 2007, 19, 1839–1844 CrossRef CAS.
- K. Kumar, K. Ramamoorthy, P. M. Koinkar, R. Chandramohan and K. Sankaranarayanan, J. Nanopart. Res., 2007, 9, 331–335 CrossRef CAS.
- L. Zou, F. Li, X. Xiang, D. G. Evans and X. Duan, Chem. Mater., 2006, 18, 5852–5859 CrossRef CAS.
- S. Mathur, J. Am. Ceram. Soc., 2001, 84, 1921–1928 CrossRef CAS.
- T. Minami, J. Alloys Compd., 2001, 315, 123–128 CrossRef.
- M. Zawadzki, Solid State Sci., 2006, 8, 14–20 CrossRef CAS.
- A. D. Ballarini, S. A. Bocanegra, A. A. Castro, S. R. de Miguel and O. A. Scelza, Catal. Lett., 2009, 129, 293–302 CrossRef CAS.
- T. Sirikajorn, O. Mekasuwandumrong, P. Praserthdam, J. G. Goodwin and J. Panpranot, Catal. Lett., 2008, 126, 313–318 CrossRef CAS.
- M. Takesada, M. Osada and T. Isobe, J. Phys. Chem. Solids, 2009, 70, 281–285 CrossRef CAS.
- M. Zawadzki, W. Mista and L. Kepinski, Vacuum, 2001, 63, 291–296 CrossRef CAS.
- J. Wrzyszcz, M. Zawadzki and J. Trawczynski, Appl. Catal., A, 2001, 210, 263–269 CrossRef CAS.
- N. A. Pakhomov, R. A. Buyanov, A. M. Moroz, E. N. Yurkenko, A. P. Chernyshev, N. A. Zaitseva and G. R. Kotelnikov, React. Kinet. Catal. Lett., 1980, 14, 329–334 CrossRef CAS.
- D. S. Bae, K. S. Han and J. H. Adair, J. Mater. Chem., 2002, 12, 3117–3120 RSC.
- Z. R. Zhu, X. Y. Li, Q. D. Zhao, H. Li, Y. Shen and G. H. Chen, Chem. Eng. J., 2010, 165, 64–70 CrossRef CAS.
- X. Y. Chen, C. Ma and Z. J. Zhang, Mater. Sci. Eng., B, 2008, 151, 224–230 CrossRef CAS.
- D. Dhak and P. Pramanik, J. Am. Ceram. Soc., 2006, 89, 1014–1021 CrossRef CAS.
- Z. X. Deng, C. Wang, X. M. Sun and Y.-D. Li, Inorg. Chem., 2002, 41, 869–873 CrossRef CAS.
- L. K. C. de Souza, J. R. Zamian, G. N. da Rocha Filho, L. E. B. Soledade, I. M. G. dos Santos, A. G. Souza, T. Scheller, R. S. Angelica and C. E. F. da Costa, Dyes Pigm., 2009, 81, 187–192 CrossRef CAS.
- M. Sangmanee and S. Maensiri, Appl. Phys. A: Mater. Sci. Process., 2009, 97, 167–177 CrossRef CAS.
- H. Liu, F. Xu and L. C. Li, React. Funct. Polymers, 2009, 69, 43–47 CrossRef CAS.
- S. B. Wang and G. Q. Lu, Ind. Eng. Chem. Res., 1999, 38, 2615–2625 CrossRef CAS.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pietotti, J. Rouquerol and T. Siemienieska, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- P. Druska, U. Steinike and V. Sepelak, J. Solid State Chem., 1999, 146, 13–21 CrossRef CAS.
- X. L. Duan, D. R. Yuan and F. P. Yu, Inorg. Chem., 2011, 50, 5460–5467 CrossRef CAS.
- K. Sakai, S. Oyama, K. Noguchi, A. Fukuyama, T. Kari and T. Okada, Physica E, 2008, 40, 2489–2493 CrossRef CAS.
- Z. Y. Liu, D. D. Sun, P. Guo and J. O. Leckie, Nano Lett., 2007, 7, 1081–1085 CrossRef CAS.
- N. Y. Konstantinov, L. V. Karaseva and V. V. Gromov, Dokl. Akad. Nauk SSSR, 1980, 228, 631–635 Search PubMed.
- V. T. Gritsyna, V. A. Kobyakov and Z. Tekh, Tech. Phys., 1985, 30, 206–210 Search PubMed.
- A. Gammard, O. Babaot, B. Jousseaucne, M. C. Rascle, T. Toupance and G. Campet, Chem. Mater., 2000, 12, 3419–3426 CrossRef.
- Y. Ohko, D. A. Tryk, K. Hashimoto and A. Fujishima, J. Phys. Chem. B, 1998, 102, 2699–2704 CrossRef CAS.
- M. I. Litter, Appl. Catal., B, 1999, 23, 89–114 CrossRef CAS.
- L. Suljo, C. Phillip and B. I. David, Nat. Mater., 2011, 10, 911–921 CrossRef.
- V. K. Prashant, J. Phys. Chem. Lett., 2012, 3, 663–672 CrossRef.
- B. I. David and L. Suljo, J. Am. Chem. Soc., 2011, 133, 5202–5205 CrossRef.
- M. S. Zhu, P. L. Chen and M. H. Liu, ACS Nano, 2011, 5, 4529–4536 CrossRef CAS.
- Z. W. Liu, W. B. Hou, P. Prathamesh, A. Mehmet and B. C. Stephen, Nano Lett., 2011, 11, 1111–1116 CrossRef CAS.
- K. Kawahara, K. Suzuki and Y. Ohko, Phys. Chem. Chem. Phys., 2005, 7, 3851–3855 RSC.
- J. G. Yu, G. P. Dai and B. B. Huang, J. Phys. Chem. C, 2009, 113, 16394–16401 CAS.
| This journal is © The Royal Society of Chemistry 2013 |
