A convenient nickel-catalysed hydrosilylation of carbonyl derivatives†
Jianxia Zheng, Christophe Darcel* and Jean-Baptiste Sortais*
Centre of Catalysis and Green Chemistry, Institut des Sciences Chimiques de Rennes, UMR 6226 CNRS-Université Rennes 1, Team “Organometallics: Materials and Catalysis” Building 10C, Campus de Beaulieu, Ave Général Leclerc, 35042 Rennes, France. E-mail: christophe.darcel@univ-rennes1.fr; jean-baptiste.sortais@univ-rennes1.fr; Fax: +33 2 23 23 69 39; Tel: +33 2 23 23 62 88
First published on 30th August 2012
Abstract
Hydrosilylation of aldehydes and ketones catalysed by nickel acetate and tricyclohexylphosphine as the catalytic system was demonstrated using polymethylhydrosiloxane as a cheap reducing reagent.
The research on alternatives to precious metal catalysts has grown rapidly over the last few years, essentially correlated with the increasing cost of raw materials.1 In the field of the hydrosilylation of unsaturated carbon–heteroatom bonds, rhodium, platinum and titanium were traditionally the metals of choice to catalyse this reaction.2 However, some limitations related to the price or the accessibility to these catalysts and to the price and/or stability of molecular hydrosilanes have led to the development of alternative systems based on earth abundant metals combined with stable inexpensive silanes.2c Among the commercially available silanes, the polymethylhydrosiloxane3 (PMHS) is the most inexpensive and abundant hydrosilane source arising from the silicone industry, which besides has the advantage to be stable to air and water, soluble in most organic solvents, which makes the hydrosilylation reaction a viable alternative to hydrogenation.4 Several earth abundant metals have been recently used as catalysts for hydrosilylation reactions, such as copper,5,6 zinc2c,d,7 and lately iron,8,9 especially with PMHS; however nickel, which is also a cheap and abundant metal, has been far less studied.10–13 In this area, for example, in 2006, the reduction of ketones with Ph2SiH2 has been reported by Kempe et al., using the combination of [Ni(0)(COD)]2 and chiral bidentate phosphines as the catalytic system.10b Recently, Guan et al.10d and Jones et al.10f have shown, respectively, that nickel PCP and PNP-pincer hydride complexes could efficiently catalyse the reduction of aldehydes in the presence of PhSiH3 under mild conditions with low catalyst loading (down to 0.2 mol%). Lately, we have developed a NHC–nickel-hydride catalyst for the reduction of carbonyl derivatives at room temperature, using Ph2SiH2 as the reducing agent.14 Thus, we decided to investigate simple catalytic systems based on air-stable nickel(II) salts using PMHS as the hydrogen source (Scheme 1 and Table 1). Interestingly, Ananikov and Beletskaya et al. have shown that the combination of Ni(acac)2 and phosphines is an excellent precursor of nickel(0) for the catalytic system.15 In the light of these results, initial studies focused on the reduction of benzaldehyde with 3 equiv. of PMHS in the presence of catalytic amounts of nickel(II) salts under various conditions to optimize the reaction parameters. (Table 1) Using 5 mol% of Ni(acac)2 and 10 mol% of PCy3 as the ligand in THF at 70 °C, a full conversion was observed. (Table 1, entry 1).
 | ||
| Scheme 1 Nickel-catalysed hydrosilylation of benzaldehyde. | ||
| Entrya | Nickel salt | Ligand | Time (h) | Conversionb (%) |
|---|---|---|---|---|
| a Typical procedure: the nickel salts (5 mol%), THF (1 mL), the ligand (10 mol%), the aldehyde (0.5 mmol) and PMHS (1.5 mmol) are added in this order and the reaction was stirred in a preheated oil bath at 70 °C.b Conversions determined by GC after methanolysis (MeOH, 2 M NaOH) and extraction with Et2O.c Reaction performed at 50 °C.d 2 mL of solvent.e Mes2NHC·HCl (5.5 mol%) deprotonated by n-BuLi (11 mol%).f Toluene as the solvent.g Ni(OAc)2·4H2O 1 mol%, PCy3 2 mol%, reaction run on the 2.5 mmol scale.h Ni(OAc)2·4H2O 0.1 mol%, PCy3 0.2 mol%, reaction run on the 12.5 mmol scale. | ||||
| 1 | Ni(acac)2 | PCy3 | 24 | 98 |
| 2 | Ni(OAc)2·4H2O | PCy3 | 16 | 97 |
| 3 | Ni(OAc)2 | PCy3 | 24 | 98 |
| 4 | NiBr2 | PCy3 | 16 | 0 |
| 5 | NiCl2 | PCy3 | 16 | 3 |
| 6c | Ni(OAc)2·4H2O | PCy3 | 22 | 52 |
| 7 | Ni(OAc)2·4H2O | PPh3 | 16 | 2 |
| 8 | Ni(OAc)2·4H2O | P(o-tolyl)3 | 16 | 0 |
| 9 | Ni(OAc)2·4H2O | dppe | 16 | 6 |
| 10 | Ni(OAc)2·4H2O | PMe2Ph | 16 | 98 |
| 11d,e | Ni(OAc)2·4H2O | Mes2NHC | 16 | 40 |
| 12f | Ni(OAc)2·4H2O | PCy3 | 16 | 30 |
| 13 | — | PCy3 | 16 | 0 |
| 14 | Ni(OAc)2·4H2O | — | 16 | 0 |
| 15g | Ni(OAc)2·4H2O | PCy3 | 16 | 98 |
| 16h | Ni(OAc)2·4H2O | PCy3 | 16 | 83 |
Encouraged by this result, we have tested other nickel(II) sources using the same ligand: with commercial Ni(OAc)2·4H2O and dried Ni(OAc)2,16 the benzaldehyde was totally reduced, whereas with either NiCl2 or NiBr2, no benzylalcohol was detected (Table 1, entries 2–5). We have then screened classical phosphines: with monodentate PPh3, P(o-tolyl)3, or with the bidentate bis-diphenylphosphinoethane (dppe) no conversion was obtained, while the more basic PMe2Ph leads to a full conversion as in the case of PCy3 (Table 1, entries 7–10). Alternatively, N-heterocyclic carbene, such as 1,3-dimesitylimidazol-2-ylidene (Mes2NHC), generated in situ by the addition of n-butyllithium17 was found to be a less active ligand than phosphine for this reaction (entry 11). Moreover, changing the solvent from THF to toluene has a deleterious effect on the conversion (entry 12). Blank experiments were also performed: Ni(OAc)2·4H2O or PCy3 alone did not promote the hydrosilylation reaction (Table 1, entries 13 and 14). Finally, the catalyst loading could be decreased and the limits of the catalytic effectiveness of the system were reached for the reduction of benzaldehyde using 0.1 mol% as only 83% conversion was obtained (entries 15 and 16).
With these optimized conditions in hand, we then examined the scope of the hydrosilylation of aldehydes with the combination of Ni(OAc)2·4H2O (5 mol%) and PCy3 (10 mol%) in refluxing THF for 16 h using 3 equiv. of PMHS.18 Benzaldehydes bearing electron donating substituents such as methoxy- and N,N-dimethylamino groups in the para-position, or a methyl group in the ortho position were fully reduced and the corresponding alcohols obtained in high yields (Table 2, entries 2, 3, and 9). The reduction of the 4-nitrobenzaldehyde was slightly more difficult, as only 83% of the corresponding alcohol was obtained. Interestingly, under such conditions, the reduction is quite selective as only traces of the over-reduced product, namely 4-aminobenzylalcohol, were detected (Table 2, entry 4). For halogenated benzaldehydes (Table 2, entries 5 and 6), under standard conditions, conversions were modest (26% and 43%, respectively, for the p-chloro and p-bromobenzaldehyde). For both the chloro- and bromo-derivatives, low but significant amounts of benzaldehyde and benzylalcohol (3–4%) were detected by GC-MS analysis in the crude mixture after reaction, which shows that reductive dehalogenation occurred during the reaction, probably leading to halogenated nickel species which are inactive for the hydrosilylation reaction. If the quantity of silane is increased to 10 equiv., the rate of the hydrosilylation is increased and the conversion rose, respectively, to 58% and 68%. It must be pointed out that a similar phenomenon was observed with a well-defined nickel-hydride catalyst.14,19 Interestingly, this system is tolerant toward amide and ester functional groups, as shown by the sole reduction of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of the aldehyde (entries 7 and 8). The hydrosilylation of 10-undecenaldehyde was completed, but a mixture of 1
O bond of the aldehyde (entries 7 and 8). The hydrosilylation of 10-undecenaldehyde was completed, but a mixture of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 undecan-1-ol and undecen-1-ols was obtained coming from either the reduction and the isomerisation of the terminal C
1 undecan-1-ol and undecen-1-ols was obtained coming from either the reduction and the isomerisation of the terminal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond.20 This result contrasts with the one obtained for the internal C
C double bond.20 This result contrasts with the one obtained for the internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond, which remained intact in the case of 2,6-dimethylhept-5-enal (entries 10 and 11).13 Heterocyclic aldehydes derived from furan and pyrrole were also nicely converted to the corresponding alcohols (entries 12 and 13). Finally, ferrocenylcarboxaldehyde was totally reduced under these conditions (entry 14).
C double bond, which remained intact in the case of 2,6-dimethylhept-5-enal (entries 10 and 11).13 Heterocyclic aldehydes derived from furan and pyrrole were also nicely converted to the corresponding alcohols (entries 12 and 13). Finally, ferrocenylcarboxaldehyde was totally reduced under these conditions (entry 14).
| Entrya | Substrate | Conversionb (%) | Yield (%) | |
|---|---|---|---|---|
a Typical procedure: Ni(OAc)2·4H2O (5 mol%), THF (4 mL), PCy3 (10 mol%), the aldehyde (2 mmol) and PMHS (3 equiv., 6 mmol) are added in this order and the reaction was stirred at 70 °C for 16 h.b Conversions determined by 1H NMR after methanolysis.c PMHS 10 equiv., 0.5 mmol scale.d 1 mmol scale.e A mixture of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 undecan-1-ol and undecen-1-ols was obtained. 1 undecan-1-ol and undecen-1-ols was obtained. | ||||
| 1 |  | R = H | >97 | 85 |
| 2 | R = OMe | >97 | 95 | |
| 3 | R = NMe2 | >97 | 90 | |
| 4 | R = NO2 | 83 | 70 | |
| 5 | R = Cl | 26 | — | |
| 58c | — | |||
| 6 | R = Br | 43 | — | |
| 68c | — | |||
| 7d | R = COOCH3 | >97 | 83 | |
| 8 | R = NHCOCH3 | >97 | 88 | |
| 9 |  | >97 | 87 | |
| 10 |  | >97 | 77e | |
| 11 | 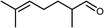 | 95 | 74 | |
| 12 |  | >97 | 70 | |
| 13 |  | 89 | 49 | |
| 14 |  | >97 | 83 | |
Encouraged by these satisfying results, we then explored the reduction of ketones with this nickel catalytic system. Using Ni(acac)2 (5 mol%), PCy3 (10 mol%) in THF at 70 °C, the conversion of acetophenone to 1-phenylethanol was only 10% after 24 h with 4 equiv. of PMHS. Nevertheless, with Ni(OAc)2·4H2O (5 mol%) and PCy3 (10 mol%) in toluene at 100 °C for 24 h, the conversion was complete. We have examined the scope of the reaction using these conditions (Table 3). The reduction of acetophenone substituted at the para position with electron donating groups such as methyl and methoxy leads to the corresponding alcohols with high conversions and good isolated yields (Table 3, entries 2 and 3). As for aldehydes, bromo and chloro ketone derivatives were not suitable for this catalytic system, and no conversion was detected (Table 3, entries 4 and 5). Interestingly, electron-deficient acetophenones were nicely converted to the corresponding alcohols (entries 6–8). Moreover, encumbered ketones, such as 2,4,6-trimethylacetophenone, isobutyrophenone or pivalophenone, are fully reduced, which demonstrated that steric hindrance does not hamper the reaction (entries 9–11). With linear and cyclic aliphatic ketones, the reaction also proceeds in good yields (entries 12–14). It is noteworthy that tetralone and indanone were more difficult substrates to be reduced as the conversions were only of 45% and 79% respectively (entries 15 and 16). With heteroaromatic ketones, the reaction proceeds well with furan derivatives (90% conversion, entry 17) and modestly with pyridine derivatives (61% conversion, entry 18). Unfortunately, with sulfur containing heterocycles, the conversion was very low (11%, entry 19). Finally, 1-ferrocenylethanol can be obtained in moderate yield (55%, entry 20).
| Entrya | Substrate | Conversionb (%) | Yield (%) | |
|---|---|---|---|---|
| a Typical procedure: Ni(OAc)2·4H2O (5 mol%), toluene (4 mL), PCy3 (10 mol%), the ketone (2 mmol) and PMHS (4 equiv., 8 mmol) are added in this order and the reaction was stirred at 100 °C for 16 h.b Conversions determined by 1H NMR after methanolysis. | ||||
| 1 |  | R = H | >97 | 86 |
| 2 | R = Me | >97 | 70 | |
| 3 | R = OMe | >97 | 68 | |
| 4 | R = Br | <5 | — | |
| 5 | R = Cl | <5 | — | |
| 6 | R = F | >97 | 65 | |
| 7 | R = CF3 | 75 | — | |
| 8 | 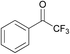 | >97 | 92 | |
| 9 |  | 85 | 79 | |
| 10 | 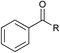 | R = iPr | >97 | 90 |
| 11 | R = tBu | >97 | 95 | |
| 12 |  | >97 | 79 | |
| 13 |  | >97 | 94 | |
| 14 |  | 92 | 70 | |
| 15 |  | 45 | 42 | |
| 16 |  | 79 | 64 | |
| 17 | 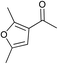 | 90 | 62 | |
| 18 |  | 61 | — | |
| 19 | 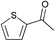 | 11 | — | |
| 20 |  | 74 | 55 | |
In conclusion, we have shown that the combination of air-stable Ni(OAc)2·4H2O and PCy3 in THF can efficiently catalyse the reduction of aldehydes and ketones using cheap PMHS as the reducing reagent. The present methodology is an appealing inexpensive and convenient methodology for the hydrosilylation reaction. We believe that nickel has high potential in homogenous catalysed reduction, thus, further developments to enlarge the scope of this catalytic system, including asymmetric reductions, are under current investigation by our group.
Acknowledgements
We are grateful to the Université de Rennes 1, CNRS, Rennes Métropole and Ministère de l'Enseignement Supérieur et de la Recherche for support, and to Axa Research Fund for a grant to J.Z.Notes and references
- Catalysis without Precious Metals, ed. M. Bullock, Wiley-VCH, Weinheim, 2010 Search PubMed.
- For general reviews about hydrosilylation, see: (a) Comprehensive Handbook on Hydrosilylation, ed. B. Marciniec, Pergamon Press, Oxford, 1992 Search PubMed; (b) Hydrosilylation: A Comprehensive Review on Recent Advances, ed. B. Marciniec, Springer, Heidelberg, 2009 Search PubMed; (c) J.-F. Carpentier and V. Bette, Curr. Org. Chem., 2002, 6, 913–936 CrossRef CAS; (d) O. Riant, N. Mostefaï and J. Courmarcel, Synthesis, 2004, 2943–2958 CrossRef CAS; (e) H. Nishiyama and K. Itoh, in Transition Metals for Organic Synthesis, ed. M. Beller and C. Bolm, Wiley-VCH, Weinheim, 2nd edn, 2004, vol. 2, pp. 182–191 Search PubMed.
- (a) H. Mimoun, J. Org. Chem., 1999, 64, 2582 CrossRef CAS; (b) L. S. Nitzsche and M. Wick, Angew. Chem., 1957, 69, 96 CrossRef; (c) N. J. Lawrence, M. D. Drew and S. M. Bushell, J. Chem. Soc., Perkin Trans. 1, 1999, 3381 RSC; (d) M. Suguro, Y. Yamamura, T. Koike and A. Mori, React. Funct. Polym., 2007, 67, 1264 CrossRef CAS; (e) H. Mimoun and S. A. Firminich, WO pat., 00/37540 A1, 2000 Search PubMed.
- D. Addis, S. Das, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 6004 CrossRef CAS.
- For representative reviews on Cu-catalysed hydrosilylation, see: (a) S. Rendler and M. Oestreich, Angew. Chem., Int. Ed., 2007, 46, 498 CrossRef CAS; (b) C. Deutsch, N. Krause and B. H. Lipshutz, Chem. Rev., 2008, 108, 2916 CrossRef CAS; (c) B. H. Lipshutz, Synlett, 2009, 509 CrossRef CAS.
- Selected examples of Cu-catalysed hydrosilylation: (a) H. Brunner and W. Mehling, J. Organomet. Chem., 1984, 275, C17 CrossRef CAS; (b) B. H. Lipshutz, K. Noson and W. Chrisman, J. Am. Chem. Soc., 2001, 123, 12917 CrossRef CAS; (c) B. H. Lipshutz, A. Lower and K. Noson, Org. Lett., 2002, 4, 4045 CrossRef CAS; (d) J. T. Issenhuth, S. Dagorne and S. Bellemin-Laponnaz, Adv. Synth. Catal., 2006, 348, 1991 CrossRef CAS.
- Selected examples of Zn-catalysed hydrosilylation: (a) H. Mimoun, J. Y. Saint Laumer, L. Giannini, R. Scopelliti and C. Floriani, J. Am. Chem. Soc., 1999, 121, 6158 CrossRef CAS; (b) V. Bette, A. Mortreux, C. W. Lehmann and J.-F. Carpentier, Chem. Commun., 2003, 332 RSC; (c) V. Bette, A. Mortreux, D. Savoia and J.-F. Carpentier, Adv. Synth. Catal., 2005, 347, 289 CrossRef CAS; (d) S. Das, D. Addis, S. Zhou, K. Junge and M. Beller, J. Am. Chem. Soc., 2010, 132, 1770 CrossRef CAS.
- For representative reviews on Fe-catalysed hydrosilylation, see: (a) M. Zhang and A. Zhang, Appl. Organomet. Chem., 2010, 24, 751 CrossRef CAS; (b) K. Junge, K. Schröder and M. Beller, Chem. Commun., 2011, 47, 4849 RSC.
- Selected examples of iron-catalysed hydrosilylation: (a) H. Brunner and K. Fisch, Angew. Chem., Int. Ed. Engl., 1990, 29, 1131 CrossRef; (b) H. Nishiyama and A. Furuta, Chem. Commun., 2007, 760 RSC; (c) N. S. Shaikh, K. Junge and M. Beller, Org. Lett., 2007, 9, 5429 CrossRef CAS; (d) A. M. Tondreau, E. Lobkovsky and P. J. Chirik, Org. Lett., 2008, 10, 2789 CrossRef CAS; (e) D. V. Gutsulyak, L. G. Kuzmina, J. A. K. Howard, S. F. Vyboishichikov and G. I. Nikonov, J. Am. Chem. Soc., 2008, 130, 3732 CrossRef CAS; (f) J. Yang and T. D. Tilley, Angew. Chem., Int. Ed., 2010, 49, 10186 CrossRef CAS; (g) V. V. K. M. Kandepi, J. M. S. Cardoso, E. Peris and B. Royo, Organometallics, 2010, 29, 2777 CrossRef CAS; (h) J. M. S. Cardoso and B. Royo, Chem. Commun., 2012, 48, 4944 RSC; (i) L. C. Misal Castro, D. Bézier, J.-B. Sortais and C. Darcel, Adv. Synth. Catal., 2011, 353, 1279 CrossRef; (j) J. Zheng, L. C. Misal Castro, T. Roisnel, C. Darcel and J.-B. Sortais, Inorg. Chim. Acta, 2012, 380, 301 CrossRef CAS; (k) L. C. Misal Castro, J.-B. Sortais and C. Darcel, Chem. Commun., 2012, 48, 151 RSC; (l) D. Bézier, G. T. Venkanna, J.-B. Sortais and C. Darcel, ChemCatChem, 2011, 3, 1747 CrossRef; (m) F. Jiang, D. Bézier, J.-B. Sortais and C. Darcel, Adv. Synth. Catal., 2011, 353, 239–244 CrossRef CAS; (n) S. Zhou, K. Junge, D. Addis, S. Das and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 9507 CrossRef CAS; (o) Y. Sunada, H. Kawakami, Y. Motoyama and H. Nagashima, Angew. Chem., Int. Ed., 2009, 48, 9511 CrossRef CAS; (p) D. Bézier, G. T. Venkanna, L. C. Misal Castro, J. Zheng, T. Roisnel, J.-B. Sortais and C. Darcel, Adv. Synth. Catal., 2012, 354, 1879 CrossRef; (q) H. Jaafar, H. Li, L. C. Misal Castro, J. Zheng, J.-B. Sortais and C. Darcel, Eur. J. Inorg. Chem., 2012, 3546 CrossRef CAS.
- Examples of Ni-catalysed hydrosilylation of carbonyls derivatives: (a) F.-G. Fontaine, R.-V. Nguyen and D. Zargarian, Can. J. Chem., 2003, 81, 1299 CrossRef CAS; (b) T. Irrgang, T. Schareina and R. Kempe, J. Mol. Catal. A: Chem., 2006, 257, 48 CrossRef CAS; (c) Y. K. Kong, J. Kim, S. Choi and S.-B. Choi, Tetrahedron Lett., 2007, 48, 2033 CrossRef CAS; (d) S. Chakraborty, J. A. Krause and H. Guan, Organometallics, 2009, 28, 582 CrossRef CAS; (e) B. L. Tran, M. Pink and D. J. Mindiola, Organometallics, 2009, 28, 2234 CrossRef CAS; (f) S. Kundu, W. W. Brennessel and W. D. Jones, Inorg. Chem., 2011, 50, 9443 CrossRef CAS.
- Example of Ni-catalysed hydrosilylation of imine derivatives: A. H. Vetter and A. Berkessel, Synthesis, 1995, 419 CrossRef CAS.
- Examples of Ni-catalysed hydrosilylation of alkenes and/or alkynes: (a) M. R. Chaulagain, G. M. Mahandru and J. Montgomery, Tetrahedron, 2006, 62, 7560 CrossRef CAS; (b) J. Berding, J. A. van Paridon, V. H. S. van Rixel and E. Bouwman, Eur. J. Inorg. Chem., 2011, 2450 CrossRef CAS; (c) M. I. Lipschutz and T. D. Tilley, Chem. Commun., 2012, 48, 7146 RSC.
- For examples of selective Ni-catalysed hydrosilylation of alkenes and/or alkynes versus C–heteroatom multiple bonds, see: (a) P. Boudjouk, B.-H. Han, J. R. Jacobsen and B. J. Hauck, J. Chem. Soc., Chem. Commun., 1991, 1424 RSC; (b) P. Boudjouk, S.-B. Choi, B. J. Hauck and A. B. Rajkumar, Tetrahedron Lett., 1998, 39, 3951 CrossRef CAS.
- L. P. Bheeter, M. Henrion, L. Brelot, C. Darcel, M. J. Chetcuti, J.-B. Sortais and V. Ritleng, Adv. Synth. Catal., 2012 DOI:10.1002/adsc.201200460.
- V. P. Ananikov, K. A. Gayduk, Z. A. Starikova and I. P. Beletskaya, Organometallics, 2010, 29, 5098 CrossRef CAS.
- Ni(OAc)2·4H2O “99.998% trace metals basis” purchased from Aldrich was used to performed this study, ICP analysis was also performed on this salt, see ESI.† For critical review about impurities in catalysis see: I. Thomé, A. Nijs and C. Bolm, Chem. Soc. Rev., 2012, 41, 979 RSC.
- (a) E. Buitrago, L. Zani and H. Adolfsson, Appl. Organomet. Chem., 2011, 25, 748 CrossRef CAS; (b) E. Buitrago, F. Tinnis and H. Adolfsson, Adv. Synth. Catal., 2012, 354, 217 CrossRef CAS.
- The use of 1 mol% of Ni(OAc)2·4H2O and 2 mol% of PCy3 led to a full conversion for the reduction of p-methoxybenzaldehyde, but only to 57% conversion for p-nitrobenzaldehyde while when using 5 mol% of nickel salt and 10 mol% of phosphine, the conversion for the nitro derivative rose to 87% (Table 2, entry 4). Indeed, these latter conditions were used as standard conditions for the scope of the reaction.
- J. Breitenfeld, R. Scopelliti and X. Hu, Organometallics, 2012, 31, 2128 CrossRef CAS.
- (a) L. W. Gosser and G. W. Parshall, Tetrahedron Lett., 1971, 12, 2555 CrossRef; (b) A. Wille, S. Tomm and H. Frauenrath, Synthesis, 1998, 305 CrossRef CAS; (c) H. J. Lim, C. R. Smith and T. V. RajanBabu, J. Org. Chem., 2009, 74, 4565 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details and NMR spectra. See DOI: 10.1039/c2cy20509b |
| This journal is © The Royal Society of Chemistry 2013 |
