Pt/C catalysed direct reductive amination of nitriles with primary amines in a continuous flow multichannel microreactor†
Sumeet K.
Sharma
ab,
James
Lynch
a,
Anna M.
Sobolewska
b,
Pawel
Plucinski
*b,
Robert J.
Watson
c and
Jonathan M. J.
Williams
*a
aDepartment of Chemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK. E-mail: j.m.j.williams@bath.ac.uk; Fax: +44 (0)1225 386231; Tel: +44 (0)1225 383942
bDepartment of Chemical Engineering, University of Bath, Claverton Down, Bath BA2 7AY, UK. E-mail: p.plucinski@bath.ac.uk; Fax: +44 (0)1225 385713; Tel: +44 (0)1225 386961
cGlaxoSmithKline Research and Development, Gunnels Wood Road, Stevenage, SG1 2NY, UK. E-mail: robert.j.watson@gsk.com
First published on 1st November 2012
Abstract
Aliphatic and aromatic secondary amines were synthesised selectively by one pot reductive amination of nitriles with primary amines using Pt/C (3% by weight) catalyst in a continuous flow multichannel microreactor. Molecular hydrogen was used as a clean reducing agent at moderate reaction conditions.
Consecutive reactions in one pot are an appealing way to reduce the number of isolation steps involved in a synthetic sequence, although the selectivity of product formation can be a major problem with such processes. Amines are important intermediates for pharmaceuticals, agrochemicals and dyes industries.1 Several methods have been developed for the synthesis of amines in batch reactors such as, amination of aryl halides,2 hydroamination,3 hydroaminomethylation of olefins,4 reduction of nitriles,5 reductive amination of aldehydes or ketones6 and reductive monoalkylation of primary amines7 using either borohydrides or molecular hydrogen as a reducing agent. The use of borohydride as a reducing agent is not atom efficient and environmental friendly. Therefore, development of atom efficient processes for the selective synthesis of substituted amines still remains a challenging task.
One pot reductive amination of nitriles can serve as a promising route for the synthesis of substituted amines in the presence of molecular hydrogen as a reducing agent. This approach eliminates the step of isolating the intermediate imine and minimises waste production due to the use of molecular hydrogen as a reducing agent. Only a few examples of reductive amination of nitriles with amines have been reported in the literature8 because nitriles are less reactive in hydrogenation conditions, therefore, selectivity control of product formation can be challenging. Most commonly Pd/C has been used as a catalyst for reductive amination of nitrile in batch reactors. To the best of our knowledge, this is the first report on the use of Pt/C as a catalyst for reductive amination of nitriles with primary amines in a continuous flow compact reactor.‡
In the present communication we report an efficient one pot reductive amination of various nitriles with aliphatic and aromatic amines for the synthesis of substituted amines (Scheme 1) in a continuous flow multichannel microreactor9 (Fig. 1, ESI†). Mesoporous microspherical carbon was used as a catalyst support in the present study which has advantages including a lack of mass transfer resistance and easy removal from the reactor channel after completion of reaction.
 | ||
| Scheme 1 Reductive amination of nitriles with amines. | ||
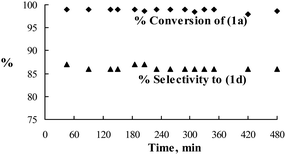 | ||
| Fig. 1 Time on stream data for reductive amination of 1a with 1c. | ||
Preliminary experiments were carried out to compare the activity of Pd/C and Pt/C catalysts under identical reaction conditions for the reductive amination of benzonitrile (1a) with butylamine (1c). The two catalysts gave comparable conversion of 1a into products, however, higher selectivity of the desired product, 1d was observed for Pt/C catalyst (Table 1, ESI†). Therefore, Pt/C was selected as a catalyst for further experiments. Choice of a suitable solvent is necessary in the flow reactor not only to avoid precipitation of reactants/products mixture which leads to blockage in the reactor but also to achieve higher conversion and selectivity. Initial experiments were carried out using methanol, 2-propanol, dichloromethane and toluene as solvents (Table 2, ESI†) for reductive amination of (1a) with (1c). Toluene and methanol gave similar results in terms of conversion and selectivity to product (1d). Lower conversion of 1a was observed in the case of 2-propanol and dichloromethane solvents. No by-products were seen other than benzylamine (1e) and dibenzylamine (1f). Therefore, toluene was selected as a solvent for further study.
| Entry | Nitrile (a) | Amine (c) | Product (d) | (a)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (c) (c) |
Conversion of (a) (%) | Selectivity (%) | ||
|---|---|---|---|---|---|---|---|---|
| (d) | (e) | (f) | ||||||
| a Catalyst (3% Pt/C) = 510 mg, concentration of (a) = 0.84 mol L−1, hydrogen flow rate = 17 mL min−1, pressure = 6 bar, T = 105 °C, solvent = toluene, flow rate of liquid = 0.1 mL min−1. | ||||||||
| 1 |

|

|

|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 | 86 | 1 | 12 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
99 | 93 | 3 | 4 | |||
| 3 |

|
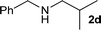
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 | 86 | 5 | 9 | |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
90 | 93 | 2 | 5 | |||
| 5 |

|

|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 | 87 | 2 | 11 | |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
96 | 95 | 2 | 1 | |||
| 7 |

|

|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97 | 92 | 2 | 5 | |
| 8 |

|

|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 | 98 | 1 | 0 | |
| 9 |

|

|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98 | 90 | 1 | 8 | |
| 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
97 | 94 | 3 | 3 | |||
| 11 |
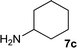
|

|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
86 | 88 | 3 | 5 | |
| 12 |

|
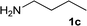
|
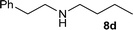
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97 | 87 | 0 | 13 |
| 13 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
90 | 96 | 0 | 4 | |||
| 14 |

|

|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 | 88 | 4 | 7 | |
| 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
88 | 90 | 3 | 7 | |||
| 16 |

|

|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97 | 87 | 1 | 12 | |
| 17 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
91 | 96 | 1 | 3 | |||
| 18 |

|
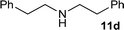
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98 | 99 | 0 | 0 | |
| 19 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
94 | 99 | 0 | 0 | |||
| 20 |

|

|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98 | 95 | 1 | 3 | |
| 21 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
96 | 96 | 2 | 1 | |||
| 22 |
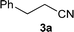
|

|

|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 | 87 | 2 | 10 |
| 23 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
85 | 90 | 2 | 8 | |||
| 24 |

|

|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 | 87 | 1 | 10 | |
| 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
91 | 87 | 3 | 1 | |||
| 26 |
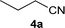
|

|

|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
65 | 72 | 0 | 22 |
| 27 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
58 | 97 | 0 | 1 | |||
| 28 |

|
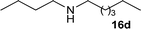
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
60 | 80 | 0 | 15 | |
| 29 |

|
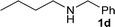
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
88 | 80 | 0 | 18 | |
| 30 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
83 | 96 | 0 | 2 | |||
| 31 |

|

|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
70 | 96 | 3 | 0 | |
| 32 |

|

|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
78 | 93 | 0 | 4 | |
| 33 |

|

|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
32 | 80 | 0 | 20 | |
| Entry | Amine product | Isolated yielda (%) |
|---|---|---|
| a Isolated yield after column chromatography. | ||
| 1 |

|
81 |
| 2 |

|
93 |
| 3 |

|
72 |
| 4 |
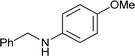
|
76 |
| 5 |
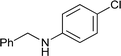
|
67 |
| 6 |

|
70 |
| 7 |
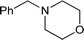
|
81 |
| 8 |
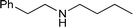
|
74 |
Initially, the one pot reductive amination of benzonitrile (1a) was achieved with almost complete conversion (99%) in the reaction with butylamine (1c) using a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The selectivity was good, although 12% of dibenzylamine was observed as a by-product. However, by increasing the amount of butylamine present to three equivalents, the amount of self-coupled product was reduced to 4%. In all cases, using an excess of the amine led to a higher selectivity for product formation. This is consistent with the intermediates identified in Scheme 1, where any benzylamine (1e) formed as a by-product from over reduction of benzonitrile (1a) is less able to compete with the excess of butylamine (1c) present for amination of the initially formed imine (1b).
1. The selectivity was good, although 12% of dibenzylamine was observed as a by-product. However, by increasing the amount of butylamine present to three equivalents, the amount of self-coupled product was reduced to 4%. In all cases, using an excess of the amine led to a higher selectivity for product formation. This is consistent with the intermediates identified in Scheme 1, where any benzylamine (1e) formed as a by-product from over reduction of benzonitrile (1a) is less able to compete with the excess of butylamine (1c) present for amination of the initially formed imine (1b).
We were pleased to find that a range of nitriles and amines underwent reductive coupling to give di-substituted amines with very good conversions and selectivities (Table 1). Benzonitrile (1a) was converted into a range of N-benzylamines (Table 1, entries 1–11), where only cyclohexylamine gave less than 90% conversion under these reaction conditions, possibly for steric reasons. The use of phenylacetonitrile (2a) and phenylpropionitrile (3a) led to similar results, with the formation of the reductively coupled products with good conversions and selectivities (Table 1, entries 12–25). Although good selectivities could be obtained using butyronitrile (4a) when an excess of amine was present, the conversions were lower.
The Pt/C catalyst is quite stable. No drop in the conversion of 1a and selectivity of 1d was observed up to 480 min time on stream data for reductive amination of 1a with 1c (Fig. 1). The main advantages of the continuous flow microreactor in the present study are high catalyst loading and low residence time (less than 10 seconds) as compared with 2–12 h in batch reactors.8
For several cases, we chose to purify the amine products by column chromatography and were pleased to find that the products could be obtained in good isolated yield. Benzonitrile was converted into a range of amines in up to 93% isolated yield (Table 2, entries 1–7) and phenylacetonitrile also underwent reductive amination with a satisfactory yield (Table 2, entry 8).
Conclusions
In summary, various substituted secondary amines were synthesised by a Pt/C catalysed one pot selective reductive amination of aromatic and aliphatic nitriles with primary amines in a continuous flow multichannel microreactor using molecular hydrogen as a reducing agent.We thank the EPSRC for funding through grant EP/G028141/1 and GlaxoSmithKline for additional support.
Notes and references
- S. A. Lawrence, Amines: Synthesis, Properties, and Application, Cambridge University Press, Cambridge, 2004 Search PubMed.
- (a) S. L. Buchwald, C. Mauger, G. Mignani and U. Scholz, Adv. Synth. Catal., 2006, 348, 23 CrossRef CAS; (b) J. F. Hartwig, Synlett, 2006, 1283 CrossRef CAS.
- (a) K. C. Hultzsch, D. V. Gribkov and F. Hampel, J. Organomet. Chem., 2005, 690, 4441 CrossRef CAS; (b) J. F. Hartwig, Pure Appl. Chem., 2004, 76, 507 CrossRef CAS; (c) T. E. Muller and M. Beller, Chem. Rev., 1998, 98, 675 CrossRef.
- (a) P. Eilbracht, L. Barfacker, C. Buss, C. Hollmann, B. E. Kitsos-Rzychon, C. L. Krannemann, T. Rische, R. Roggenbuck and A. Schmidt, Chem. Rev., 1999, 99, 3329 CrossRef CAS; (b) A. Moballigh, A. Seayad, R. Jackstell and M. Beller, J. Am. Chem. Soc., 2003, 125, 10311 CrossRef; (c) A. Seayad, M. Ahmed, H. Klein, R. Jackstell, T. Gross and M. Beller, Science, 2002, 297, 1676 CrossRef CAS.
- (a) H. Greenfield, Ind. Eng. Chem. Prod. Res. Dev., 1976, 15, 156 CrossRef CAS; (b) Y. Huang and W. M. H. Sachtler, J. Catal., 1999, 188, 215 CrossRef CAS; (c) S. Enthaler, D. Addis, K. Junge, G. Erre and M. Beller, Chem.–Eur. J., 2008, 14, 9491 CrossRef CAS; (d) L. Hegedus and T. Mathe, Appl. Catal., A, 2005, 296, 209 CrossRef CAS.
- (a) S. Gomez, J. Peters and T. Maschmeyer, Adv. Synth. Catal., 2002, 344, 1037 CrossRef CAS; (b) A. Abdel-Magid and S. Mehrman, Org. Process Res. Dev., 2006, 10, 971 CrossRef CAS.
- (a) H. Sajiki, T. Ikawa and K. Hirota, Org. Lett., 2004, 6, 4977–4980 CrossRef CAS , and references cited therein; (b) R. Nacario, S. Kotakonda, D. M. D. Fouchard, L. M. V. Tillekeratne and R. A. Hudson, Org. Lett., 2005, 7, 471 CrossRef CAS; (c) E. Byun, B. Hong, K. D. Castro, M. Lim and H. Rhee, J. Org. Chem., 2007, 72, 9815 CrossRef CAS; (d) M. H. S. A. Hamid and J. M. J. Williams, Chem. Commun., 2007, 725 RSC; (e) X. Cui, Y. Zhang, F. Shi and Y. Deng, Chem.–Eur. J., 2011, 17, 2587 CrossRef CAS.
- (a) P. N. Rylander, L. Hasbrouck and I. Karpenko, Ann. N. Y. Acad. Sci., 1973, 214, 100 CrossRef CAS; (b) M. K. S. Vink, C. A. Schortinghuis, A. Mackova-Zabelinskaja, M. Fechter, P. Pochlauer, A. M. C. F. Castelijns, J. H. van Maarseveen, H. Hiemstra, H. Griengl, H. E. Schoemaker and F. P. J. T. Rutjes, Adv. Synth. Catal., 2003, 345, 483 CrossRef CAS; (c) R. Juday and H. Adkins, J. Am. Chem. Soc., 1955, 77, 4559 CrossRef CAS; (d) T. Witzel and E. Fuchs, US Patent, 5 557 011, 1996, to BASF Germany Search PubMed; (e) W. Reif, M. Hesse, H. Zimmermann and H. Lingk, US Patent, 5 648 545, 1997, to BASF Germany Search PubMed.
- (a) P. K. Plucinski, D. V. Bavykin, S. T. Kolaczkowski and A. A. Lapkin, Ind. Eng. Chem. Res., 2005, 44, 9683 CrossRef CAS; (b) X. Fan, A. A. Lapkin and P. K. Plucinski, Catal. Today, 2009, 147S, S313 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental rig and procedure, results for screening of catalysts and solvents, GC chromatograms. See DOI: 10.1039/c2cy20431b |
| ‡ Brief experimental procedure: in a typical experimental run, nitrile and primary amine were premixed at the desired concentration in toluene and charged to the feed vessel. Pt/C (3 wt% Pt) as a catalyst was filled in the 3 × 3 mm channel. The reaction mixture from the feed vessel was fed into the reactor using an HPLC pump (Kontron) at 0.1 mL min−1 flowrate. Hydrogen gas was supplied to the reactor using mass flow controller (Brooks) at 17 mL min−1 flowrate and 6 bar pressure. Constant temperature (105 °C) of the reactor was maintained by circulating heat transfer fluid through the micro-heat exchangers using a recirculating bath (Haake). The pressure of the reactor was controlled by a back pressure regulator (Brooks) and pressure drop across the reactor was monitored using a differential pressure transducer (Bronkhorst). After completion of the reaction, the reaction mixture was collected by a high pressure valve. Analysis of reaction mixture was carried out by gas chromatography (Varian 3900) equipped with CP-Sil 5CB capillary column (15 m length and 0.25 mm dia.) and GC-MS. |
| This journal is © The Royal Society of Chemistry 2013 |

