An overview of catalytic conversion of vegetable oils/fats into middle distillates
J. K.
Satyarthi
,
T.
Chiranjeevi
*,
D. T.
Gokak
and
P. S.
Viswanathan
Corporate R&D Centre, Bharat Petroleum Corporation Ltd, India. E-mail: chiranjeevit@bharatpetroleum.in; chiranjeevi13@yahoo.co.in; Fax: +91 120-2354172; Tel: +91 120-2354118
First published on 4th September 2012
Abstract
Due to scarce conventional petroleum fuel, there is a need to search for alternative renewable fuel that can be adopted for use in place of fossil fuel oil. Even the ester-based biodiesel has many limitations, such as low energy content, oxidative stability etc. Renewable hydrocarbon-based diesel obtained by hydroprocessing of vegetable oils/fats (2nd generation biodiesel) is one of the alternatives that is getting attention from both academia and industry. The present paper reviews the emerging processes, catalysts and operating conditions for the catalytic conversion of vegetable oils/fats into hydrocarbon-based diesel via hydroprocessing. Hydroprocessing of vegetable oils is similar to petroleum hydroprocessing, but requires slight modification and optimization in the catalyst, as well as process parameters. The current status and future scope of the catalyst and process for hydroprocessing of vegetable oil are discussed.
1. Introduction
The main sources of fuel and energy for transportation and manufacturing sectors are natural gas, petroleum and coal. These are non-renewable fossil fuels with limited sources and are depleting fast under the pressure of high energy and fuel demand. Besides this, carbon dioxide emission from fossil fuels is another issue. Fulfilling energy demand while keeping the emission under control is a major challenge. Improving energy efficiency, reducing carbon dioxide emission, energy saving and developing clean and fuel efficient vehicles are a few options in this direction. Besides these, alternative renewable energy sources are the other option. Among renewable energy resources, vegetable oils/fats are a good source for liquid transportation fuel due to their high energy density. Vegetable oil occupies a prominent position in the development of alternative fuels. India, being an agriculture-based country, produces around 6.7 × 106 tons of non-edible oils, such as Jatropha, linseed, castor, karanj (Pongamia Pinnata), Neem (Azadirachta indica), Palash (Butea monosperma) and Kusum (Schelchera Trijuga). These oils can be used as alternative fuels in compression ignition engines after modifying the fuel structure or properties or modifying the engine.1–5In the literature, mainly the research work is aimed at ester-based biodiesel, which is obtained by transesterification of vegetable oils/fats with methanol.6–11 Oxidative stability, low energy content and cold-flow properties are the major factors that work against the use of ester-based biodiesel. A different processing route to convert vegetable oils into a high quality diesel fuel or diesel blend stock that is fully compatible with petroleum-derived diesel fuel is desired.12 The new types of bio-renewable fuels entering the fuel market must satisfy the following conditions:13
(1) It can be used in existing engines without any modification.
(2) Engine power should not be affected.
(3) It must be blended with petro-diesel in any proportion and can be easily integrated into the existing market structure.
(4) It should be of high quality and should form environmentally cleaner combustion products.
Conversion of vegetable oils directly into deoxygenated liquid fuel addresses most of the above discussed issues. Hydrodeoxygenation or hydroconverion of triglycerides directly results in diesel-like hydrocarbon fuel. Hence, it is most ideal and also the shortest route to arrive at biofuel that will have identical properties to those of conventional diesel fuels. Hydrocarbons can be prepared from vegetable oils by the following pathways:14
(a) Conversion of vegetable oils to soaps (sodium salt) by hydrolysis, followed by dry distillation of the soap in the presence of soda lime15
(b) Hydrolysis of vegetable oils into fatty acids and then decarboxylation of the fatty acids in the presence of catalysts
(c) Thermal/catalytic pyrolysis
(d) Hydroprocessing of vegetable oils.
Routes generally used for obtaining deoxygenated biofuel from triglycerides include thermal and catalytic pyrolysis,16–19 but these processes provide low atom economy, poor selectivity due to uncontrollable side reactions, such as cracking or polymerization of the hydrocarbons, and formation of undesired hydrocarbon gases coupled with a poor yield of diesel range product.20
The emerging technology for the conversion of triglycerides into hydrocarbon-based diesel fuel is the hydroprocessing of vegetable oils. The hydroprocessing process requires hydrogen gas to selectively eliminate the linkage oxygen as water and saturate the double bonds present in vegetable oils. The resulting products are the respective diesel-like hydrocarbons. Paraffins obtained by hydroprocessing of vegetable oil and fats is sometimes termed as second generation biodiesel or green diesel.21 Unlike the first generation (ester-based biodiesel), this new generation biodiesel (hydrocarbons) is more favorable for industrial applications, being compatible with the current engines, even avoiding the need to be blended with diesel if isomerised.22,23
2. Production of deoxygenated biofuel from vegetable oil
2.1. Thermal/catalytic pyrolysis
Pyrolysis of vegetable oil is well-studied and its use to produce fuels dates back 100 years.24,25 Similar to biodiesel, pyrolysis will work on a variety of feedstocks, both edible and non-edible. However, pyrolysis only requires a pre-drying step for use with soybean soapstock.26 The fuel obtained by pyrolysis of different triglycerides was used in different countries during the First and Second World Wars. For instance, a Tung oil pyrolysis batch system was used in China as a hydrocarbon supply during World War II.27 These hydrocarbons were used as raw materials for gasoline and diesel-like fuel production in a cracking system similar to the petroleum process now in use. Since then, several studies on vegetable oil pyrolysis as an alternative method to obtain chemicals and fuels have been reported in the literature.28–32 Pyrolysis of triglycerides can be divided into direct thermal cracking and catalytic cracking. Complex mixtures of products are obtained by thermal cracking. In thermal cracking, distribution of product constituents significantly depends upon reaction variables such as the reactor type, residence time, the composition of feedstock and reaction temperature, as well as the collection procedure and analytical techniques used.33 The results of these studies provide an idea about the type of products can be achieved from this process. It is evident that liquid mixtures with high percentages of hydrocarbons can be obtained, but in many of these studies, the presence of undesirable oxygenated compounds, such as carboxylic acids and ketones, was also reported.33 The effect of reaction conditions on the final product distribution needs to be studied more extensively so that the reaction may be optimized to obtain the desired product.Vegetable and plant oils contain a complex mixture of unsaturated and saturated triglycerides. Idem et al. studied the thermal cracking of canola oil in the presence and absence of steam and postulated a reaction scheme to account for the thermal cracking of both the unsaturated and saturated components.32 The reaction in Scheme 1 proposed by Chang et al.34 consists of various steps and reactions involved in thermal pyrolysis. Other researchers, such as Alencar,35 Kitamura,36 Nawar,37 Nichols and Holman,38 have also proposed the mechanisms for this reaction. The mechanism proposed by Schwab et al.28 also accounts for the formation of heavy oxygenated compounds, including ketones, aldehydes and esters, carbon monoxide and carbon dioxide, C1–C5 straight and branched chain hydrocarbons, alcohols and dimethyl ether, diolefinic, cyclic and acetylenic hydrocarbon gases, C6+ aliphatic hydrocarbons, aromatics, heavy hydrocarbons, coke and hydrogen during the cracking of canola oil in the absence of a catalyst. The degree of unsaturation of the triglyceride has a significant effect on the cracking behavior. Decarboxylation and decarbonylation can occur before or after the C–C bond cleavage. If the triglyceride is unsaturated, the cleavage most likely occurs before the decarboxylation and decarbonylation. It has also been shown that C–C bond cleavage for unsaturated and saturated molecules results in different products.32 Thermal decomposition of triglycerides produces different compounds, which include alkanes, alkenes, alkadienes, aromatics and carboxylic acids. Different types of vegetable oils produce large differences in the composition of the thermally decomposed oil. Scheme 2 outlines a scheme that accounts for the formation of alkanes, alkenes, alkadienes, aromatics and carboxylic acids from pyrolysis of triglycerides.28 Mechanisms for the thermal decomposition of triglycerides are likely to be complex because of the many structures and multiplicity of possible reactions of mixed triglycerides. Generally, thermal decomposition of these structures proceeds through either a free-radical or carbonium ion mechanism.35 Formation of a homologous series of alkanes and alkenes can be accounted for by the generation of the “RCOO˙” radical from the triglyceride cleavage and subsequent loss of carbon dioxide. The ‘R˙’ radical, upon disproportionation and ethylene elimination, gives the odd-numbered carbon alkanes and alkenes. The presence of unsaturation enhances cleavage at a position α, β to the unsaturation. Positional isomerization and subsequent cleavage could account for the higher amounts of C5–C10 alkanes obtained from safflower compared with soybean oil.40 The formation of aromatics is supported by a Diels–Alder addition of ethylene to a conjugated diene formed in the pyrolysis reaction.37 Carboxylic acids formed during the pyrolysis of vegetable oils probably result from cleavage of the glyceride moiety.
 | ||
| Scheme 1 Decomposition of vegetable oils.33 | ||
Pyrolysis studies were carried out in the absence of a catalyst using oil from soybean,28 palm tree,35 babassu,35 pequi,35 macauba,30,31 and canola32 as raw materials. Thermal cracking of vegetable oils is generally carried out at temperatures of 350–500 °C or higher and at moderate pressures (atmospheric to several bar) to yield around 70% hydrocarbons. As a rule, an increase in pyrolysis temperature leads to an increase in the selectivity towards the formation of light hydrocarbons. The liquid fractions of the thermally decomposed vegetable oil are similar to diesel fuels. The pyrolyzed soybean oil contains 79% carbon and 12% hydrogen.28,40
To improve the yield and selectivity of the desired product, triglycerides are treated at high temperature in the presence of a catalyst. Processing of vegetable oil at high temperature is divided into two categories; in the presence of hydrogen or in the absence of hydrogen. Processing in the presence of hydrogen is known as hydroprocessing or hydrotreatment of vegetable oils, which will be discussed in detail in the next section. By processing in the absence of hydrogen, more gasoline-type products are obtained containing significant amount of aromatics and gaseous products. Pyrolyzed oil has low viscosity and a high cetane number compared to pure vegetable oils. The cetane number of pyrolyzed soybean oil is enhanced to 43 from 37.9 and the viscosity is reduced to 10.2 cSt from 32.6 at 38 °C,28,41 but it exceeds the specified value of 4.5 cSt (IS 1460: 2005). The pyrolysed vegetable oils possess acceptable amounts of sulphur, water and sediment and give acceptable copper corrosion values, but unacceptable ash, carbon residue amounts and pour point. Gas and liquid products characterization has been reported in the literature30–32,40 and some reaction pathways have also been proposed. Many studies have focused on improving the performance of standard catalytic cracking procedures for application to vegetable oils using standard or modified cracking catalysts, such as those based on basic oxides,42 activated alumina,43 aluminosilicates,44–46 zeolites47–49 and pillared clays.50 In recent years, some new types of catalysts based on H-ZSM-551 and MCM-4152,53 have been developed and used in a pyrolysis reaction. It was observed that the product in this case has lower viscosity and a higher cetane number (>50) compared to pure soybean oil.54 It is worth mentioning that the pyrolytic oils exhibited high carboxylic acid content (acid number 116–133). These undesired products have great effects on the corrosion value, cold filter plugging point and freezing point of the biofuel. Pyrolyzed oil also contains significant amount of olefins, which increase the oxidative instability and it needs to be upgraded by hydrogenation. Low yield 45–65% and selectivity to the desired hydrocarbon range are the major issues with pyrolysis.55
2.2. Hydroprocessing
Renewable hydrocarbon-based diesel obtained by hydroprocessing is also termed as second generation biodiesel or green diesel. Table 1 compares various properties of ester-based and hydrocarbon-based renewable diesel. Hydroprocessing of vegetable oils was proposed several years ago, using processes and catalysts similar to those used for middle distillate hydrotreatment.56 There is renewed interest for this route for several reasons. The primary product is straight-chain paraffins, which can be hydroisomerized to high quality diesel or hydrocracked to kerosene. The gas by-product propane is feedstock for the petrochemical industry and also used as motor fuel.57 A large variety of vegetable oils, including edible, non-edible and animal fats, can be processed to yield the same high quality diesel product. Finally, co-processing with crude oil-derived middle distillates is possible and even favorable.| Properties | Biodiesel | Green diesel |
|---|---|---|
| Chemical composition | Methyl or ethyl ester of fatty acids | Saturated hydrocarbons |
| Oxygen content | 10–12% | Nil |
| Density (g ml−1) | 0.880 | 0.780 |
| Heating value (KJ g−1) | 38 | 45 |
| Cloud point (°C) | −5 | −5 to +30 |
| Sulfur content (ppm) | <1 | <1 |
| Oxidative stability | Unstable | Stable |
| Cetane number | 50–65 | 70–90 |
| Feedstock flexibility | Good quality | More feedstock flexibility |
| Production facility | Production require new facility | Can be produced in existing refinery |
| Engine modification | Some changes required in existing diesel engine | No change is required |
This is an emerging area and several authors have reviewed the work in this area58,59 and have tried to elucidate the mechanism of the hydroprocessing of vegetable oils into diesel. It is generally acknowledged that the triglycerides are first get saturated on their side chain, followed by scission of the C–O bond, leading to the formation of diglycerides, monoglycerides, carboxylic acids and waxes. Then, these are transformed into hydrocarbons by hydrotreatment. Removal of oxygen is accomplished through hydrodeoxygenation (HDO) and other direct mechanisms, such as hydrodecarbonylation (HDCN), hydrodecarboxylation (HDCX) and hydrogenation (Scheme 3).39
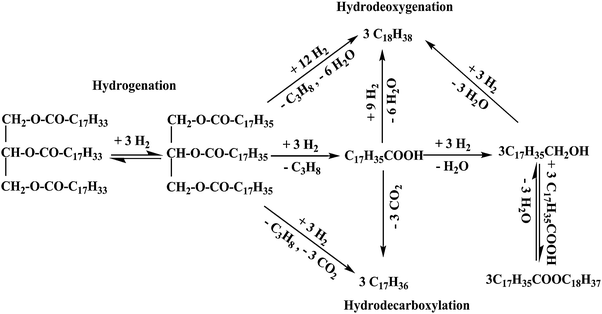 | ||
| Scheme 3 Hydroprocessing of vegetable oils.38 | ||
The predominant hydroconverted products are n-heptadecane and n-octadecane and byproducts, carbon monoxide, propane, carbon dioxide and water, are obtained. Due to the acid function of the catalyst, isomerization and cyclization of the olefin intermediates can occur, leading to the formation of isoparaffins and naphthenes. Aromatics may also be observed when low severity hydrogenation reactions are carried out. Dehydrogenation is favored at high temperature and low pressure.60
Hydroprocessing is used in the refinery to remove S, N and metals from petroleum-derived feedstock, including heavy gas–oil or vacuum gas–oil. These processes and existing refinery facilities can be used for vegetable oils and fats hydroprocessing. These processes can be sometimes called hydrodeoxygenation when applied to oxygenated hydrocarbons, such as vegetable oils or FFAs, as they lead to the removal of oxygen by hydrogenation, decarboxylation and decarbonylation. Therefore, fully deoxygenated high cetane hydrocarbons suitable for use in diesel engines are formed. In hydroprocessing, maximum hydrocarbon yield is obtained via hydrodeoxygenation as compared to decarboxylation, where one carbon is lost as carbon dioxide. With respect to the fractional composition, the products of thermo-catalytic cracking represent a suitable alternative to gasoline rather than to diesel. Even if the catalytic cracking process is optimized, the yields of biodiesel are relatively low. Furthermore, these products often contain carboxylic acids and other oxygenates, which could complicate their use with respect to storage and distribution and also with respect to their combustion in engines. On the other hand, hydroprocessing as compared to pyrolysis provides high conversion of vegetable oils, as well as high yields of hydrocarbon middle distillates, which can be used as an alternative diesel fuel.
| S. no. | Catalyst | Feed | Conditions | Ref. |
|---|---|---|---|---|
| 1 | 5% Pd/C (Aldrich) | Oleic acid | P = 15–27 bar, T = 573–633 K, time = 6 h | 61 |
| 2 | 4% Pd/C mesoporous | Stearic and palmitic acid | T = 573 K, P = 17 bar (5% H2 in Ar), | 62 |
| 3 | 1% Pt-Re/H-ZSM-5 | Vegetable oil | P = 65 bar (85% H2 in N2), T = 543 K, time =12 h | 63 |
| 4 | 1% Pt/Al2O3 | Methyl stearate | P = 6.9 bar (H2), T = 598 K, time = 5 h | 64 |
| 5 | Ni–Mo/SiO2 or Al2O3 | Jatropha oil | H2/oil = 800 mL/mL, LHSV = 7.6 h−1, T = 623 K | 65 |
| 6 | Topsøe TK-575 Ni–Mo/γ-alumina | Rapeseed (15% in n-heptane) | P = 45 bar, T = 623 K, LHSV = 1.5 h−1, | 66 |
| 7 | PtSnK/silica PtSn/silica Pt/silica | Methyl ester and triglyceride | P = 24.1 bar, T = 350 °C, | 67 |
| 8 | Pd/SAPO-31 | Sunflower oil | T = 310–360 °C, P = 20 bar, WHSV = 0.9–1.6 h−1. | 68 |
| 9 | Ni–Mo/Al2O3 | Waste cooking oil | T = 25–450 °C, P = 0–150 bar, LHSV = 1 h−1. | 69 |
| 10 | Pt/Zeolite (HY & H-ZSM-5), sulfided Ni–Mo/Al2O3 | Rapeseed oil | T = 300–400 °C, P = 50–110 bar, time 3 h | 70 |
| 11 | Mo, W, V nitrides/Al2O3 | Oleic acid and canola oil | T = 380–410 °C, P = 71.5 bar, LHSV = 0.45 h−1 | 71 |
| 12 | Sulfide Ni–W supported on mesoporous SiO2–Al2O3 and Ni–Mo/Al2O3 | Waste soybean oil and refinery oil mixtures | T = 340–380 °C, P = 50 bar, LHSV = 2,4 h−1 | 72 |
| 13 | Sulfided Co–Mo/Al2O3 | Rapeseed oil | T = 310 °C, P = 35 bar, WHSV = 2 h−1 | 73 |
| 14 | Sulfided Co–Mo/Al2O3 | Cotton seed oil in petroleum diesel | T = 305–345 °C, P = 30 bar, WHSV = 5–25 h−1. | 74 |
| 15 | Ni–Mo,NiW and Co–Mo/Al2O3 | Waste cooking oil trapped grease | T = 30–350 °C, P = 70 bar, time-3 h | 75 |
| 16 | Ni–Mo/Al2O3 | Rapeseed oil | T = 260–340 °C, P = 70 bar, WHSV = 1 h−1. | 76 |
| 17 | Ni–Mo/Al2O3 | Crude Palm oil | T = 260–340 °C, P = 40–90 bar, LHSV = 2 h−1. | 77 |
| 18 | Pd/mesoporous C | Stearic acid | T = 360 °C, P = 10 bar, WHSV = 0.45 h−1. | 78 |
| 19 | Co–Mo/Al2O3 | Gas oil and palm oil | T = 310–350 °C, P = 33 bar, WHSV = 0.7–1.4 h−1. | 79 |
| 20 | Co–Mo/MCM-41 | Rapeseed oil | T = 300 and 320 °C, P = 20–110 bar, WHSV = 1–4 h−1. | 80 |
| 21 | Co–Mo/mesoporous alumina | Rapeseed oil | T = 250–350 °C, P = 7–70 bar, WHSV = 1.5 h−1. | 81 |
| 22 | Sulfided commercial hydrocracking catalyst | Sunflower oil | T = 360–420 °C, P = 180 bar, WHSV = 1.5 h−1. | 82 |
| 23 | Commercial hydrotreating catalyst | Waste cooking oil | T = 330–398 °C, P = 82 bar, WHSV = 1 h−1 | 83, 84 |
| 24 | Ni–Mo/Al2O3 | Waste cooking oil | T = 300–400 °C, P = 35 bar, W/F = 0.7 h−1. | 85 |
| 25 | Ni–Mo/Al2O3 | Sunflower oil–heavy vacuum oil (HVO) mixtures | T = 300–450 °C, P = 50 bar, LHSV = 5 h-1 | 86 |
| 26 | Ni–Mo/Al2O3 | 10 and 20% rapeseed oil in diesel oil | T = 300–380 °C, P = 30 and 50 bar, LHSV = 2 h−1 | 87 |
| 27 | Ni–Mo/Al2O3 and NiW/Al2O3 | 6.5 Vegetable oil + gas oil | T = 320–360 °C, P = 35–55 bar, LHSV = 1 h−1 | 88 |
| 28 | Three commercial hydrocracking catalysts | Vacuum gas oil (VGO) – vegetable oil mixtures | T = 250–450 °C, P = 0–150 bar, liquid flow = 6.5–50 mL min−1 | 89 |
| 29 | Sulfided Ni–Mo/γ-Al2O3 sulfided CoMo/γ-Al2O3, Ni/SiO2–Al2O3, Pd/γ-Al2O3, Pt/γ-Al2O3, Ru/γ-Al2O3 | Soybean oil | T = 400 °C, P = 92 bar, catalyst/oil weight ratio of 0.044 batch mode | 90 |
| 30 | Ni/SiO2 and sulphided Ni–Mo/γ-Al2O3 | Vegetable oils | T = 350–400 °C, P = 10–200 bar, | 91 |
| 31 | Co–Mo/SBA-15, Co–Mo/HMS, Co–Mo/SBA-16, Co–Mo/DMS-1 | Olive oil | T = 250 °C, P = 30 bar, TOS-5 h | 92 |
Supported metal catalysts61–64,70 have been reported to convert fatty acids and their esters into hydrocarbons mainly by decarboxylation and, because of this, the product contains more odd number hydrocarbons as compared to even ones, while sulfided catalysts provide more even number hydrocarbons resulting from hydrodeoxygenation of fatty acid feedstock. Despite their high cost, noble metal catalysts possess several advantages over conventional sulfide catalysts: (1) high reactivity at moderate temperature, achieving an energy efficient HDO process, (2) no sulfur stripping and (3) flexible catalyst design by tailoring the active phase and support.63,64 Zeolites have also been used for the hydroprocessing of vegetable oils, but a small pore diameter and cracking due to acidity lead to lower yield.70
Mesoporous materials show great potential as catalyst supports.78,80,81 Moreover, mesoporous supports, such as mesoporous molecular sieves (MCM-41, SBA-15) or organized mesoporous alumina (OMA), offer several advantages over the alumina supports used in traditional hydrodesulfurization catalysts. In contrast to standard alumina supports and similar to microporous molecular sieves (zeolites), organized mesoporous aluminas have very high specific surface areas that can be used to achieve very high dispersions of the supported active phase80 and high loadings of the active phase. Moreover, larger pore diameters as compared to zeolites make mesoporous supports excellent candidates for applications where large organic molecules have access to the well dispersed active sites located inside the pores. The molecular size of triglycerides suggests that mesoporous supports could be used advantageously in catalytic transformations of triglycerides. Nava et al. recently investigated the effect of mesoporous silicate supports (SBA-15, HMS, SBA-16, DMS-1) on the hydroprocessing of an olive oil by-product.92 The selectivity for paraffin production for the reported catalysts was found to decrease in the following order: CoMo/SBA-15 > CoMo/HMS > CoMo/SBA-16 ∼ CoMo/DMS-1. The SBA-15-, SBA-16- and DMS-1-based catalysts were reported to be more active in hydrodeoxygenation than a HMS-supported catalyst.92
Besides the above mentioned catalysts, nitrides of molybdenum, tungsten and vanadium supported on γ-Al2O371 and metals supported on carbon61,62,78 have also been reported to catalyze hydrodeoxygenation of fatty acids and vegetable oils.
In hydrotreatment of vegetable oils, hydrogen has to be taken in excess to avoid decarboxylation. Excess hydrogen flow helps in removing water (a by-product of hydrodeoxygenation) and thereby avoids deactivation of the catalyst. The excess hydrogen can be recycled after purification. Since hydrogen also removes the sulfur from the catalyst, therefore keeping the active metals in sulfided form, up to 1–2% sulfiding agent is added to the feed, which generates H2S by decomposition at the reaction temperature in the presence of hydrogen and keeps the catalyst activity.
Product yield and its composition depend on various factors, such as nature and quality of the feedstock, operating conditions (flow, temperature, pressure etc.) and the type of catalyst. Boda et al.93 showed that hydrotreatment of a model compound for vegetable oils, tricaprin (TC), proceeds in consecutive steps: hydrogenolysis (HYS) of TC to capric acid (CA) and propane, followed by hydrodeoxygenation (HDO) of the CA intermediate. The overall reaction rate was governed by the rate of the HDO reaction. Over Pd/C the prevailing reaction route of CA hydroconversion was the decarbonylation giving mainly C7 alkane and CO, whereas the HDO over the NiMo/γ-Al2O3 catalysts proceeded in consecutive H2 addition and dehydration steps giving predominantly C18 alkanes and water. In other side reactions, alcohol and traces of aldehyde are also detected as acid-to-alkane intermediates. Hydrodeoxygenation is the preferred scheme for the production of hydrocarbons as in this path there is no loss of carbon as carbon dioxide, which results in higher yield (86 wt%) as compared to decarboxylation (81 wt%) only (Scheme 4).
 | ||
| Scheme 4 Hydrodeoxygenation of vegetable oils. | ||
Influence of sulfidation. Sulfided catalysts are the most active and widely used catalysts for the hydroprocessing of the vegetable oils. However, the presence of oxygenates tends to remove the sulfur from the catalysts, thereby making it inactive or less active. To keep the catalysts in active sulfided form some sulfiding agents have to be added into the vegetable oil feedstock as the sulfur content of these bio feedstocks is very low. Various studies have indicated that sulfiding agents affect the HDO process differently depending on feed composition and process conditions. For example, the addition of a sulfiding agent to a bio-oil has generally been found to suppress the HDO of phenolic and furanic compounds on sulfided NiMo/Al2O3 and CoMo/Al2O3 catalysts,94,95 but a promoting effect has been demonstrated in HDO studies with aliphatic oxygenates, such as esters and carboxylic acids on similar catalysts.96,97 As water forms as the by-product in hydrodeoxygenation and inhibits the catalyst’s activity, Senol et al. reported that addition of H2S (or H2S generated from sulfiding agent) not only keep the catalysts in sulfided state, but also compensates for activity loss due to water.97
Influence of hydrogen pressure and flow. Hydrogen pressure and flow is a very crucial in hydrotreatment of vegetable oils as it is not only a reactant, but also helps in removal of water from the catalyst, thereby preventing it from deactivation. Relative activities of catalysts towards decarboxylation and hydrodeoxygenation are very important in hydroprocessing of triglycerides as these reactions decide the hydrogen consumption, product yields, heat balance and catalyst inhibition.66 Higher hydrogen pressure decreases decarboxylation and favors hydrodeoxygenation of triglycerides and also suppresses cracking of the products, thereby helping to obtain higher yield. A higher H2/oil ratio also favors the hydrodeoxygenation over decarboxylation, which can be confirmed by more even number hydrocarbons being obtained in the products.66
Influence of temperature. One of the most dominant effects of hydrotreatment temperature is on the product yields. At low temperatures, the oxygen removal is low and higher temperatures favor the oxygen removal from the final products. With the increase in temperature, a decrease in diesel range product is observed, which is expected as increasing temperature favors the undesirable hydrocracking reactions competing with the desirable hydrotreatment reactions. An increase in alkanes with an odd number of carbon atoms was also observed as the reaction temperature was raised. As the hydrotreating temperature increases, diesel selectivity gradually drops. The cold flow properties, such as cloud point and pour point, were found to improve with increasing temperature, as expected from the increase in iso-alkanes content. But it should be noted that higher reactor temperatures are more attractive when gasoline production is of interest, while lower reaction temperatures are more suitable when diesel production is important. Sulfur and nitrogen are most effectively removed in all cases (over 99.4%), while oxygen removal is favored by hydrotreating temperature.83,84
Influence of feedstocks. The fatty acid composition of different vegetable oils is shown in Table 3.29,98,99 Generally, vegetable oil contains fatty acids that have carbon numbers from C14 to C18 with few exceptions, such as coconut oil. Hydroprocessing studies have shown that the product alkanes contain equivalent or one less carbon than the parent fatty acids. The renewable diesel product composition of the vegetable oil hydroprocessing reaction can thereby be roughly estimated based on the fatty acid composition of the feed. However, depending on the chain length and degree of saturation of the vegetable oils, some differences have been observed in the product yields. The soybean, buriti and maracuja oils contain fatty acids that have a longer chain length and are more unsaturated compared to tucuma and babassu oils.100,101 Gaseous products were found to increase with a decrease in chain length and degree of unsaturation of the vegetable oils. Some authors have also reported getting some amount of cycloalkane and aromatics.102 This generally happens in cases of low hydrogen pressure or low hydrogen to oil ratio, then side reactions, such as cracking, cyclization and aromatization, increase. Cycloalkane also increases when there is a higher degree of unsaturation in the feedstock and alkane selectivity increases with an increase in the degree of saturation.103 But these products are not generally found in hydrotreating, where at process conditions of temperature and pressure most of the unsaturation becomes saturated and does not lead to cyclisation or aromatization.
| Vegetable oil | Iodine value | Fatty acid composition (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C12 | C14 | C16 | C18 | C18:1 | C18:2 | C18:3 | Other | ||
| a C18:3(ZEE) – 9Z,11E,13E – Octadecatrienoic (α-eleostearic) acid. | |||||||||
| Edible oils | |||||||||
| Soybean oil | 128–143 | — | — | 12 | 3 | 27 | 52 | 6 | — |
| Sunflower oil | 125–140 | — | — | 7 | 5 | 19 | 68 | 1 | — |
| Rapeseed oil (mustard oil) | 98–110 | — | — | 4 | 1 | 65 | 22 | 8 | — |
| Palm oil | 48–58 | — | 1 | 45 | 4 | 40 | 10 | — | — |
| Cotton seed oil | 103–115 | — | 1 | 22 | 3 | 19 | 54 | 1 | — |
| Coconut oil | 7–11 | 51 | 18 | 9 | 3 | 6 | 2 | — | C10:0 (6), C8:0 (5) |
| Rice bran oil | 90–108 | — | — | 15 | 2 | 43 | 39 | 1 | — |
| Corn (maize) oil | 103–128 | — | — | 2 | 11 | 28 | 58 | 1 | — |
| Peanut/ground nut/arachis oil | 84–100 | — | — | 11 | 2 | 48 | 30 | 2 | — |
| Tucuma oil | 12–17 | 47 | 26 | 6 | 3 | 13 | 3 | — | C8:0 (1), C10:0 (1) |
| Buriti oil | 81–90 | — | 1 | — | 4 | 72 | 2 | 2 | C16:1 (19) |
| Babassu oil | 14–20 | 50 | 20 | 11 | 4 | 15 | — | — | — |
| Non-edible oils | |||||||||
| Karanja oil | 81–90 | — | — | 6 | 7 | 62 | 17 | — | C20:0 (3), C22:0 (4) |
| Jatropha oil | 82–98 | — | 1 | 15 | 6 | 55 | 23 | — | |
| Rubber seed oil | 132–148 | — | — | 10 | 9 | 25 | 40 | 16 | — |
| Mahua oil | 58–70 | — | — | 28 | 23 | 50 | 9 | — | — |
| Tung oil | 160–175 | — | — | 4 | 1 | 8 | 4 | 3 | C18:3(ZEE) (80)a |
| Maracuja oil | 105–145 | 0 | 8 | 2 | 12 | 77 | 1 | — | — |
| Neem oil | 65–80 | — | — | 16 | 10 | 50 | 16 | 8 | — |
| Animal Fats | |||||||||
| Chicken fat (poultry fat) | 75 | — | 1 | 23 | 6 | 42 | 17 | 1 | C16:1 (8), C20:1 (2) |
| Fish oil (menhaden) | 167 | — | 11 | 20 | 3 | 15 | 2 | 1 | C16:1 (15), C20:1 (1), C18:4 (4), C20:5 (5), C20:6 (12) |
| Beef tallow | 35–48 | 1 | 3 | 27 | 17 | 42 | 3 | 1 | C16:1 (4), C17:0 (2) |
3. Co-processing of vegetable oil with petroleum feed
Renewable diesel that is produced when an oil company adds small amounts vegetable oils or animal fats to the traditional petroleum refining process while producing diesel fuel is termed co-processing. Co-processing is a relatively cheap option to produce renewable fuels because it avoids setting up new units. Advantages of co-processing are: (1) increases cetane number of the diesel fraction, (2) reduction in CO and hydrocarbon emission, (3) reduction in sulfur content and (4) reduces dependence on petroleum. While some studies have considered co-processing of vegetable oils with vacuum gas oil (VGO) boiling range petroleum, others have investigated co-processing with light gas oil (LGO) fractions. Corma and co-workers have reported that the kinetics of oxygen removal from sunflower oil was much faster than that of sulfur removal from VGO.101 The conversion of sunflower oil at 350 °C was 100%, while the corresponding sulfur conversion was only 41%, and up to 50% sunflower oil has no influence on the rate of desulfurization over the sulfided NiMo/Al2O3 catalyst. In general, it has been observed that an increase in vegetable oil content is detrimental to the cold flow properties of the diesel product. An increase in the rapeseed oil content in the feed from 0% to 25% resulted in an increase in the cloud point product from 4.4 °C to 10.5 °C.66 Knudsen et al. also reported that in LGO–rapeseed oil co-processing studies, decarboxylation selectivity and hydrogen consumption increases with an increase in rapeseed oil content, but a decrease in aromatic conversion was observed due to inhibition of the hydrogenation of mono-ring aromatic compounds by CO formed from the rapeseed oil conversion.4. Commercial units
Industrial hydrogenation plants are under development and presented in Table 4.104 Neste Oil105,106 has developed the NExBTL® process for which the first commercial plant has started in Finland. UOP/Eni Ecofining™ is another commercialized process to convert non-edible, second generation natural oils to Honeywell Green Diesel™, a drop-in diesel fuel for use in any percentage.107 Co-processing of vegetable oils with middle distillates, the H-Bio process (Fig. 1),108 has been started in Brazil by Petrobras. However, several problems still need to be addressed. The presence of a large amount of water in the hydrotreatment reactor could have adverse effects on the sulfided catalyst performance. To make this process attractive, it should be optimized to consume minimum hydrogen and the catalyst should be robust enough to tolerate high concentrations of free fatty acids present in non-edible oils and water produced as a by-product. Even hydrogen can be produced by steam reforming from light fraction produced as a side reaction or from biomass.107 Companies are concentrating more on non-edible oil sources to avoid the food vs. fuel issue and reduce the production cost. Renewable diesel is becoming more attractive than conventional biodiesel due to its higher feedstock flexibility, lower cost, high oxidative stability, high blendability and used in existing fuel distribution systems.107,108 For stand-alone processing, since vegetable oils do not have a high sulfur content, new non-sulfided hydrotreating catalysts could be used.| Company | Feedstock | Country/plant location |
|---|---|---|
| Neste oil | Vegetable oils/animal fats | Finland, Singapore, Netherlands |
| UOP and ENI | Vegetable oils | Italy |
| Dynamic Fuels (Syntroleum/Tyson) | Non-food grade animal fats and greases | USA |
| Petrobras | Vegetable oils/animal fats | Brazil |
| British Petroleum | Vegetable oils/animal fats co-processed with petroleum diesel | Australia |
| ConocoPhillips/Tyson | Vegetable oils/animal fats co-processed with petroleum diesel | Ireland |
 | ||
| Fig. 1 Hydroconversion in hydrotreating units (HDT) developed by Petrobras (H-bio process).104 | ||
For co-processing, carbon monoxide, formed by hydrogenolysis of vegetable oils, has an inhibiting effect on hydrodesulfurization activity. In the hydrotreatment conditions, unsaturated fatty chains are hydrogenated. The resulting straight chains, mainly C12–C18, are completely paraffinic. Such fully saturated compounds have an excellent cetane index, but generally bad cold flow properties compared to corresponding esters,109,110 which may require an additional hydroisomerization step. In this type of process, there are no longer any constraints on the chemical composition of the vegetable oils or fats (length of the fatty chains, degree of unsaturation).
If, as it seems today (average crude oil price in 2012 is >$100/barrel), oil prices stay at a high level, then the incentive for manufacturing fuels from renewable resources will become attractive, especially from non-edible vegetable oils/fats and biodiesel will gradually emerge in the market place. This essentially sulfur-free fuel could, eventually, have a moderating effect on the growth of the hydrotreating industry. The use of bio-based feeds will lead to the presence of oxygen atoms in feed molecules and hydrodeoxygenation (HDO) or any other technique for the removal of oxygen may be needed. In that case, more basic knowledge of HDO is necessary.
5. Conclusions
Hydroprocessing of vegetable oils to obtain hydrocarbon-based diesel fuel is becoming more attractive due to its stability and feedstock (low quality high FFA) flexibility over ester-based biodiesel and compatibility with the petroleum distribution system and diesel engine. Although many reported studies advanced the knowledge of vegetable oil hydroprocessing, still the stability of the catalyst in long duration runs with various feedstocks and optimization of process parameters need more detailed understanding for its wider commercial use. High hydrogen consumption in the process is another issue to be addressed as it is crucial to develop and optimize quality catalysts and process conditions. This process is a two-step process that results in a higher operating cost and lower yield. Therefore, a single step process for conversion of vegetable oil into hydrocarbons would be more desirable.Acknowledgements
JKS thanks BPCL management for a post doctoral fellowship. The authors also thank BPCL management for the permission to publish this article.References
- R. Altin and S. Cetinkaya, Energy Convers. Manage., 2001, 42, 529 CrossRef CAS.
- A. B. Chhetri, M. S. Tango, S. M. Budge, K. C. Watts and M. R. Islam, Int. J. Mol. Sci., 2009, 8, 169 Search PubMed.
- D. Agarwal and A. K. Agarwal, Appl. Therm. Eng., 2007, 27, 2314 CrossRef CAS.
- C. D. Rakopoulos, D. C. Rakopoulos, D. T. Hountalas, E. G. Giakoumis and E. C. Andritsakis, Fuel, 2008, 87, 147 CrossRef CAS.
- G. Fontarasl, T. Tzamkiozis, E. Hatziemmanouil and Z. Samaras, Process Saf. Environ. Prot., 2007, 85, 396 CrossRef.
- A. Sivasamy, K. Y. Cheah, P. Fornasiero, F. Kemausuor, S. Zinoviev and S. Miertus, ChemSusChem, 2009, 2, 278 CrossRef CAS.
- M. Di serio, R. Tesser, L. Pengmei and E. Santacesaria, Energy Fuels, 2008, 22, 207 CrossRef CAS.
- A. A. Kiss, A. C. Dimian and G. Rothenberg, Adv. Synth. Catal., 2006, 348, 75 CrossRef CAS.
- D. Srinivas and J. K. Satyarthi, Catal. Surv. Asia, 2011, 15, 145 CrossRef CAS.
- J. A. Melero, J. Iglesias and G. Morales, Green Chem., 2009, 11, 1285 RSC.
- D. Srinivas and J. K. Satyarthi, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 2012, 51A, 174 CAS.
- B. Smith, H. C. Greenwell and A. Whiting, Energy Environ. Sci., 2009, 2, 262 CAS.
- T. A. Mamedova, N. K. Andryushchenko, E. N. Askerova, K. R. Veliev, V. M. Abbasov and M. I. Rustamov, Chem. Technol. Fuels Oils, 2010, 46, 149 CrossRef CAS.
- K. V. C. Rao, US Patent 4102938, 25 July 1978 Search PubMed.
- http://www.askiitians.com/iit-jee-chemistry/organic-chemistry/general-methods-of-preparation-of-alkanes.aspx .
- J. T. Kloprogge, L. V. Duong and R. L. Frost, Environ. Geol., 2005, 47, 967 CrossRef CAS.
- H. Lappi and R. Alén, J. Anal. Appl. Pyrolysis, 2011, 91, 154 CrossRef CAS.
- J. D. Adjaye and N. N. Bakhshi, Fuel Process. Technol., 1995, 45, 185 CrossRef CAS.
- Y. S. Ooi and S. Bhatia, Microporous Mesoporous Mater., 2007, 102, 310 CrossRef CAS.
- J. O. Olusola, M. M. Adediran, A. K. Oluseyi and U. L. Ajao, Energy Environ. Sci., 2009 Search PubMed 20/2010, 21, 1325.
- T. Kalnes, T. Marker and D. R. Shonnard, Int. J. Chem. React. Eng., 2007, 5, A48 Search PubMed.
- J. Holmgren, C. Gosling, K. Couch, T. Kalnes, T. Marker, M. McCall and R. Marinangeli, Pet. Technol., 2007, 3, 119 Search PubMed.
- J. Holmgren, C. Gosling, R. Marinangeli, T. Marker, G. Faraci and C. Perego, Hydrocarbon Process., 2007, 9, 67 Search PubMed.
- N. N. A. N. Yusuf, S. K. Kamarudin and Z. Yaakub, Energy Convers. Manage., 2011, 52, 2741 CrossRef CAS.
- H. Lappi and R. Alén, J. Anal. Appl. Pyrolysis, 2009, 86, 274 CrossRef CAS.
- K. M. Doll, B. K. Sharma, P. A. Z. Suarez and S. Z. Erhan, Energy Fuels, 2008, 22, 2061 CrossRef CAS.
- C. C. Chang and S. W. Wan, Ind. Eng. Chem., 1942, 39, 1543 CrossRef.
- A. W. Schwab, G. J. Dykstra, E. Selke, S. C. Sorenson and E. H. Pryde, J. Am. Oil Chem. Soc., 1988, 65, 1781 CrossRef CAS.
- A. Demirbaş, Biodiesel: a realistic fuel alternative for diesel engines, Springer, London, 2008 Search PubMed.
- I. C. P. Fortes and P. J. Baugh, J. Anal. Appl. Pyrolysis, 1994, 29, 153 CrossRef CAS.
- I. C. P. Fortes and P. J. Baugh, J. Braz. Chem. Soc., 1999, 10, 469 CrossRef CAS.
- R. O. Idem, S. P. R. Katikaneni and N. N. Bakhshi, Energy Fuels, 1996, 10, 1150 CrossRef CAS.
- K. D. Maher and D. C. Bressler, Bioresour. Technol., 2007, 98, 2351 CrossRef CAS.
- C. C. Chang and S. W. Wan, Ind. Eng. Chem., 1947, 39, 1543 CrossRef CAS.
- J. W. Alencar, P. B. Alves and A. A. Craveiro, J. Agric. Food Chem., 1983, 31, 1268 CrossRef CAS.
- K. Kitamura, Bull. Chem. Soc. Jpn., 1971, 44, 606 CrossRef.
- W. W. Nawar, J. Agric. Food Chem., 1969, 17, 18 CrossRef CAS.
- P. C. Nichols and R. T. Holman, Lipids, 1972, 7, 773 CrossRef CAS.
- D. Kubička and L. Kaluza, Appl. Catal., A, 2010, 372, 199 CrossRef.
- A. Srivastava and R. Prasad, Renewable Sustainable Energy Rev., 2000, 4, 111 CrossRef CAS.
- M. O. Bagby, Vegetable oils for diesel fuel: opportunities for development, International Winter Meeting of the ASAE, Hyatt Regency Chicago, 15–18 December, 1987 Search PubMed.
- H. Tani, T. Hasegawa, M. Shimouchi, K. Asami and K. Fujimoto, Catal. Today, 2011, 164, 410 CrossRef CAS.
- D. G. B. Boocock, S. K. Konar, A. Mackay, P. T. C. Cheung and J. Liu, Fuel, 1992, 71, 1291 CrossRef CAS.
- N. Taufiqurrahmi and S. Bhatia, Energy Environ. Sci., 2011, 4, 1087 CAS.
- Y. S. Ooi, R. Zakaria, A. R. Mohamed and S. Bhatia, Energy Fuels, 2005, 19, 736 CrossRef CAS.
- Y. S. Ooi, R. Zakaria, A. R. Mohamed and S. Bhatia, Catal. Commun., 2004, 5, 441 CrossRef CAS.
- J. D. Adjaye and N. N. Bakhshi, Fuel Process. Technol., 1995, 45, 185 CrossRef CAS.
- S. P. R. Katikaneni, J. D. Adjaye and N. N. Bakhshi, Energy Fuels, 1995, 9, 1065 CrossRef CAS.
- R. K. Sharma and N. N. Bakhshi, Energy Fuels, 1993, 7, 306 CrossRef CAS.
- J. T. Kloprogge, L. V. Duong and R. L. Frost, Environ. Geol., 2005, 47, 967 CrossRef CAS.
- F. A. A. Twaiq, A. R. Mohamad and S. Bhatia, Fuel Process. Technol., 2004, 85, 1283 CrossRef CAS.
- O. Y. Sang, F. Twaiq, R. Zakaria, A. R. Mohamed and S. Bhatia, Energy Sources, 2003, 25, 859 CrossRef CAS.
- O. Y. Sang, F. Twaiq, R. Zakaria, A. R. Mohamed and S. Bhatia, Biomass Bioenergy, 2004, 27, 477 CrossRef.
- D. G. Lima, V. C. D. Soares, E. B. Ribeiro, D. A. Carvalho, E. C. V. Cardoso, F. C. Rassi, K. C. Mundim, J. C. Rubim and P. A. Z. Suarez, J. Anal. Appl. Pyrolysis, 2004, 71, 987 CrossRef CAS.
- X. Junming, J. Jianchun, L. Yanju and C. Jie, Bioresour. Technol., 2009, 100, 4867 CrossRef.
- J. Monnier, G. Tourigny, D. W. Soveran, A. Wong, E. Hogan and M. Stumborg, U. S. Patent 5705722, Natural Resources, Canada, 1998 Search PubMed.
- D. Casanave, J.-L. Duplan and E. Freund, Pure Appl. Chem., 2007, 79, 2071 CrossRef CAS.
- I. Kubičkova and D. Kubička, Waste Biomass Valoriz., 2010, 1, 293 CrossRef.
- S. Lestari, P. M. Arvela, J. Beltramini, G. Q. M. Lu and D. Y. Murzin, ChemSusChem, 2009, 2, 1109 CrossRef CAS.
- D. Kubička and L. Kaluza, Appl. Catal., A, 2010, 372, 199 CrossRef.
- M. Snåre, I. Kubičková, P. Mäki-Arvela, D. Chichova, K. Eränen and D. Yu. Murzin, Fuel, 2008, 87, 933 CrossRef.
- S. Lestari, P. Mäki-Arvela, I. Simakova, J. Beltramini, G. Q. Max Lu and D. Yu. Murzin, Catal. Lett., 2009, 130, 48 CrossRef CAS.
- K. Murata, Y. Liu, M. Inaba and I. Takahara, Energy Fuels, 2010, 24, 2404 CrossRef CAS.
- P. T. Do, M. Chiappero, L. L. Lobban and D. E. Resasco, Catal. Lett., 2009, 130, 9 CrossRef CAS.
- Y. Liu, R. Sotelo-Boyás, K. Murata, T. Minowa and K. Sakanishi, Chem. Lett., 2009, 552 CrossRef.
- B. Donnis, R. G. Egeberg, P. Blom and K. G. Knudsen, Top. Catal., 2009, 52, 229 CrossRef CAS.
- M. Chiappero, P. T. M. Do, S. Crossley, L. L. Lobban and D. E. Resasco, Fuel, 2011, 90, 1155 CrossRef CAS.
- O. V. Kikhtyanin, A. E. Rubanov, A. B. Ayupov and G. V. Echevsky, Fuel, 2010, 89, 3085 CrossRef CAS.
- S. Bezergianni, A. Dimitriadis, A. Kalogianni and K. G. Knudsen, Ind. Eng. Chem. Res., 2011, 50, 3874 CrossRef CAS.
- R. S. Boyás, Y. Liu and T. Minowa, Ind. Eng. Chem. Res., 2011, 50, 2791 CrossRef.
- J. Monnier, H. Sulimma, A. Dalai and G. Caravaggio, Appl. Catal., A, 2010, 382, 176 CrossRef CAS.
- R. Tiwari, B. S. Rana, R. Kumar, D. Verma, R. Kumar, R. K. Joshi, M. O. Garg and A. K. Sinha, Catal. Commun., 2011, 12, 559 CrossRef CAS.
- D. Kubička and J. Horáček, Appl. Catal., A, 2011, 394, 9 CrossRef.
- I. Sebos, A. Matsoukas, V. Apostolopoulos and N. Papayannakos, Fuel, 2009, 88, 145 CrossRef CAS.
- M. Toba, Y. Abe, H. Kuramochi, M. Osako, T. Mochizuki and Y. Yoshimura, Catal. Today, 2011, 164, 533 CrossRef CAS.
- P. Šimáček, D. Kubička, G. Šebor and M. Pospíšil, Fuel, 2009, 88, 456 CrossRef.
- A. Guzman, J. E. Torres, L. P. Prada and M. L. Nunez, Catal. Today, 2010, 156, 38 CrossRef CAS.
- S. Lestari, P. M. Arvela, H. Bernas, O. Simakova, R. Sjöholm, J. Beltramini, G. Q. M. Lu, J. Myllyoja, I. Simakova and D. Y. Murzin, Energy Fuels, 2009, 23, 3842 CrossRef CAS.
- C. Templis, A. Vonortas, I. Sebos and N. Papayannakos, Appl. Catal., B, 2011, 104, 324 CrossRef CAS.
- D. Kubička, M. Bejblová and J. Vlk, Top. Catal., 2010, 53, 168 CrossRef.
- D. Kubička, P. Šimáček and N. Žilková, Top. Catal., 2009, 52, 161 CrossRef.
- P. Šimáček, D. Kubička, I. Kubičková, F. Homol, M. Pospišil and J. Chudoba, Fuel, 2011, 90, 2473 CrossRef.
- S. Bezergianni, A. Dimitriadis, A. Kalogianni and P. A. Pilavachi, Bioresour. Technol., 2010, 101, 6651 CrossRef CAS.
- S. Bezergianni, A. Dimitriadis, T. Sfetsas and A. Kalogianni, Bioresour. Technol., 2010, 101, 7658 CrossRef CAS.
- M. Santikunaporn and S. Danphitak, Thammasat Int. J. Sci. Technol., 2010, 15, 1 Search PubMed.
- G. W. Huber, P. O'Connor and A. Corma, Appl. Catal., A, 2007, 329, 120 CrossRef CAS.
- J. Walendziewski, M. Stolarski, R. Łużny and B. Klimek, Fuel Process. Technol., 2009, 90, 686 CrossRef CAS.
- J. Mikulec, J. Cvengroš, L. Joríková, M. Banič and A. Kleinová, J. Cleaner Prod., 2010, 18, 917 CrossRef CAS.
- S. Bezergianni, A. Kalogianni and I. A. Vasalos, Bioresour. Technol., 2009, 100, 3036 CrossRef CAS.
- B. Veriansyah, J. Y. Han, S. K. Kim, S.-A. Hong, Y. J. Kim, J. S. Lim, Y.-W. Shu, S.-G. Oh and J. Kim, Fuel, 2012, 94, 578 CrossRef CAS.
- J. Gusmão, D. Brodzki, G. Djéga-Mariadassou and R. Frety, Catal. Today, 1989, 5, 533 CrossRef.
- R. Nava, B. Pawelec, P. Castano, M. C. Alvarez-Galvan, C. V. Loricera and J. L. G. Fierro, Appl. Catal., B, 2009, 92, 154 CrossRef CAS.
- L. Boda, G. Onyestyak, H. Solt, F. Lonyi, J. Valyon and A. Thernesz, Appl. Catal., A, 2010, 374, 158 CrossRef CAS.
- E. Laurent and B. Delmon, Ind. Eng. Chem. Res., 1993, 32, 2516 CrossRef CAS.
- T.-R. Viljava and A. O. I. Krause, Stud. Surf. Sci. Catal., 1997, 106, 343 CrossRef CAS.
- E. Laurent and B. Delmon, Appl. Catal., A, 1994, 109, 97 CrossRef CAS.
- O. İ. Şenol, E. M. Ryymin, T. R. Viljava and A. O. Krause, J. Mol. Catal. A: Chem., 2007, 277, 107 CrossRef.
- G. N. da Rocha Filho, D. Brodzki and G. Djega-Mariadassou, Fuel, 1993, 72, 543 CrossRef CAS.
- A. Thomas, Fats and Fatty Oils, Ullmann's Encyclopaedia of Industrial Chemistry, 2000, http://onlinelibrary.wiley.com/doi/10.1002/14356007.a10_173/pdf Search PubMed.
- M. Mittelbach and C. Remschmidt, Biodiesel - The Comprehensive Handbook, Boersedruck Ges.m.b.H, Vienna, 3rd edn, 2006 Search PubMed.
- T. V. Choudhary and C. B. Phillips, Appl. Catal., A, 2011, 397, 1 CrossRef CAS.
- S. Gong, A. Shinozaki, M. Shi and E. W. Qian, Energy Fuels, 2012, 26, 2394 CrossRef CAS.
- G. Knothe, Prog. Energy Combust. Sci., 2010, 36, 364 CrossRef CAS.
- http://agr.wa.gov/bioenergy/docs/RenewableDieselWhitePaperFINAL.pdf as on 6th January 2012.
- http://www.nesteoil.com .
- L. Rantanen, R. Linnaila, P. Aakko and T. Harju, SAE [Tech. Pap.], 2005, 1, 3771 Search PubMed.
- http://www.uop.com, http:// www.uop.com/processing-solutions/biofuels/green-diesel/ .
- http://www.petrobras.com.br/en/about-us/profile/activities/biofuel-production/ .
- J. Monnier, G. Tourigny, D. W. Soveran, A. Wong, E. Hogan and M. Stumborg, U. S. Patent 5705722, Natural Resources Canada, 1998 Search PubMed.
- D. Casanave, J.-L. Duplan and E. Freund, Pure Appl. Chem., 2007, 79, 2071 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |

