Esterification of 2-keto-L-gulonic acid catalyzed by a solid heteropoly acid
Thu Ha Thi
Vu
*,
Hang Thi
Au
,
Tuyet Mai Thi
Nguyen
,
Minh Tu
Pham
,
Tam Thi
Bach
and
Hong Nhan
Nong
National Key Laboratory for Petrochemical and Refinery Technologies, Hanoi, Vietnam. E-mail: ptntd2004@yahoo.fr; Fax: +84 439335410; Tel: +84 422189067
First published on 26th October 2012
Abstract
The efficacy of a potassium 12-phosphotungstate (KPW) catalyst in the synthesis of methyl 2-keto-L-gulonat from 2-keto-L-gulonic acid (2-KLGA) and methanol is investigated. The KPW catalyst gives high yields in short reaction times. The present procedure represents a clean, efficient, practical, simple, mild, time-saving and eco-friendly method for the synthesis of methyl 2-keto-L-gulonat. The KPW catalyst is found to be a truly heterogeneous catalyst, highly efficient and reusable in the synthesis of methyl 2-keto-L-gulonat.
1. Introduction
The 2-keto-L-gulonic acid (2-KLGA) esterification reaction plays an important role in the industrial manufacture of vitamin C.1–3 Homogeneous acid catalysts, such as sulfuric acid and heteropoly acids (HPAs), have been used for the esterification reaction.4–6 However, the use of homogeneous acid catalysts for esterification causes difficulties in recovery after the reaction has taken place and produces toxic wastewater.7 To overcome this shortcoming of homogeneous catalysts, heterogeneous catalysts have been used in esterification reactions in recent years,8 although the catalytic activity of heterogeneous catalysts is often lower than that of homogeneous catalysts.9Heterogeneous acid catalysis by a HPA with a Keggin structure is one of the most important and growing areas of research in recent years because of its potential economic rewards and green benefits.10,11 HPAs with Keggin structures possess special characteristics that allow for their use as catalysts in esterification reactions as they have a very high intrinsic acidity.12 The catalytic acidity of HPAs is stronger than that of conventional solid acid catalysts such as acidic oxides and zeolites. The acid strength of Keggin HPAs decreases in the order: H3PW12O40 > H4SiW12O40 > H3PMo12O40 > H4SiMo12O40.13 In addition, the acid sites in HPA are more uniform and easier to control than those in other solid acid catalysts.14 Usually, phosphotungstic acid (HPW) is the catalyst of choice because of its stronger acidity, higher thermal stability and lower oxidation potential compared to phosphomolybdic acid.15 However, due to the low surface area and difficulty in the reutilization of the homogeneous tungsten HPAs, it is advisable to support tungsten HPAs on a carrier with a high surface area, such as silica, activated carbon, alumina or clays.16 Another alternative approach is to prepare HPA-salts by partially exchanging protons of the parent HPAs with large cations, such as K+ and Cs+, which have high porosity.9,15 Thus, the HPA-salt not only has a high surface area but can also be reused in the esterification of methyl 2-keto-L-gulonat.
In our present studies, potassium 12-phosphotungstate (KPW) was prepared by the partially exchanging protons process. The Keggin structure of KPW was examined by X-ray Diffraction Spectra analysis (XRD). An automated BET sorptometer was used to study the increasing surface area of the HPA-salt compared with that of HPA. The surface area of Amberlyst 15 was also characterized by a BET sorptometer. The number of acid sites in the catalyst was examined by TPD-NH3. Fourier Transform Infrared Spectroscopy (FTIR) was used to study the Keggin structure of the KPW catalyst. The catalytic activity of KPW was tested in the esterification of methyl 2-keto-L-gulonat. The aim of the present work is to compare the KPW catalyst performance with other known catalyst systems, such as the homogeneous catalyst 12-phosphotungstic acid and heterogeneous catalyst Amberlyst 15 on the esterification reaction.
2. Experimental
2.1. Materials and catalysts
Monohydrous 2-keto-L-gulonic acid (90 wt%), 12-phosphotungstic acid (H3PW12O40·21H2O) and methanol (99.5 wt%) were purchased from Sigma Aldrich. Amberlyst 15 resin was obtained from Rohm & Hass Co.The acidic heteropoly salt (KPW) was prepared according to the literature procedure18 by adding dropwise the required amount of an aqueous solution of H3PW12O40 (0.1 M) to an aqueous saturated solution of KCl at 40 °C with stirring. The precipitate obtained was filtrated and washed with distilled water and then dried at 70 °C for 10 hours and after that in an oven at 120 °C for 5 hours.
2.2. Techniques
Powder X-ray diffraction (XRD) spectra of KPW and H3PW12O40 (HPW) were recorded on a D8 Advance diffractometer (XRD) with monochromatic Cu–Kα radiation using a Brucker Tensor 37.The nature of the acid sites of these catalysts was determined by NH3-TPD on an Autochem 2020 (micrometric) at the temperature range of 100 °C to 600 °C at a rate of 10 °C min−1, using nitrogen as a gas carrier.
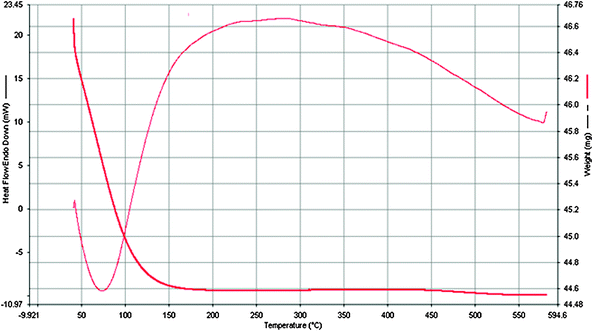
The thermal stability of the catalyst was examined by thermogravimetric/differential thermal analysis (TG/DTA) using a Perkin Elmer instrument. The samples were heated at a rate of 10 °C min−1. The TG and DTA curves were recorded.
The Brunauer–Emmett–Teller (BET) surface area and porosity of the catalysts were measured from the N2 adsorption isotherm at 77 K using a BET Sorptometer (Automated BET Sorptometer 201-A, USA).
Infrared spectra were recorded on a Brucker FTIR spectrometer with samples prepared as KBr disks in the 400–4000 cm−1 range.
The yield of the esterification reaction was determined by HPLC chromatography. HPLC was performed using a Model 1200 Agilent, a model UV (210 nm) and a C18 column.
The chemical composition of the KPW catalyst was analysed using X-ray fluorescence, XRF (Model: Bruker S4 Pioneer, USA).
2.3. Catalytic reactions
The catalytic activity of KPW was tested in 2-keto-L-gulonic acid esterification, which was carried out under atmospheric pressure in a 250 ml bottom glass reactor. The 2-KLGA was mixed with methanol at various molar ratios of 2-KLGA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol: 1
methanol: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24, 1
24, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48, 1
48, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96, 1
96, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 192, 1
192, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 384, 1
384, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 720, 1
720, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1440 (mol
1440 (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol−1). The mixtures were heated to 65 °C and then the catalyst was added to the 2-KLGA–methanol solutions with the weight ratio of catalyst to 2-KLGA of 1
mol−1). The mixtures were heated to 65 °C and then the catalyst was added to the 2-KLGA–methanol solutions with the weight ratio of catalyst to 2-KLGA of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (g
10 (g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g−1). The esterification reaction time was started by charging the catalyst. The product of the reaction was periodically taken out at 5 min, 15 min, 30 min, 60 min, 120 min, 180 min, 240 min, 300 min, 360 min and 420 min and analyzed by HPLC analysis.
g−1). The esterification reaction time was started by charging the catalyst. The product of the reaction was periodically taken out at 5 min, 15 min, 30 min, 60 min, 120 min, 180 min, 240 min, 300 min, 360 min and 420 min and analyzed by HPLC analysis.
Catalyst recycling experiments were performed as follows: after the reaction, the heterogeneous KPW catalyst was recovered from solution by simple filtration. The catalyst was washed with copious amounts of methanol solvent. Then the catalyst was reused in the reaction. The efficacy on the esterification reaction when using the KPW catalyst was determined after each recycling.
2.4. Scale up reaction
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol was 1
methanol was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 (mol mol−1), which is the highest concentration of 2-KLGA in methanol at 65 °C. A mixture solution was prepared by mixing 200 g of a 2-keto-L-gulonic acid solution with 1000 ml of a CH3OH solution. The mixture was stirred at a rate of 500 r min−1 and heated to 65 °C. 20 g of the catalyst was then added to the mixture. The esterification reaction time was started by charging the catalyst. The products of the reaction were periodically taken out at 5 min, 15 min, 30 min, 60 min, 120 min, 180 min, 240 min, 300 min, 360 min and 420 min. The products were diluted 30 times by methanol and were analyzed by HPLC analysis.
24 (mol mol−1), which is the highest concentration of 2-KLGA in methanol at 65 °C. A mixture solution was prepared by mixing 200 g of a 2-keto-L-gulonic acid solution with 1000 ml of a CH3OH solution. The mixture was stirred at a rate of 500 r min−1 and heated to 65 °C. 20 g of the catalyst was then added to the mixture. The esterification reaction time was started by charging the catalyst. The products of the reaction were periodically taken out at 5 min, 15 min, 30 min, 60 min, 120 min, 180 min, 240 min, 300 min, 360 min and 420 min. The products were diluted 30 times by methanol and were analyzed by HPLC analysis.
3. Results and discussion
3.1. Chemical composition
The chemical composition of the KPW catalyst was determined by XRF analysis (as shown in Table 1). The results confirm that the potassium element is present in the catalyst structure. The presence of potassium in the catalyst structure can change the physicochemical properties of the catalyst from a homogeneous catalyst to a heterogeneous catalysts.15 From the results in Table 1, we can calculate the content of potassium ions in KPW. The structure formula of KPW is K2.2H0.8PW12O40.| Element | Chemical composition (%) |
|---|---|
| W | 94.6 |
| K | 3.67 |
| P | 1.04 |
| Se | 0.20 |
| As | 0.14 |
| Si | 0.122 |
| Al | 0.106 |
| Ge | 590 (ppm) |
| V | 430 (ppm) |
3.2. Catalyst characterization
| KPW | HPW | Amberlyst 15 dry | |
|---|---|---|---|
| S BET (m2 g−1) | 96.37 | 11.55 | 45.00 |
| S pores (m2 g−1) | 43.07 | — | — |
| V pores (cm3 g−1) | 0.06 | — | — |
| ∅ pores (nm) | 5.20 | <2 | 25 |
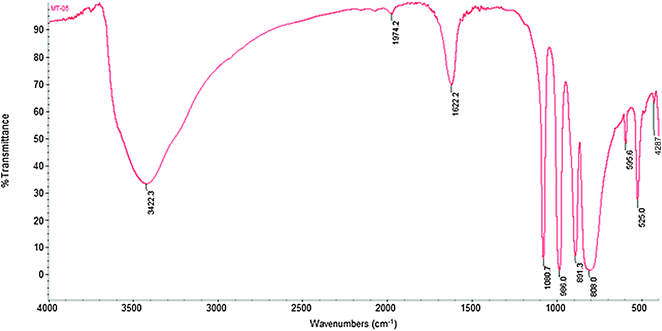 | ||
| Fig. 2 FTIR spectra of the KPW catalyst. | ||
 | ||
| Fig. 3 Keggin structure. | ||
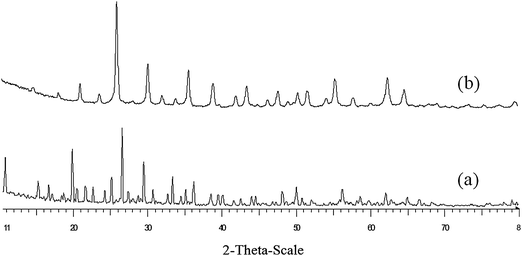 | ||
| Fig. 4 Wide-angle XRD pattern of (a) HPW catalyst, (b) KPW catalyst before using. | ||
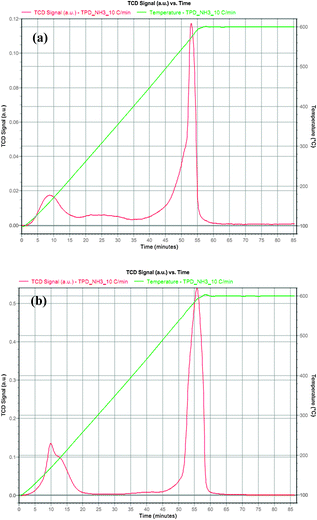 | ||
| Fig. 5 NH3 temperature-programmed-desorption profiles of (a) KPW and (b) HPW. | ||
3.3 Catalyst performance
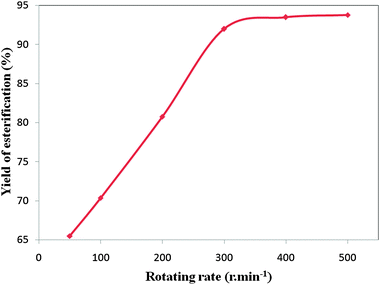 | ||
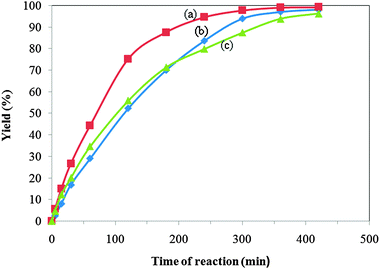
Fig. 6 The effect of the rotating rate on the yield of the esterification reaction (the molar ratio of 2-KLGA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol of 1 methanol of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1440 (mol mol−1), reaction time: 180 min, 65 °C). 1440 (mol mol−1), reaction time: 180 min, 65 °C). | ||
In the concept of internal diffusion, Xu et al. demonstrated that the internal diffusion can be considered negligible in the esterification reaction of 2-KLGA when using the Amberlyst catalyst with particle sizes of 0.7 mm at a stirrer speed of 500 r min−1.23 The smaller the particle size, the lower the effect of internal diffusion on the esterification reaction. In our case, the size of the KPW powder catalyst is much smaller than that of the Amberlyst catalyst. Therefore, the internal diffusion can be considered negligible in the esterification reaction of 2-KLGA.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 (mol
24 (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol−1). Therefore, the effect of the molar ratios of 2-KLGA
mol−1). Therefore, the effect of the molar ratios of 2-KLGA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol on the equilibrium yield of the esterification reaction was investigated at various molar ratios of 2-KLGA
methanol on the equilibrium yield of the esterification reaction was investigated at various molar ratios of 2-KLGA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol: 1
methanol: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24, 1
24, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48, 1
48, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96, 1
96, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 192, 1
192, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 384, 1
384, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 720 and 1
720 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1440 (mol
1440 (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol−1). The results are presented in Fig. 7. It was found that identical results were observed with each fraction obtained. This indicated that the molar ratios of 2-KLGA
mol−1). The results are presented in Fig. 7. It was found that identical results were observed with each fraction obtained. This indicated that the molar ratios of 2-KLGA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol have no effect on the equilibrium yield of the esterification reaction with ratios up to 1
methanol have no effect on the equilibrium yield of the esterification reaction with ratios up to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 (mol mol−1). In addition, by using a large excess of alcohol, the products of the esterification reaction can be directly pumped into the column of the HPLC to determine the yield of the reaction without diluting with methanol. Thus, further experiments were performed at a 2-KLGA
24 (mol mol−1). In addition, by using a large excess of alcohol, the products of the esterification reaction can be directly pumped into the column of the HPLC to determine the yield of the reaction without diluting with methanol. Thus, further experiments were performed at a 2-KLGA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol molar ratio of 1
methanol molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1440 (mol
1440 (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol−1).
mol−1).
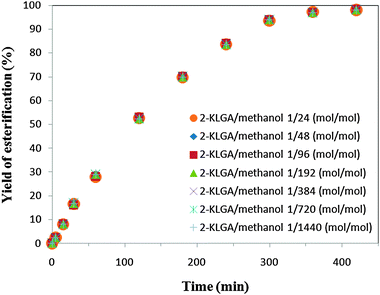 | ||
| Fig. 7 The yield of the esterification reaction at various molar ratios of 2-KLGA to methanol. | ||
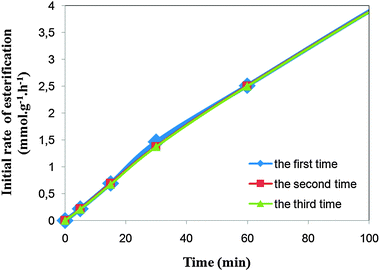 | ||
| Fig. 10 The effect of time on the initial rate of esterification in three cycles. | ||
Fig. 11 shows the XRD patterns of the KPW catalyst after recycling 3 times. It was found that the characteristic peaks of KPW after recycling 3 times also tended to be the same as those of the fresh KPW. There was no difference in the reflections between the KPW catalyst before and after recycling 3 times, indicated that the KPW catalyst can be recycled in the esterification reaction of 2-KLGA.
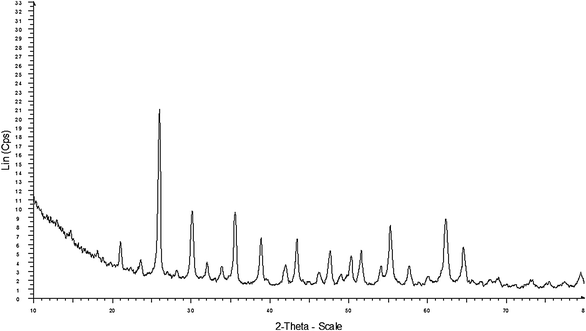 | ||
| Fig. 11 XRD pattern of the KPW catalyst after recycling 3 times. | ||
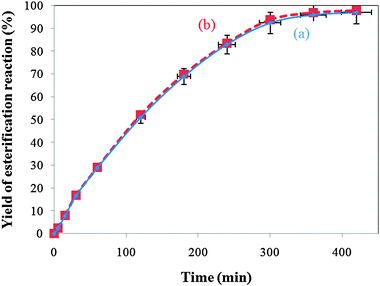 | ||
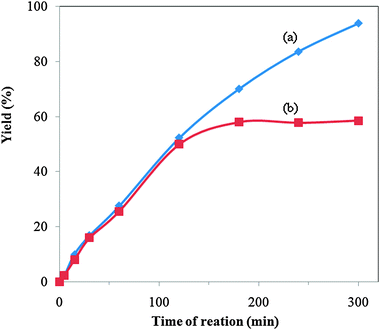
| Fig. 12 Yield of the esterification reaction using the KPW catalyst with and without scale up: (a) with scale up; (b) without scale up. | ||
4. Conclusion
The KPW catalyst was successfully synthesised by an ion exchange process between a potassium ion and proton. It was found that the KPW catalyst shows a high efficiency for the esterification of 2-KLGA. The KPW catalyst is a real heterogeneous catalysts in the esterification reaction. The catalytic activity of the heterogeneous KPW catalyst is slightly lower than that of the homogeneous HPW catalyst and is approximate to that of the commercial heterogeneous Amberlyst catalyst. The KPW catalysts are effective, environmentally friendly and recyclable. The present procedure also represents a clean, practical, simple and mild method for the esterification of 2-KLGA with excellent equilibrium yields of approximately 96% at 360 min.Acknowledgements
This work was supported by the National Key Programs on Research of the sciences and technology for developing the pharmaceutical industry to 2020 of the Ministry of Industry and Trade, Vietnam.References
- A. C. Blaga and T. Malutan, J. Chem. Eng. Data, 2011, 57, 431 CrossRef.
- J. J. Giovanno, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 9109 CrossRef.
- J. C. Hubbs, Patent US, No. 6,022,719, 2000 Search PubMed.
- M. N. Timofeeva, R. I. Maksimovskaya, E. A. Paukshtis and I. V. Kozhevnikov, J. Mol. Catal. A: Chem., 1995, 102, 73 CrossRef CAS.
- Y. Liu, E. Lotero and J. G. Goodwin, Jr., J. Catal., 2006, 242, 278 CrossRef CAS.
- J. M. Marchetti and A. F. Errazu, Biomass Bioenergy, 2008, 32, 892 CrossRef CAS.
- B. Y. Xu, H. J. Shang, X. M. Zheng and J. Hebei, Indian J. Sci. Technol., 2006, 23, 193 CAS.
- A. P. L. de Meireles, A. K. da Silva Rocha, V. I. Kozhevnikov and V. E. Gusevskaya, Appl. Catal., A, 2011, 409–410, 82 CrossRef CAS.
- M. A. Alsalme, V. P. Wiper, Z. Y. Khimyak, F. E. Kozhevnikova and V. I. Kozhevnikov, J. Catal., 2010, 276, 181 CrossRef.
- F. Bamoharram, Molecules, 2009, 14, 3214 CrossRef CAS.
- C. Rocchiccioli-Deltcheff, R. Thouvenot and R. Franck, Spectrochim. Acta, Part A, 1976, 32, 587 CrossRef.
- V. I. Kozhevnikov, Catal. Rev. Sci. Eng., 1995, 37, 311 Search PubMed.
- T. Okuhara, N. Mizuno and M. Misono, Adv. Catal., 1996, 41, 113 CrossRef CAS.
- I. V. Kozhevnikov, J. Mol. Catal. A: Chem., 2009, 305, 104 CrossRef CAS.
- E. Rafiee, M. Joshaghani, F. Tork, A. Fakhri and S. Eavani, J. Mol. Catal. A: Chem., 2008, 283, 1 CrossRef CAS.
- R. L. Pizzio, V. C. Cáceres and N. M. Blanco, Appl. Catal., A, 1998, 167, 283 CrossRef.
- B. B. Bardin and J. R. Davis, Appl. Catal., A, 2000, 200, 219 CrossRef CAS.
- Y. Zhang and S. Yang, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2008, 23, 346 CrossRef CAS.
- G. M. Brown, M. R. Noe-Spirlet, W. R. Busing and H. A. Levy, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1977, 1038 CrossRef CAS.
- G. Sunita, M. B. Devassy, A. Vinu, P. D. Sawant, V. V. Balasubramanian and B. S. Halligudi, Catal. Commun., 2008, 9, 696 CrossRef CAS.
- Z. Zhu and W. Yang, J. Phys. Chem. C, 2009, 113, 17025 CAS.
- F. Chambon, F. Rataboul, C. Pinel, A. Cabiac, E. Guillon and N. Essayem, Appl. Catal., B, 2011, 105, 171 CrossRef CAS.
- B. Xu, X. Zheng, W. Zhang, X. Zhang, Z. Zhang and H. Shang, Transactions of Tianjin University, 2008, 14, 37 CrossRef CAS.
- M. J. Marchetti and F. A. Errazu, Fuel, 2008, 87, 3477 CrossRef.
- W. Zhang, Y. Leng, D. Zhu, Y. Wu and J. Wang, Catal. Commun., 2009, 11, 151 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |