Symmetrical and unsymmetrical Brønsted acidic ionic liquids for the effective conversion of fructose to 5-hydroxymethyl furfural†
Deepali A.
Kotadia
and
Saurabh S.
Soni
*
Department of Chemistry, Sardar Patel University, Vallabh Vidyanagar, 388120, Gujarat, India. E-mail: soni_b21@yahoo.co.in; Fax: +91 2692 236475; Tel: +91 2692 226857-Ext.216
First published on 12th September 2012
Abstract
Symmetrical and unsymmetrical Brønsted acidic Ionic Liquids (ILs) have been synthesized via a one-pot halogen-free route and characterized by TGA-DTA and DSC to compare their stability and activity. The acidities of the four ILs have also been tested by the Hammett method. These ILs were utilised as catalysts for the dehydration of fructose to 5-hydroxymethylfurfural (HMF) in the presence of dimethylsulphoxide (DMSO) as solvent. The effects of reaction time, temperature and catalyst concentration were studied. The ILs are very efficient for the reaction at relatively lower temperature with very good HMF yield and selectivities. The used ILs could be easily recycled without any significant loss in fructose conversion and HMF yield.
1 Introduction
We are entering an epoch of diminishing availability of petrochemical resources used to produce the energy and chemical materials needed by society. A sustainable future for the chemical industry requires feedstocks based on renewable rather than steadily depleting sources. Such demands can be made by a shift from the petrochemical industry to one based on biomass resources.1–3 The conversion of renewable biomass resources into non-petroleum derived fuel and chemicals is becoming increasingly attractive to avoid escalation of global warming and diversify energy sources.4–6 One of the most attractive directions for this research is towards furan derivatives, such as 5-hydroxymethylfurfural (HMF), a sustainable precursor for the preparation of non-petroleum derived polymeric materials and fine chemicals.7–10 Dehydration of fructose to HMF has been carried out using a variety of organic acids (oxalic and maleic acid),11 inorganic acids (H2SO4, HCl),11 organic and inorganic salts,12,13 solid acids (ion exchange resins),14 zeolites15,16 and VOPO4.17 Dehydration was carried out in many solvents including water, organic solvents, mixed systems and more advanced reaction media such as sub- or supercritical solvents and their mixtures.18,19Recent studies have demonstrated the use of ionic liquids (ILs) on the selective dehydration of fructose into HMF with or without catalysts.7,20–29 Considering a few examples, it was found that the metal halides in 1-alkyl-3-imidazolium chloride are effective catalysts for converting fructose to HMF, among which chromium(II) chloride was uniquely effective, leading to conversion of glucose to HMF with very good yield.7 Moreau et al. reported that 1-H-3-methylimidazolium chloride can be used as both solvent and catalyst for the conversion of fructose to HMF with satisfactory yield.21 Use of a catalytic amount of acidic ILs for the efficient dehydration of fructose has also been reported earlier by Tong et al.30 and Valente et al.31 To date, various forms of ILs have been synthesized and studied for their catalytic activity.
Production of HMF from fructose with higher efficiency and greener routes at mild conditions is a challenging topic of great importance. As already seen, various types of ILs are used for dehydration reactions as solvent or as catalyst. But very few have focused on the nature and type of IL used for the catalysis. Most studies include ILs of routine usage with routine synthetic procedures and are less focused on their physicochemical properties. In a recent review by Riisager et al.32 there are a few points to focus on regarding the chemistry and engineering of fructose dehydration in IL, such as the influence of both the cation and anion of the IL on the reaction, the mechanism of dehydration (interaction of IL with fructose) and a study on the physicochemical properties of ILs. In this work, symmetrical and unsymmetrical Brønsted acidic ILs have been synthesized and characterized by their physicochemical properties, comparing their stability and utilising them as effective catalysts for the conversion of fructose to HMF. We have found that both the symmetrical ([MMIM]HSO4 and [MMBIM]HSO4) and unsymmetrical ILs ([PSMBIM]HSO4 and [HMBIM]HSO4) (Fig. 1) are very efficient for the conversion of fructose to 5-hydroxymethyl furfural. [PSMBIM]HSO4 showed the highest catalytic activity in the dehydration of fructose reaction.
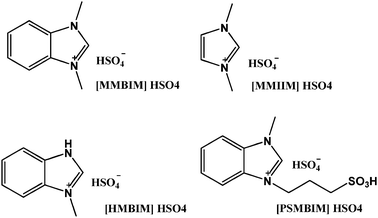 | ||
| Fig. 1 Structures of ionic liquids prepared and used in this paper. | ||
2 Results and discussion
2.1 Catalyst preparation and characterization
The fresh symmetrical ILs [MMIM]HSO4 and [MMBIM]HSO4 are liquid at room temperature; however, they solidify after a long time—a phenomenon that is consistent with the behaviour of other ILs.33 Additionally, the unsymmetrical IL [PSMBIM]HSO4 is a viscous liquid and [HMBIM]HSO4 is a waxy solid at room temperature. All the ILs are miscible with water and alcohols and immiscible with esters, alkanes and aromatic hydrocarbons. The synthesized ILs were characterized by 1H and 13C NMR spectroscopy, IR spectroscopy, Mass spectroscopy, DSC and TGA-DTA analysis. The thermogravimetric analyses (TGA-DTA) of ILs are shown in (Fig. S5–S8, ESI†). The TGA of [MMIM]HSO4 shows an initial weight loss of 11% which is followed by a steep shouldering from 290 to 400 °C indicating complete loss of hydrocarbons. The TGA of [MMBIM]HSO4 shows a gradual weight loss of 14% between 50 and 350 °C, followed by the decomposition of the IL cation at around 420 °C. The data shows slow thermal degradation of [MMBIM]HSO4 which in turn increases its stability from the similar kind of symmetrical IL [MMIM]HSO4. The TGA graph for [PSMBIM]HSO4 shows a sudden weight loss of about 20% which might be due to the alkane side chain degradation and moisture loss. The constant weight loss was observed with a shouldering at 235 °C, and at 400 °C, complete decomposition of hydrocarbons was observed. In the case of [HMBIM]HSO4, the TGA graph shows complete decomposition of the organic moiety at 420 °C. The thermal degradation of benzimidazolium based ILs viz. [PSMBIM]HSO4, [MMBIM]HSO4, and [HMBIM] HSO4 is slow compared to the imidazolium-based IL [MMIM]HSO4, thus showing higher stability. Differential scanning calorimetry (DSC) of [MMIM]HSO4, [MMBIM]HSO4, and [HMBIM]HSO4 was carried out to determine their melting point behaviours which were found to be 60, 25 and 122 °C, respectively, with a single first order transition. No other phase transitions were observed. From the result it can be seen that the symmetrical ILs [MMBIM]HSO4 and [MMIM]HSO4 have lower melting points than the two unsymmetrical ones. This property is very useful in organic synthesis for the effective separation of ILs from the reaction system by cooling which leads to solidification of the IL and decantation of the resultant material. Little attention has been given to the low melting properties of ILs, which needs more focus from the separation point of view. As the size and shapes of cations are important in determining the melting points and thermal stabilities of salts, the difference in the melting point and the higher stability of [MMBIM]HSO4 compared to [MMIM]HSO4 might be due to the large cation with delocalized charge and to weakly coordinating anions.34The acidity measurements of these ILs were conducted on UV-visible spectrophotometer with p-nitroaniline as the basic indicator according to the literature reported previously.35 Herein, the ILs and indicator were dissolved in water with concentrations of 50 and 0.1 mmol, respectively, and then their UV spectras were recorded. Their Hammett acidity functions (H0) were calculated using the equation H0 = pKa(I)aq+log ([I]/[IH+]) where pKa(I) is the pKa value of the p-nitroaniline(0.99) indicator solution, and [I] and [IH+] are the molar concentrations of the protonated and unprotonated forms of the indicator, respectively. The maximum absorbance of the unprotonated form of the indicator was observed at 380 nm in water. When the IL was added, the absorbance of the unprotonated form of the indicator decreased. As can be seen from the results (Table 1), [PSMBIM]HSO4 has the highest acidity among the four ILs followed by [HMBIM]HSO4, [MMBIM]HSO4 and [MMIM]HSO4.
| Ionic liquid | Absorbance [AU] | [I]% | [HI+]% | H 0 |
|---|---|---|---|---|
| Base | 1.66 | 100 | 0 | — |
| [MMIM]HSO4 | 1.45 | 87.45 | 12.55 | 1.84 |
| [MMBIM]HSO4 | 1.39 | 83.79 | 16.21 | 1.71 |
| [HMBIM]HSO4 | 1.24 | 74.61 | 25.39 | 1.47 |
| [PSMBIM]HSO4 | 1.10 | 66.45 | 33.55 | 1.29 |
2.2 Fructose dehydration reaction and recycling experiment
Dehydration of fructose to 5-hydroxymethylfurfural (Scheme 1) involves removal of three water molecules per molecule of fructose in the presence of an acid catalyst. Thus we started our work by testing the dehydration reaction using all the four ILs as catalyst. The results are shown in Fig. 2 which indicates the improved catalytic activity of [PSMBIM]HSO4 compared with the other three ILs. [PSMBIM]HSO4 gives 72.8% yield with 93% fructose conversion, while [MMBIM]HSO4, [HMBIM]HSO4 and [MMBIM]HSO4 show 70.7, 68.1 and 55.6% yield with 89.1, 87.1 and 74.4% respective fructose conversion. The results are in agreement with the Hammett acidity order of the ILs. As explained earlier, the characteristic low melting points of symmetrical ILs will lead to a convenient separation of the product. We attempted such a process for the separation of HMF from reaction mass; however, the problem we face is that both the IL and HMF have very close melting point ranges (∼26 °C); thus, on cooling, both the IL and HMF become solidified, leading to a more tedious work up. Among the ILs, [MMBIM]HSO4 and [PSMBIM]HSO4 both show comparable catalytic activity for the dehydration reaction, leading us to opt [PSMBIM]HSO4 for further studies. In this reaction DMSO was used as solvent as it avoids the formation of by-products such as levulinic and humic acid, giving higher selectivity for HMF.36,37 The yellow color of the reaction mixture is an indicator of the completion of reaction, thus the formation of a polymeric substance (humins) is avoided. Furthermore, for optimization of the reaction conditions, three parameters such as catalyst loading, reaction time, and reaction temperature were tested using [PSMBIM]HSO4 as a catalyst. Fig. 3 shows the effect of catalyst dosage with respect to the fructose conversion and HMF yield. It was found that the HMF yield increases gradually on increasing the catalyst amount from 0.02 to 0.1 g while the selectivity was maintained during the reaction. On further increasing the catalyst, a slight decrease in HMF yield was observed, which might be due to the increased availability of acidic sites, favouring the rehydration of HMF into levulinic acid. The dehydration reaction is next optimised with respect to reaction temperature as shown in Fig. 4. When the reaction temperature was 40 °C, 30.3% HMF yield was obtained for 1h reaction time. When the temperature was increased to 100 °C, 72.1% HMF yield with 93.6% fructose conversion was obtained in the same duration. At 120 °C, the fructose conversion rate was much higher but a decrease in the HMF yield was observed. Thus, from the results, a reaction temperature of 80 °C was chosen as the best temperature to perform the reaction. Furthermore, the effect of reaction time was optimized for the reaction which is shown in Fig. 5. It was found that on increasing the time from 0.5 to 2 h, the HMF yield and fructose conversion increased gradually during the reaction. On further increasing the reaction time to 3 h, a decrease in the yield and conversion was observed, which indicates that by-product formation increases at this stage. Thus, we consider 1h to be the optimized reaction time for the dehydration of fructose using [PSMBIM]HSO4.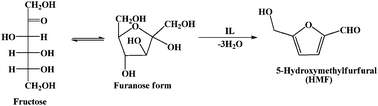 | ||
| Scheme 1 Reaction for dehydration of fructose. | ||
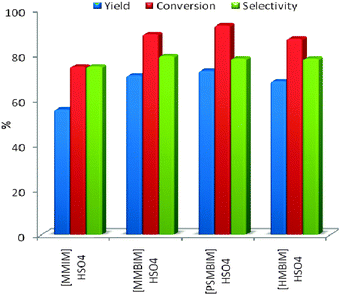 | ||
| Fig. 2 Fructose conversion, HMF yield and selectivity in different ILs. Reaction of fructose was performed on a 1.0 g scale (5.5 mmol), in the presence of various ILs in DMSO (10 ml); reaction time = 1 h, temperature = 80 °C. Yield and selectivity of HMF were obtained by HPLC analysis. | ||
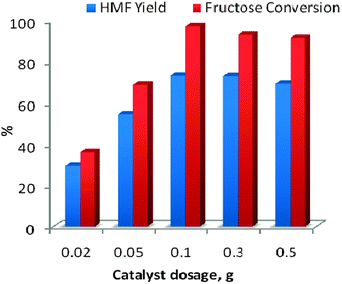 | ||
| Fig. 3 Effect of catalyst amount on HMF yield and fructose conversion. Reaction of fructose was performed on a 1.0 g scale (5.5 mmol), in the presence of various amounts of ILs in DMSO (10 ml); reaction time = 1 h, temperature = 80 °C. | ||
![Effect of temperature on HMF yield and fructose conversion. Reaction of fructose was performed on a 1.0 g scale (5.5 mmol) at various reaction temperatures in the presence of [PSMBIM]HSO4 (0.1 g) in DMSO (10 ml); reaction time = 1 h.](/image/article/2013/CY/c2cy20493b/c2cy20493b-f4.gif) | ||
| Fig. 4 Effect of temperature on HMF yield and fructose conversion. Reaction of fructose was performed on a 1.0 g scale (5.5 mmol) at various reaction temperatures in the presence of [PSMBIM]HSO4 (0.1 g) in DMSO (10 ml); reaction time = 1 h. | ||
![Effect of time on HMF yield and fructose conversion. Reaction of fructose was performed on a 1.0 g scale (5.5 mmol) at various reaction times in the presence of [PSMBIM]HSO4 (0.1 g) in DMSO (10 ml); reaction temperature = 80 °C.](/image/article/2013/CY/c2cy20493b/c2cy20493b-f5.gif) | ||
| Fig. 5 Effect of time on HMF yield and fructose conversion. Reaction of fructose was performed on a 1.0 g scale (5.5 mmol) at various reaction times in the presence of [PSMBIM]HSO4 (0.1 g) in DMSO (10 ml); reaction temperature = 80 °C. | ||
In green chemistry, the recycling of catalyst is very important. Thus, the recycling of [PSMBIM]HSO4 was carried out for five runs of the dehydration reaction (Fig. 6). Experiments were conducted at 80 °C for a reaction time of 1 h with 0.1 g of catalyst loading. After completion of the reaction, DMSO was removed and collected by distilling the reaction mixture under reduced pressure and the remaining mixture was extracted with ethyl acetate. Fructose and the IL were found to be insoluble in ethyl acetate and so can be easily separated, while HMF was the sole product in ethyl acetate phase and can be extracted easily. The remaining IL containing mixture was then vaccum dried at 80 °C for 6 h and then reused as such for the next run by adding an equal amount of fructose. Recycling of the IL shows comparable results, giving a good-to-moderate HMF yield after five catalytic runs.
![Recycling of [PSMBIM]HSO4 for the dehydration reaction. Reaction conditions for each run: Fructose (1 g, 5.5 mmol), [PSMBIM]HSO4 (0.1 g, recycled), DMSO (10 ml), reaction time = 1 h, temperature =80 °C.](/image/article/2013/CY/c2cy20493b/c2cy20493b-f6.gif) | ||
| Fig. 6 Recycling of [PSMBIM]HSO4 for the dehydration reaction. Reaction conditions for each run: Fructose (1 g, 5.5 mmol), [PSMBIM]HSO4 (0.1 g, recycled), DMSO (10 ml), reaction time = 1 h, temperature =80 °C. | ||
After optimising the reaction conditions and testing the recyclability of the catalyst, we next attempted to understand the possible mechanism of the reaction in the presence of IL [PSMBIM]HSO4 (Scheme 2). The conversion of fructose to HMF is via a sequence of consecutive steps leading to the elimination of three water molecules. The sulfonic acid part of the cation of [PSMBIM]HSO4 (acting as an electrophile) activates the C–OH bond of the anomeric carbon of fructose while the anion HSO4 (acting as nucleophile) activates the C–H of the C1 of fructose via hydrogen bonding. This activation results in the removal of the first water molecule and the formation of an enol intermediate, which is the rate determining step.38 The subsequent elimination of a second and third water molecule is driven by HSO4 (which is more accessible), with the hydrogen of the –OH groups at C1 and at C5 of the intermediate, respectively. As a whole [PSMBIM]HSO4 does not act as a proton reservoir. The concerted catalysis by the [PSMBIM] cation and HSO4 anion might be responsible for the observed catalytic results. Thus, the catalytic action of the IL [PSMBIM]HSO4 in the dehydration reaction will be enhanced by the accessibility of the cation and the hydrogen bonding strength of the anion. The comparative or lower performance of other ILs might be due to the reduced accessibility of the cation in facilitating the enolisation step. In symmetrical ILs, the acidity or the polarization of the substrate would be achieved using the C2 atom.39 The acidity of the C2 proton will be highly dependent upon the delocalisation of the positive charge borne by the imidazolium and benzimidazolium cations. The delocalisation will be increased in the case of the benzimidazolium cation which might lead to an increase in C–H proton polarity which in turn leads to the higher acidity of the C2 proton. Meanwhile, due to reduced delocalisation, the acidic character of the imidazolium cation will be reduced and hence, it is less efficient.
To compare the applicability and the efficiency of our catalysts with the reported catalysts for the reaction, we have tabulated the results of the fructose dehydration reaction in Table 2. As it is shown in Table 2, [PSMBIM]HSO4 remarkably improved the dehydration reaction in terms of reaction time, reaction temperature, HMF yield and fructose conversion.
| Entry | Catalyst used | T/°C | t/min | Conversion% | Yield% | Ref. |
|---|---|---|---|---|---|---|
| a IER corresponds to ion exchange resin. | ||||||
| 1 | SO3H-IER/[BMIM]Cla | 80 | 10 | 98.6 | 83.3 | 23 |
| 2 | PTA/ML-101 | 130 | 30 | 84 | 63 | 24 |
| 3 | B(OH)3/NaCl | 150 | 45 | 92 | 60 | 25 |
| 4 | ChoCl/Citric acid | 80 | 60 | 97.6 | 91.4 | 26 |
| 5 | Si-3-IL-HSO4 | 130 | 30 | 99.9 | 63 | 28 |
| 6 | Cr[(DS)H2PW12O40] | 150 | 120 | 77.1 | 52.7 | 29 |
| 7 | [NMP]+[CH3SO3]- | 90 | 120 | 83 | 72.3 | 30 |
| 8 | [PSMBIM]HSO4 | 80 | 60 | 93 | 72.8 | Our work |
3 Conclusions
In this paper, four acidic ionic liquids—symmetrical ILs [MMIM]HSO4 and [MMBIM]HSO4, and unsymmetrical ILs [PSMBIM]HSO4, [HMBIM]HSO4—have been effectively synthesized and characterized by TGA-DTA and DSC, and their hammett acidity function was also calculated. Of all the ILs used for fructose dehydration, [MMBIM]HSO4 and [PSMBIM]HSO4 showed comparable results. Among the two, [PSMBIM]HSO4 shows very high stability, acidity and catalytic activity for the dehydration of fructose, and thus was further used for the optimisation of the reaction with respect to different parameters such as the effect of catalyst loading, reaction time, and reaction temperature on the HMF yield. The process developed was shown to be efficient with excellent fructose conversion giving very good HMF yield at 80 °C in a reaction time of 1 h. The catalyst was effectively recycled five times with little loss of activity. From the above discussion, it is clear that unsymmetrical ILs show comparatively better activities than symmetrical ILs for the dehydration of fructose.4 Experimental
4.1 Materials, instrumentation and analysis methods
Imidazole, benzimidazole and dimethyl sulphate were purchased from Spectrochem, India. 5-Hydroxymethylfurfural and fructose were purchased from Sigma-Aldrich, India. The synthesized ILs were characterized by IR (ABB FTIR, Canada), 1H and 13C NMR (400 MHz, Bruker Scientific, Switzerland, advance), and MS (Thermoscientific, USA). The TGA data were obtained at a heating rate of 5 °C min−1 on a TGA-DTA instrument (TA instruments model 5000/2960 thermogravimetric analyser, USA). DSC data were obtained in a sealed aluminium pan with a heating rate of 2 °C min−1 on a DSC instrument (TA instrument, USA). UV-vis spectra were recorded on a UV-160A spectrophotometer (Shimadzu Corporation, Japan) in water. For the dehydration reaction, the amount of HMF was calculated using an external standard analysed by an Agilent 1260 series HPLC (Agilent Technologies, USA) with a ZORBAX Eclipse plus C18 column (4.6 (I.D.) × 100 mm (L); 3.5 μm particle size) using methanol/water (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 v/v) as the mobile phase at a flow rate of 0.7 ml min−1. The amount of fructose was calculated using an external standard analysed using a Shodex RI 701 HPLC with an RI detector (Merck, Hitachi LC100, isocratic pump) and water 250 × 4.6 5 μ amino column using milliQ water as the mobile phase at a flow rate of 0.6 ml min−1. HMF was identified by 1H and 13C NMR spectra and by GC-MS (GC-mass spectrometry, Perkin Elmer, Auto system XL, GC+).
80 v/v) as the mobile phase at a flow rate of 0.7 ml min−1. The amount of fructose was calculated using an external standard analysed using a Shodex RI 701 HPLC with an RI detector (Merck, Hitachi LC100, isocratic pump) and water 250 × 4.6 5 μ amino column using milliQ water as the mobile phase at a flow rate of 0.6 ml min−1. HMF was identified by 1H and 13C NMR spectra and by GC-MS (GC-mass spectrometry, Perkin Elmer, Auto system XL, GC+).
4.2 Synthesis of Brønsted acidic ionic liquids
Spectral data [MMBIM]HSO4: 1H NMR (D2O, δ/ppm relative to TMS) = 7.71–7.73 (m 2H), 7.56–7.58 (m 2H), 3.97 (s 6H); 13C NMR (D2O δ/ppm) = 142.3, 132.1, 126.7, 112.9, 32.8; MS: m/z (+) 146.7, m/z (−) 194.69; IR (KBr) (ν cm−1) = 754, 886, 1177, 1285, 1453, 1574, 2487, 2604, 3061, 3416.
Spectral data [MMIM]HSO4: 1H NMR (D2O, δ/ppm relative to TMS) = 8.51 (s 1H), 7.28–7.29 (t 2H), 3.77 (s 6H); 13C NMR (D2O δ/ppm) = 136.5, 123.4, 55.4, 35.6; MS: m/z (+) 97.2, m/z (−) 194.69; IR (KBr) (ν cm−1) = 756, 849, 1038, 1169, 1574,3117, 3433.
Spectral data [PSMBIM]HSO4: 1H NMR (DMSO, δ/ppm relative to TMS) = 9.65 (s 1H), 7.97–8.06 (d 2H), 7.64–7.68 (d 2H), 4.59–4.63 (t 2H), 4.05 (s 3H), 2.58–2.62 (t 2H), 2.50 (s OH), 2.17–2.24 (m 2H); 13C NMR (D2O δ/ppm) = 143.3, 132.3, 131.3, 126.96, 126.92, 114.0, 113.8, 48.0, 45.8, 33.6, 25.5; MS: m/z (+) 255.11, m/z (−) 9787; IR (KBr) (ν cm−1) = 563, 748, 868, 1022, 1142, 1169, 1215, 1466, 1578, 1717, 3009, 3171, 3352.
Spectral data [HMBIM]HSO4: 1H NMR (D2O, δ/ppm relative to TMS) = 8.71 (s 1H), 7.23–7.27 (m 2H), 7.14–7.17 (m 2H), 3.65 (s 3H); 13C NMR (D2O δ/ppm) = 140.0, 130.9, 129.6, 126.5, 126.1, 113.9, 112.1, 32.6; MS: m/z (+) 133.7, m/z (−) 97.86; IR (KBr) (ν cm−1) = 570, 750, 850, 1040, 1170, 1360, 1460, 1560, 2920, 3050, 3140, 3410.
4.3 Dehydration reaction for fructose
In a typical reaction, a 50 ml round-bottom flask was charged with fructose (1 g), ionic liquid (0.1 g) and DMSO (10 ml) and heated at 80 °C in a water bath for 1 h. After reaction completion, the sample was diluted with ultrapure water before analysis. For the recycling of the IL, HMF was extracted from the mixture with ethyl acetate (4 × 10 ml). After extraction, the IL was vaccum dried at 80 °C for 6 h.Acknowledgements
The authors are thankful to Prof. V. K. Parmar, Mr Saumil V. Patel and Mr Tushar J. Vaja for the HPLC measurements. DAK is thankful to UGC-Delhi for financial support in terms of a meritorious fellowship.Notes and references
- B. Kamm and M. Kamm, Appl. Microbiol. Biotechnol., 2004, 64, 137–145 ( Adv. Biochem. Eng./Biotechnol. , 2007 , 105 , 175–204 ) CrossRef CAS.
- C. H. Christensen, J. Rass-Hansen, C. C. Marsden, E. Taarning and K. Egeblad, ChemSusChem, 2008, 1, 283–289 CrossRef CAS.
- Y. Roman-Leshkov, C. J. Barrett, Z. Y. Liu and J. A. Dumesic, Nature, 2007, 447, 982–985 CrossRef CAS.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS.
- P. Gallezot, Green Chem., 2007, 9, 295–302 RSC.
- H. B. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS.
- G. Yong, Y. G. Zhang and J. Y. Ying, Angew. Chem., Int. Ed., 2008, 47, 9345–9348 CrossRef CAS.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS.
- Y. Roman-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933–1937 ( Green Chem. , 2007 , 9 , 342–350 ) CrossRef CAS.
- F. S. Asghari and H. Yoshida, Ind. Eng. Chem. Res., 2006, 45, 257–263 CrossRef.
- C. Fayet and J. Gelas, Carbohydr. Res., 1983, 122, 59–68 CrossRef.
- S. K. Tyrlik, D. Szerszen, M. Olejnik and W. Danikiewicz, Carbohydr. Res., 1999, 315, 268–272 CrossRef CAS.
- D. Mercadier, L. Rigal, A. Gaset and J. P. Gorrichon, J. Chem. Technol. Biotechnol., 1981, 31, 489–496 ( J. Chem. Technol. Biotechnol. , 1981 , 31 , 497–502 ) CrossRef CAS.
- C. Moreau, R. Durand, C. Pourcheron and S. Razigade, Ind. Crops Prod., 1994, 3, 85–90 CrossRef CAS.
- C. Moreau, R. Durand, S. Razigade, J. Duhamet, P. Faugeras, P. Rivalier, P. Ros and G. Avignon, Appl. Catal., A, 1996, 145, 211–224 CrossRef CAS.
- C. Carlini, P. Patrono, A. M. R. Galletti, A. Maria and G. Sbrana, Appl. Catal., A, 2004, 275, 111–118 CrossRef CAS.
- F. W. Lichtenthaler, Acc. Chem. Res., 2002, 35, 728–737 CrossRef CAS.
- B. F. M. Kuster, Starch/Staerke, 1990, 42, 314–321 CrossRef CAS.
- C. Lansalot-Matras and C. Moreau, Catal. Commun., 2003, 4, 517–520 CrossRef CAS.
- C. Moreau, A. Finiels and L. Vanoye, J. Mol. Catal. A: Chem., 2006, 253, 165–169 CrossRef CAS.
- Q. Bao, K. Qiao, D. Tomida and C. Yokoyama, Catal. Commun., 2008, 9, 1383–1388 CrossRef CAS.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith Jr., Green Chem., 2009, 11, 1327–1331 RSC.
- Y. Zhang, V. Degirmenci, C. Li and E. J. M. Hensen, ChemSusChem, 2011, 4, 59–64 CrossRef CAS.
- T. S. Hansen, J. Mielby and A. Riisager, Green Chem., 2011, 13, 109–114 RSC.
- S. Hu, Z. Zhang, Y. Zhou, B. Han, H. Fan, W. Li, J. Song and Y. Xie, Green Chem., 2008, 10, 1280–1283 RSC.
- C. Sievers, I. Musin, T. Marzialetti, O. M. B. Valenzuela, P. K. Agrawal and C. W. Jones, ChemSusChem, 2009, 2, 665–671 CrossRef CAS.
- K. B. Sidhpuria, A. L. D. da Silva, T. Trindade and J. A. P. Coutinho, Green Chem., 2011, 13, 340–349 RSC.
- S. Zhao, M. Cheng, J. Li, J. Tian and X. Wang, Chem. Commun., 2011, 47, 2176–2178 RSC.
- X. Tong and Y. Li, ChemSusChem, 2010, 3, 350–355 CrossRef CAS.
- S. Lima, P. Neves, M. M. Antunes, M. Pillinger, N. Ignatyev and A. A. Valente, Appl. Catal., A, 2009, 363, 93–99 CrossRef CAS.
- T. Stahlberg, W. Fu, J. M. Woodley and A. Riisager, ChemSusChem, 2011, 4, 451–458 CrossRef CAS.
- C. P. Fredlake, J. M. Crosthwaitte, D. G. Hert, S. N. V. K. Aki and J. F. Brennecke, J. Chem. Eng. Data, 2004, 49, 954–964 CrossRef CAS.
- P. Wassercheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, 2002 Search PubMed.
- C. Thomazeau, H. O. Bourbigou, L. Magna, S. Luts and B. Gilbert, J. Am. Chem. Soc., 2003, 125, 5264–5265 CrossRef CAS.
- Y. Nakamura and S. Morikawa, Bull. Chem. Soc. Jpn., 1980, 53, 3705–3706 CrossRef CAS.
- R. M. Musau and R. M. Munavu, Biomass, 1987, 13, 67–74 CrossRef CAS.
- O. O. James, S. Maity, L. A. Usman, K. O. Ajanaku, O. O. Ajani, T. O. Siyanbola, S. Sahu and R. Chaubey, Energy Environ. Sci., 2010, 3, 1833–1850 CAS.
- T. L. Amyes, S. T. Diver, J. P. Richard, F. M. Rivas and K. Toth, J. Am. Chem. Soc., 2004, 126, 4366–4374 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: ESI includes 1H, 13C NMR and TGA of all the ILs and along with characterization of HMF using GC-MS, HPLC and 1H and 13C NMR. See DOI: 10.1039/c2cy20493b |
| This journal is © The Royal Society of Chemistry 2013 |

![Plausible mechanism for fructose dehydration using [PSMBIM]HSO4 as catalyst.](/image/article/2013/CY/c2cy20493b/c2cy20493b-s2.gif)