Graphite oxide activated zeolite NaY: applications in alcohol dehydration†
Alexander D.
Todd
a and
Christopher W.
Bielawski
*ab
aDepartment of Chemistry and Biochemistry, The University of Texas at Austin, 1 University Station, A1590, Austin, TX, 78712, USA. E-mail: bielawski@cm.utexas.edu
bWorld Class University (WCU) Program of Chemical Convergence for Energy & Environment (C2E2), School of Chemical and Biological Engineering, Seoul National University, Seoul 151-742, Korea
First published on 19th October 2012
Abstract
A mixture of graphite oxide (GO) and the zeolite NaY (Si/Al = 5.1) was used to dehydrate various alcohols to their respective olefinic products. Using conditions optimized for 4-heptanol (15 wt% GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt/wt), 150 °C, 30 min), a series of secondary and tertiary aliphatic alcohols were cleanly dehydrated in moderate to excellent conversions (27.5–97.2%). Several primary alcohols were also dehydrated, although higher catalyst loadings (200 wt% GO–NaY (1
1 wt/wt), 150 °C, 30 min), a series of secondary and tertiary aliphatic alcohols were cleanly dehydrated in moderate to excellent conversions (27.5–97.2%). Several primary alcohols were also dehydrated, although higher catalyst loadings (200 wt% GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and longer reaction times (3 h) were required. The enhanced dehydration activity was attributed to the ability of GO to convert NaY to an acidic form and without the need for ammonium cation exchange and/or high temperature calcination.
1) and longer reaction times (3 h) were required. The enhanced dehydration activity was attributed to the ability of GO to convert NaY to an acidic form and without the need for ammonium cation exchange and/or high temperature calcination.
Introduction
The use of solid acid catalysts (SACs) has become increasingly important for applications in industry as well as academia. The benign nature of SACs has been shown to be an effective alternative to conventional mineral acids such as H2SO4, HCl, HF, and H3PO4.1 Moreover, SACs possess advantages over their homogeneous counterparts, particularly in terms of separation, adaptation to flow reactor systems, and negating the need for corrosion resistant equipment.1,2 The composition and structure of SACs is diverse and includes alumina and modified alumina,3,4 modified zirconia,5 reactive clays,6 sulfonated polymer resins,7–9 carbon-based materials,10–15 and zeolites.16–19 Zeolites, which are high-surface area aluminosilicates with a well-defined structure, are among the most widely used SACs largely because they are employed in the petroleum industry as fluid cracking catalysts.20 Although zeolites can assume a variety of structures and compositions, the protic form (i.e., H+ as the counter cation) has shown remarkable utility in the dehydration of alcohols as well as other organic transformations that require acid catalysts (e.g., deprotections,21 transesterifications,22 macrolactonizations of hydroxyacids,23 and cyclizations24).There are two widely used methods for preparing the protic form of zeolites: (1) ion exchange or (2) high temperature (>400 °C) calcination of an NH4+ exchanged precursor. For the former process, only high silica zeolites effectively undergo ion exchange with protons via mineral acid washing. Zeolites with low silica content (e.g., NaY) are prone to structural damage via dealumination when exposed to strong mineral acids.25
Carbon-based SACs have also been used in various catalytic applications. For example, activated carbons treated with different chemical reagents (HNO3, Cl2, NH3, air, etc.) have been examined for their abilities to dehydrate/dehydrogenate isopropanol.13 Although good conversions were observed in these reactions, high catalyst loadings (>1000 wt%) were required. Sulfonated pyrolyzed carbon sources such as olive stone, sucrose and starch have also been utilized as SACs for the dehydration of ethanol, the esterification of glycerol, and the hydration of 2,3-dimethyl-2-butene, respectively.11,12,14 These sulfonated carbon materials maintain a high level of acidity even in the presence of water which offers advantages over traditional inorganic oxides such as zeolites, whose acidity can be compromised under aqueous conditions.11 Unfortunately, the pyrolysis step required to prepare these sulfonated catalysts utilizes temperatures that commonly exceed 400 °C.
Recently, graphite oxide (GO), an acidic, insoluble carbon-based material known for more than 150 years,26 was found to mediate a variety of acid catalyzed transformations,27–29 including the ring opening polymerization of lactones and the dehydration polymerization of benzyl alcohol.28,29 GO is an ideal candidate for use in solid acid catalysis due to its relatively low cost, heterogenous state, and ease of preparation.30 The material is typically synthesized in one step by treating natural flake graphite with KMnO4 in H2SO4 at room temperature followed by filtration. As a result of the oxidation process, various functional groups are installed (e.g., hydroxyl, epoxide, carboxylic acid, etc.) and enable the material with catalytic properties.31
Although heterogenous composites comprised of carbons and zeolites have gained attention for their applications in separation, gas absorption, templating agents, and photocatalysis,32–36 the catalytic activities of such composites are relatively unexplored. Considering the complementary acidic characteristics displayed by zeolites and GO, we hypothesized that the two materials may function in a synergistic manner to promote their intrinsic catalytic activities. Herein we explore the abilities of mixtures of NaY and GO to facilitate the dehydration of alcohols, a reaction of significant industrial importance.37 We show that the GO exchanges its protons with the zeolite’s sodium ions and that the resulting mixture facilitates the dehydration of primary, secondary, and tertiary alcohols under mild conditions.
Results and discussion
As summarized in Table 1, initial studies focused on the dehydration of 1-octanol using a mixture of either GO–NaY or GO–NH4Y. In a preliminary experiment, 1-octanol (50.0 mg), GO (50 mg), zeolite (50 mg) and CDCl3 (0.5 mL) were added to a pressure tube which was sealed and then mixed with constant stirring at 150 °C for 3 h. Upon cooling to room temperature, the contents of the reaction vessel were filtered through a 0.2 μm PTFE syringe filter into a NMR tube containing a standard (mesitylene, 10 μL) and the conversion of starting material to product were measured by quantitative 1H NMR spectroscopy. The highest conversion of 1-octanol to its dehydration products (46.9% to a mixture of olefins and 32.3% to di-n-octyl ether) was achieved using a combination of GO and NaY (entry 1). A similar reaction that utilized GO and NH4Y as the catalyst was also performed but only a small amount of the desired products was obtained under otherwise identical conditions (entry 2). Likewise, neither GO nor NaY independently afforded appreciable quantities of desired dehydration products (entries 3 and 4). Although the treatment of 1-octanol with 200 wt% GO (entry 3) resulted in a relatively high substrate conversion, the majority of the products were intractable, possibly due to the oxidation of the 1-octanol starting material.‡ Based on these preliminary results, we surmised that GO and the NaY operated in a synergistic manner to facilitate the aforementioned alcohol dehydration reaction.| Entry | Catalystb | Olefinsc (% conv.) | Ether (% conv.) | Total (% conv.) |
|---|---|---|---|---|
a Commercial Y zeolites were obtained from Zeolyst International (Si/Al = 5.1) and used as received. The products consisted of a mixture of C8 olefins and di-n-octyl ether which were identified by 1H and 13C NMR spectroscopy. Because the products were not separable by gas chromatography (GC), the catalyst selectivities were not quantified.
b The catalyst loading was based on the mass of 1-octanol and the GO and zeolite were used in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (wt/wt) ratio (200 wt% total).
c The conversions were determined by 1H NMR spectroscopy using mesitylene as an internal standard.
d A series of unidentified products were obtained. Mass spectrometry showed the presence of a species with an m/z = 287 consistent with the MH+ for di-n-octyl carbonate and a species with an m/z = 159 consistent with protonated methyl octanoate. An unidentified species possessing a m/z = 314 was also present. 1 (wt/wt) ratio (200 wt% total).
c The conversions were determined by 1H NMR spectroscopy using mesitylene as an internal standard.
d A series of unidentified products were obtained. Mass spectrometry showed the presence of a species with an m/z = 287 consistent with the MH+ for di-n-octyl carbonate and a species with an m/z = 159 consistent with protonated methyl octanoate. An unidentified species possessing a m/z = 314 was also present.
|
||||
| 1 | GO–NaY | 46.9 | 32.3 | 83.9 |
| 2 | GO–NH4Y | 4.4 | <1.0 | 15.8 |
| 3d | GO | 3.7 | 5.0 | 77.2 |
| 4 | NaY | 6.1 | <1.0 | 10.0 |
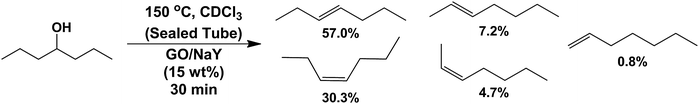
To optimize the dehydration conditions, 4-heptanol was chosen as a model substrate and numerous reaction parameters were varied, including: catalyst loading (5–50 wt% with respect to the alcohol), time (10–60 min), temperature (100–180 °C), and catalyst composition (e.g., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 GO–NaY wt/wt). The results of the optimization study are summarized in Table 2. The conversion of the alcohol dehydration reaction as well as the total alcohol conversion were measured using 1H NMR spectroscopy. Entry 1 in Table 2 showed that GO (15 wt%) was capable of dehydrating 4-heptanol to its heptene isomers in high conversions (88.5%); however, further investigation revealed that loadings could be decreased (7.5 wt% GO) when paired with NaY (7.5 wt%) without detriment to the outcome of the reaction (entry 10). As previously observed with 1-octanol, NaY was ineffective for facilitating the dehydration of 4-heptanol (entry 2) as was graphite–NaY (entry 18), which indicated the functionality and acidity of the GO were critical for the observed dehydrations. Entries 13 and 14, which summarize experiments that involved varying the weight ratio of GO and NaY, revealed evidence for an interaction between the two materials. For instance, when the weight ratio of GO–NaY was 3
1 GO–NaY wt/wt). The results of the optimization study are summarized in Table 2. The conversion of the alcohol dehydration reaction as well as the total alcohol conversion were measured using 1H NMR spectroscopy. Entry 1 in Table 2 showed that GO (15 wt%) was capable of dehydrating 4-heptanol to its heptene isomers in high conversions (88.5%); however, further investigation revealed that loadings could be decreased (7.5 wt% GO) when paired with NaY (7.5 wt%) without detriment to the outcome of the reaction (entry 10). As previously observed with 1-octanol, NaY was ineffective for facilitating the dehydration of 4-heptanol (entry 2) as was graphite–NaY (entry 18), which indicated the functionality and acidity of the GO were critical for the observed dehydrations. Entries 13 and 14, which summarize experiments that involved varying the weight ratio of GO and NaY, revealed evidence for an interaction between the two materials. For instance, when the weight ratio of GO–NaY was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entry 13), only a 15.8% conversion to product was measured. Conversely, when a 1
1 (entry 13), only a 15.8% conversion to product was measured. Conversely, when a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 weight ratio mixture of GO–NaY was used, the conversion to product was 86.6% (entry 14). Collectively, these results demonstrated that the zeolite had a relatively greater influence than the GO on the catalytic activity of the GO–NaY mixture.
3 weight ratio mixture of GO–NaY was used, the conversion to product was 86.6% (entry 14). Collectively, these results demonstrated that the zeolite had a relatively greater influence than the GO on the catalytic activity of the GO–NaY mixture.
| Entry | Catalyst | Loading (wt%) | Temperature (°C) | Time (min) | Olefinsa (% conv.) | Totalb (% conv.) |
|---|---|---|---|---|---|---|
| a The product selectivities were determined by GC and the substrate conversions were determined by quantitative 1H NMR spectroscopy using mesitylene as the internal standard. b The total conversion reflects the disappearance of the 4-heptanol pentet at 3.58 ppm in the 1H NMR spectrum of the reaction mixture after being subjected to the indicated conditions. c Used as received from Bay Carbon SP-1. | ||||||
| 1 | GO | 15 | 150 | 30 | 88.5 | >99.0 |
| 2 | NaY | 15 | 150 | 30 | 3.8 | 12.0 |
| 3 | GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
5 | 150 | 40 | 50.6 | 60.8 |
| 4 | GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
10 | 150 | 40 | 78.1 | 84.4 |
| 5 | GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
15 | 150 | 40 | 88.9 | >99.0 |
| 6 | GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
20 | 150 | 40 | 60.3 | >99.0 |
| 7 | GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
50 | 150 | 40 | 68.3 | >99.0 |
| 8 | GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
15 | 150 | 10 | 72.9 | 73.4 |
| 9 | GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
15 | 150 | 20 | 81.4 | 82.8 |
| 10 | GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
15 | 150 | 30 | 97.0 | >99.0 |
| 11 | GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
15 | 150 | 45 | 77.8 | >99.0 |
| 12 | GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
15 | 150 | 60 | 87.3 | >99.0 |
| 13 | GO–NaY (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
15 | 150 | 30 | 15.8 | 20.8 |
| 14 | GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
15 | 150 | 30 | 86.6 | 89.8 |
| 15 | GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
15 | 100 | 30 | 2.7 | 13.5 |
| 16 | GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
15 | 130 | 30 | 40.3 | 50.9 |
| 17 | GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
15 | 180 | 30 | 73.5 | >99.0 |
| 18c | Graphite–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
15 | 150 | 30 | <1.0 | 8.2 |
To further investigate the nature of the interaction between GO and NaY during the aforementioned dehydration reactions, a series of experiments were performed; the results summarized in Table 3. The protic form of zeolite Y (HY) displayed good conversion (79.2%) of 4-heptanol to the desired mixture of olefinic products at low loadings (entry 1). This result led us to postulate that a protic form of NaY was the active catalyst in the GO–NaY mixture. Thus, studies were undertaken to determine if Na+–H+ exchange was occurring between the two materials. Entries 4–7 summarizes a series of experiments where GO (7.5 wt%) was placed in a reaction vessel then heated at 40 or 80 °C in the presence of CDCl3 and 4-heptanol for 10 or 30 min, respectively. The supernatant was then filtered into a different reaction vessel containing NaY (7.5 wt%; entries 4 and 6) or no zeolite (entries 5 and 7), then heated to 150 °C for 30 min. The experiments summarized in entries 4 and 6 show that 26.3% and 79.8% of the alcohol converted to an olefinic product, respectively. In contrast, when the zeolite was not included in the reaction mixture (entries 5 and 7), less than 2% of the alcohol underwent dehydration. Collectively, the results suggested to us that upon dispersion of GO in the alcohol and solvent, H+ was released from GO which then exchanged with Na+ to convert the zeolite into a protic form.§ Additionally, the protic form of the zeolite proved to be a more effective dehydration catalyst than a mineral acid (cf., entry 6 vs. entry 1).¶
| Entry | Catalyst | Loading (wt%) | Temperature (°C) | Time (min) | Olefins (% conv.) | Total (% conv.) |
|---|---|---|---|---|---|---|
| a GO (7.5 wt% with respect to 4-heptanol) was heated in a CDCl3–4-heptanol solution for 10 min at 40 °C, and the supernatant was filtered through a 0.2 μm PTFE syringe filter into a separate reaction vessel containing 7.5 wt% NaY. The resulting mixture was then heated at 150 °C for 30 min. b A procedure similar to the one used in entry 4 was used with the exception that the CDCl3–4-heptanol supernatant was filtered into an empty reaction vessel and heated to 150 °C for 30 min. c GO (7.5 wt% with respect to 4-heptanol) was heated in a CDCl3–4-heptanol solution for 30 min at 80 °C, and the supernatant was filtered into a separate reaction vessel containing NaY (7.5 wt%). The resulting mixture was then heated to 150 °C for 30 min. Heating a mixture of 7.5 wt% GO and 4-heptanol at 80 °C for 30 min resulted in no conversion to C7 olefins as determined by quantitative 1H NMR spectroscopy. d The reaction was performed in a manner similar to the one that described in entry 6 with the exception that the CDCl3–4-heptanol supernatant was filtered into an empty reaction vessel and heated to 150 °C for 30 min. | ||||||
| 1 | HY | 5 | 150 | 30 | 79.2 | >99.0 |
| 2 | GO–HY | 15 | 150 | 30 | 94.3 | >99.0 |
| 3 | GO–NH4Y | 15 | 150 | 30 | 25.2 | 30.1 |
| 4a | GO then NaY | 7.5 | 150 | 30 | 26.3 | 35.6 |
| 5b | – | N/A | 150 | 30 | <1.0 | 3.6 |
| 6c | GO then NaY | 7.5 | 150 | 30 | 79.8 | >99.0 |
| 7d | – | N/A | 150 | 30 | 1.7 | 3.3 |
The recyclability of the catalyst mixture was also examined under the optimized conditions (15 wt% GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 150 °C, 30 min) using 4-heptanol as the starting material; the results are summarized in Fig. 1. The total conversion of alcohol to product steadily decreased with each subsequent cycle by approximately 20%, and may be due the deactivation of GO or NaY, or a combination thereof. GO has been shown to undergo thermal deoxygenation/reduction38–40 as well as chemical reduction in the presence of alcohols41–43 at elevated temperatures. Likewise, the deactivation of zeolites for alcohol dehydration has been attributed to the accumulation of byproducts as a result of side reactions which blocks the substrate from active sites within the pores of the material.44
1), 150 °C, 30 min) using 4-heptanol as the starting material; the results are summarized in Fig. 1. The total conversion of alcohol to product steadily decreased with each subsequent cycle by approximately 20%, and may be due the deactivation of GO or NaY, or a combination thereof. GO has been shown to undergo thermal deoxygenation/reduction38–40 as well as chemical reduction in the presence of alcohols41–43 at elevated temperatures. Likewise, the deactivation of zeolites for alcohol dehydration has been attributed to the accumulation of byproducts as a result of side reactions which blocks the substrate from active sites within the pores of the material.44
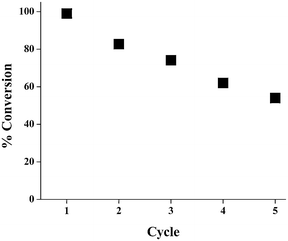 | ||
Fig. 1 Plot of the percent conversion of starting material to product versus reaction cycle for the dehydration of 4-heptanol using GO–NaY as the catalyst. Conditions: 15 wt% GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt/wt), 150 °C. Each reaction cycle was performed for a total of 30 min. 1 wt/wt), 150 °C. Each reaction cycle was performed for a total of 30 min. | ||
To examine the deactivation process in more detail, the surface area of the GO–NaY mixture was analyzed via the Brunauer–Emmett–Teller (BET) method before and after introduction of 4-heptanol. The BET surface areas of the pre and post-treated mixture of GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt/wt) were measured to be 130.6 m2 g−1 and 118.0 m2 g−1 respectively.|| The fresh and spent GO–NaY were also subjected to powder X-ray diffraction (XRD) to determine if the NaY structure was altered (see Fig. S2 in the ESI†). XRD analysis of the spent GO–NaY mixture showed that the zeolite retained its crystallinity after the reaction with 4-heptanol, while the characteristic peak of GO (2θ = 11.8°) was greatly diminished. However, based on the absence of the graphitic peak (2θ = 26.5°), the data revealed that the carbon did not undergo graphitization, even though the GO underwent deoxygenation/reduction.** From the BET and XRD analyses, it was reasoned that the presence of reduced GO, which could prevent the alcohol from reaching the active sites in the zeolite, was the primary cause of activity loss.
1 wt/wt) were measured to be 130.6 m2 g−1 and 118.0 m2 g−1 respectively.|| The fresh and spent GO–NaY were also subjected to powder X-ray diffraction (XRD) to determine if the NaY structure was altered (see Fig. S2 in the ESI†). XRD analysis of the spent GO–NaY mixture showed that the zeolite retained its crystallinity after the reaction with 4-heptanol, while the characteristic peak of GO (2θ = 11.8°) was greatly diminished. However, based on the absence of the graphitic peak (2θ = 26.5°), the data revealed that the carbon did not undergo graphitization, even though the GO underwent deoxygenation/reduction.** From the BET and XRD analyses, it was reasoned that the presence of reduced GO, which could prevent the alcohol from reaching the active sites in the zeolite, was the primary cause of activity loss.
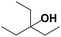
Finally, as summarized in Table 4, the substrate scope of the aforementioned catalyst under optimized conditions was explored. Overall, the GO–NaY catalyst displayed good activity for secondary (entries 1 and 9) and tertiary alcohols (entries 2–4, 6, and 8) as moderate to excellent conversions of the corresponding dehydration products were obtained. The reactions summarized in entries 1–4, 6 and 9 all proceeded cleanly under the optimized conditions with minimal byproducts according to the 1H NMR spectra recorded for the products. The dehydration of 2-heptanol (entry 1) resulted in a mixture of C7 olefins whereas the dehydration of 7-tridecanol (entry 9) resulted in only two isomers (cis/trans-6-tridecene). In general, aliphatic primary alcohols showed low conversions under the optimized conditions; however, the previously described preliminary studies with 1-octanol indicate that higher conversions may be obtained by increasing the catalyst loading and reaction time.†† Based on 1H NMR spectroscopic data collected, the low conversion of 3-methylbuten-2-en-1-ol and 2-methylbut-3-en-2-ol (entries 7 and 8) was a likely result of oligomerization. Similarly, α-aryl alcohols, such as 1-phenylethanol and 2-phenyl-2-propanol (not shown), suffered low conversion to the corresponding olefins despite high total conversion; oligomerization was observed by 1H NMR spectroscopy for these substrates.
| Entry | Starting material | Product(s) | Olefinb (% conv.) | Selectivity (%) |
|---|---|---|---|---|
a Conditions: 15 wt% GO–NaY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 150 °C, 30 min.
b Unless otherwise noted, the conversions and selectivities were by determined by 1H NMR spectroscopy and/or GC using mesitylene as the internal standard. For entries 1–4 and 6–9, a >99.0% conversion of starting material was observed. For entry 5, a 90.9% conversion of starting material was observed.
c The product selectivities were determined by GC.
d The low conversion to product (tetrahydrofuran) was the result of oligomerization as broad signals consistent with polytetrahydrofuran were observed in the 1H NMR spectrum of the product mixture. 1), 150 °C, 30 min.
b Unless otherwise noted, the conversions and selectivities were by determined by 1H NMR spectroscopy and/or GC using mesitylene as the internal standard. For entries 1–4 and 6–9, a >99.0% conversion of starting material was observed. For entry 5, a 90.9% conversion of starting material was observed.
c The product selectivities were determined by GC.
d The low conversion to product (tetrahydrofuran) was the result of oligomerization as broad signals consistent with polytetrahydrofuran were observed in the 1H NMR spectrum of the product mixture.
|
||||
| 1 |
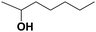
|
(E)-2-Heptene | 82.7 | 36.5c |
| (E)-3-Heptene | 32.0 | |||
| (Z)-2-Heptene | 14.6 | |||
| (Z)-3-Heptene | 13.8 | |||
| 1-Heptene | 3.1 | |||
| 2 |
|
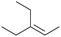
|
56.7 | N/A |
| 3 |

|
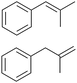
|
97.2 | 90.5 |
| 9.5 | ||||
| 4 |
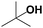
|
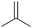
|
81.2 | N/A |
| 5 |

|

|
34.1d | N/A |
| 6 |
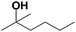
|
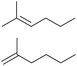
|
68.1 | 84.9 |
| 15.1 | ||||
| 7 |
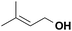
|
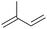
|
27.5 | N/A |
| 8 |
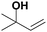
|
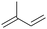
|
44.5 | N/A |
| 9 | 7-Tridecanol | (E)-6-Tridecene | 80.1 | 69.1 |
| (Z)-6-Tridecene | 30.9 | |||
Conclusions
In summary, GO was used in tandem with the zeolite NaY to effectively dehydrate a variety of alcohols to their corresponding olefinic or ethereal products. The dehydration of alcohols which do not readily dimerize or oligomerize under acidic conditions proceeded cleanly and rapidly (30 min) in moderate to excellent conversions (27.5–97.2%) to their corresponding products. The acidic nature of the GO transformed the zeolite to a protic form without the need for ammonium exchange or high temperature calcination. Moreover, the structure of the zeolite was largely retained, as determined by powder XRD analysis. Collectively, these results demonstrate that GO may be used in a synergistic manner with other heterogenous materials, such as zeolites, to promote the intrinsic catalytic properties displayed by the latter.Acknowledgements
We gratefully acknowledge the NSF (DMR-0907324), the Robert A. Welch Foundation (F-1621) and the WCU program through the NRF of Korea funded by the Ministry of Education, Science, and Technology (R31-10013) for their generous support.Notes and references
- G. Busca, Chem. Rev., 2007, 107, 5366–5410 CrossRef CAS.
- H. Hattori, Top. Catal., 2010, 53, 432–438 CrossRef CAS.
- H. Knozine, Angew. Chem., Int. Ed. Engl., 1968, 7, 791–805 CrossRef.
- J. M. Campelo, A. Garcia, J. F. Herencia, D. Luna, J. M. Marinas and A. A. Romero, J. Catal., 1995, 151, 307–314 CrossRef CAS.
- G. Larsen, E. Lotero, L. M. Petkovic and D. S. Shobe, J. Catal., 1997, 169, 67–75 CrossRef CAS.
- M. L. Kantam, P. L. Santhi and M. F. Siddiqui, Tetrahedron Lett., 1993, 34, 1185–1186 CrossRef CAS.
- R. Bringue, J. Tejero, M. Iborra, J. F. Izquierdo, C. Fite and F. Cunill, Chem. Eng. J., 2008, 145, 135–141 CrossRef CAS.
- G. A. Olah, T. Shamma and G. K. S. Prakash, Catal. Lett., 1997, 46, 1–4 CrossRef CAS.
- C. Casas, R. Bringue, E. Ramirez, M. Iborra and J. Tejero, Appl. Catal., A, 2011, 396, 129–139 CrossRef CAS.
- F. Carrasco-Marin, A. Mueden and C. Moreno-Castilla, J. Phys. Chem. B, 1998, 102, 9239–9244 CrossRef CAS.
- M. Okamura, A. Takagaki, M. Toda, J. N. Kondo, K. Domen, T. Tatsumi, M. Hara and S. Hayashi, Chem. Mater., 2006, 18, 3039–3045 CrossRef CAS.
- K. Nakajima, M. Hara and S. Hayashi, J. Am. Ceram. Soc., 2007, 90, 3725–3734 CAS.
- E. Jasinska, B. Krzyzynska and M. Kozlowski, Catal. Lett., 2008, 125, 145–153 CrossRef CAS.
- J. A. Sanchez, D. L. Hernandez, J. A. Moreno, F. Mondragon and J. J. Fernandez, Appl. Catal., A, 2011, 405, 55–60 CrossRef CAS.
- F. Liu, J. Sun, L. Zhu, X. Meng, C. Qi and F.-S. Xiao, J. Mater. Chem., 2012, 22, 5495–5502 RSC.
- P. A. Jacobs, M. Tielen and J. B. Uytterhoeven, J. Catal., 1977, 50, 98–108 CrossRef CAS.
- M. Anbazhagan, G. Kumaran and M. Sasidharan, J. Chem. Res., Synop., 1997, 336–337 RSC.
- C. Park and M. A. Keane, J. Mol. Catal. A: Chem., 2001, 166, 303–322 CrossRef CAS.
- Q. J. Zhu, J. N. Kondo, S. Inagaki and T. Tatsumi, Top. Catal., 2009, 52, 1272–1280 CrossRef CAS.
- B. Yilmaz and U. Müller, Top. Catal., 2009, 52, 888–895 CrossRef CAS.
- C. Moreau, J. Lecomte, S. Mseddi and N. Zmimita, J. Mol. Catal. A: Chem., 1997, 125, 143–149 CrossRef CAS.
- B. S. Balaji, M. Sasidharan, R. Kumar and B. Chanda, Chem. Commun., 1996, 707–708 RSC.
- T. Ookoshi and M. Onaka, Tetrahedron Lett., 1998, 39, 293–296 CrossRef CAS.
- F. Bigi, S. Carloni, R. Maggi, C. Muchetti and G. Sartori, J. Org. Chem., 1997, 62, 7024–7027 CrossRef CAS.
- R. Szostak, in Stud. Surf. Sci. Catal., ed. H. van Bekkum, E. M. Flanigen, P. A. Jacobs and J. C. Jansen, Elsevier, 2001, vol. 137, pp. 261–297 Search PubMed.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228–240 RSC.
- H. P. Jia, D. R. Dreyer and C. W. Bielawski, Adv. Synth. Catal., 2011, 353, 528–532 CrossRef CAS.
- D. R. Dreyer, K. A. Jarvis, P. J. Ferreira and C. W. Bielawski, Macromolecules, 2011, 44, 7659–7667 CrossRef CAS.
- D. R. Dreyer, K. A. Jarvis, P. J. Ferreira and C. W. Bielawski, Polym. Chem., 2012, 3, 757–766 RSC.
- D. R. Dreyer, H. P. Jia and C. W. Bielawski, Angew. Chem., Int. Ed., 2010, 49, 6813–6816 CAS.
- A. Lerf, H. He, M. Forster and J. Klinowski, J. Phys. Chem. B, 1998, 102, 4477–4482 CrossRef CAS.
- Q. L. Liu, T. H. Wang, C. H. Liang, B. Zhang, S. L. Liu, Y. M. Cao and J. S. Qiu, Chem. Mater., 2006, 18, 6283–6288 CrossRef CAS.
- C. J. H. Jacobsen, C. Madsen, J. Houzvicka, I. Schmidt and A. Carlsson, J. Am. Chem. Soc., 2000, 122, 7116–7117 CrossRef CAS.
- A. H. Janssen, I. Schmidt, C. J. H. Jacobsen, A. J. Koster and K. P. de Jong, Microporous Mesoporous Mater., 2003, 65, 59–75 CrossRef CAS.
- Z. Ren, E. Kim, S. W. Pattinson, K. S. Subrahmanyam, C. N. R. Rao, A. K. Cheetham and D. Eder, Chem. Sci., 2012, 3, 209–216 RSC.
- D. Li, L. Qiu, K. Wang, Y. Zeng, T. Williams, Y. Huang, M. Tsapatsis and H. Wang, Chem. Commun., 2012, 48, 2249–2251 RSC.
- A. Molnar and M. Bartok, in Fine Chemicals Through Heterogenous Catalysis, Wiley, 2001, pp. 295–307 Search PubMed.
- X. Gao, J. Jang and S. Nagase, J. Phys. Chem. C, 2009, 114, 832–842 Search PubMed.
- M. Acik, G. Lee, C. Mattevi, A. Pirkle, R. M. Wallace, M. Chhowalla, K. Cho and Y. Chabal, J. Phys. Chem. C, 2011, 115, 19761–19781 CAS.
- R. Larciprete, S. Fabris, T. Sun, P. Lacovig, A. Baraldi and S. Lizzit, J. Am. Chem. Soc., 2011, 133, 17315–17321 CrossRef CAS.
- D. R. Dreyer, S. Murali, Y. Zhu, R. S. Ruoff and C. W. Bielawski, J. Mater. Chem., 2011, 21, 3443–3447 RSC.
- C. Y. Su, Y. P. Xu, W. J. Zhang, J. W. Zhao, A. P. Liu, X. H. Tang, C. H. Tsai, Y. Z. Huang and L. J. Li, ACS Nano, 2011, 4, 5285–5292 CrossRef.
- E. B. Nursanto, A. Nugroho, S. A. Hong, S. J. Kim, K. Y. Chung and J. Kim, Green Chem., 2011, 13, 2714–2718 RSC.
- M. R. Guisnet, Acc. Chem. Res., 1990, 23, 392–398 CrossRef CAS.
- T. Szabo, E. Tombacz, E. Illes and I. Dekany, Carbon, 2006, 44, 537–545 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic and characterization procedures, spectroscopic data (IR, XRD, and NMR), and additional surface analysis data. See DOI: 10.1039/c2cy20474f |
| ‡ To determine if the olefinic product underwent oxidation, 1-octene was heated at 150 °C in the presence of GO (200 wt%) for 3 h. 1H NMR spectroscopy revealed that 1-octene was isomerized to the disubstituted C8 olefinic isomers (>99%); no oxidized products were observed. |
| § It was previously shown45 that aqueous GO dispersions at 1 mg mL−1 have a pH of ca. 4.5. Under the optimized dehydration reactions (25 mg mL−1), the pH of an aqueous dispersion was measured to be 3.3. |
| ¶ 4-heptanol was dehydrated using a catalytic amount (5 drops) of conc. H2SO4 at 150 °C, and within 10 min the reaction mixture turned black in color. 1H NMR analysis of the product mixture showed heptene isomers (59.0%) as well as several unidentified byproducts. |
| || The surface area of GO and NaY were determined independently to be 2.5 m2 g−1 and 286.7 m2 g−1 respectively. The surface area of the carbon material obtained after the dehydration reaction was measured to be 4.2 m2 g−1, and the surface area of the NaY from entry 6 in Table 3 was measured to be 217.9 m2 g−1. |
| ** GO was used as the catalyst and 4-heptanol as the starting material (15 wt% GO, 150 °C, 30 min). The FT-IR spectrum (Fig. S1, ESI†) recorded for the recovered carbonaceous material revealed that a substantial number of the oxygen containing groups were removed. |
| †† 1-Octanol showed <1% conversion to the corresponding olefinic products under the reaction conditions optimized for 4-heptanol. |
| This journal is © The Royal Society of Chemistry 2013 |