Enhanced catalytic methane coupling using novel ceramic foams with bimodal porosity†
B.
Neumann
a,
T. W.
Elkins
b,
W.
Dreher
c,
H.
Hagelin-Weaver
b,
J. C.
Nino
d and
M.
Bäumer
*a
aInstitute of Applied and Physical Chemistry, University Bremen, Leobener Str. NW2, 28359 Bremen, Germany. E-mail: mbaeumer@uni-bremen.de
bDepartment of Chemical Engineering, University of Florida, Gainesville, Florida 32611, USA
cDepartment of Chemistry, University Bremen, Leobener Str. NW2-C, 28359 Bremen, Germany
dDepartment of Materials Science and Engineering, University of Florida, Gainesville, Florida 32611, USA
First published on 5th October 2012
Abstract
Monolithic reactor concepts are currently intensively discussed in the literature. For the oxidative coupling of methane relying on a balance of surface and gas phase reactions, such concepts have previously been claimed to have beneficial effects with respect to obtainable C2 yields. In order to verify the superior performance in the case of a foam catalyst, ceria and samaria foams were fabricated by a direct foaming process. In both cases, mechanically stable, homogeneous open-cell foams were obtained as revealed by 3D magnetic resonance imaging, Hg-porosimetry, and scanning electron microscopy. As a characteristic feature of the introduced foaming methodology the process resulted in bimodal pore size distributions, ensuring low pressure drops on the one hand and sufficiently large surface areas on the other hand. Oxidative coupling of methane was carried out over the samaria foams. It was possible to obtain C2 yields that were indeed higher than those obtained with the samaria powder, in contrast to honeycomb monoliths previously studied in the literature.
Introduction
Although fixed bed reactors consisting of packed beds of pellets or particles are established and largely used in industrial catalytic processes, monolithic catalysts increasingly attract attention as novel reactor concepts.1 Various advantages are discussed in recent literature, such as lower pressure drops, high safety, easy catalyst separation and fixed geometry avoiding the danger of channel and dead zone formation.1,2Whereas in the past honeycomb structures dominated the field of monolithic catalyst designs, ceramic foams are meanwhile an interesting alternative. Due to a wealth of preparation techniques, different porosities in combination with high mechanical strengths are achievable.3 In many cases, the foam porosity can be tuned so that surface areas, pore sizes, and pore distributions can be adjusted to optimize contact times and mass transfer4–6 for a given catalytic application.1,7
A reaction which could particularly benefit from monolithic reactor concepts is the oxidative coupling of methane (OCM) to ethane and ethylene being a potentially important step in the utilization of natural gas for the production of chemicals. It is an example of a reaction where heterogeneous surface reactions and homogenous gas phase reactions play a role. On basic oxides, such as (lithium doped) magnesia or samaria, methane is activated by the abstraction of a proton leading to the emanation of methyl radicals into the gas phase. These radicals can combine to form ethane which may undergo secondary dehydrogenation to ethylene.8 Because of the need to balance the heterogeneous and homogeneous parts of the reaction network, the reactor type and in particular the configuration of the catalyst bed play an important role. Various reactor concepts have been studied in the past.9 Yet, monolithic reactors have rarely been considered so far,10–13 although the adiabatic situation in a ceramic monolith, the low pressue drop (also meaning that low pressures can be realized favouring C2 selectivity) and the higher free (gas) volume have been claimed to be particularly favourable in view of high C2 yields.10
As pioneers in the field of OCM, Aigler and Lunsford used honey comb monoliths,10 as they are extensively employed in, for example, the field of car exhaust catalysis.1,14 Surprisingly at first sight, they found an inferior performance of their magnesia monoliths for OCM as compared to conventional fixed bed reactors. They concluded that the disadvantageous flow pattern resulting from the laminar flow conditions, associated with such honeycombs due to the assembly of straight channels, was the reason.
In our study, we pursued the question whether a ceramic foam would yield better results and bring about the proposed advantages. To this end, an approach was tested to synthesize foams catalytically active for OCM by a direct foaming technique. As discussed extensively elsewhere,3 direct foaming is based on air bubbles which are generated in a liquid or suspension of primary particles and are consolidated by a setting agent before extensive coalescence and growth of the bubbles can set in. In a subsequent step, the structures are sintered at high temperatures so that the setting agent, mostly a polymer, is burned and the structure transformed into a mechanically stable monolith. By controlling foam stability and setting kinetics, pore sizes in the range between 30 μm and 1 mm are achievable.
We chose samaria as a catalytically important rare earth oxide (REO), since it is the most commonly studied lanthanide in the oxidative coupling of methane (OCM), and the C2 production rate for samaria is almost 3 times greater than the next best REO catalyst.15,16 Since a comparative study of identical materials in the form of monoliths and particle beds is missing at this point,17 we studied OCM over samaria foams and over samaria powder as used for the foam synthesis in a fixed bed configuration under exactly the same conditions. In view of other applications, such as exhaust catalysis, and to elucidate the potential of the synthesis on a broader basis we also synthesized ceria foams.18 Our results show that the obtained porosities in the case of samaria are particularly suited for OCM.
Results and discussion
Foam preparation and characterization
Ceria and samaria foams were prepared using polyurethane as a gas releasing and setting agent in the foaming process.19 To obtain a homogeneous foam, it was essential to mix the components (isocyanate, surfactant and the catalyst) intensively by means of an electrical drill. To increase the amount of CO2 which is released during the polymerisation process a proper amount of water was added shortly before the end of the mixing.Although the composition and the mixing of the ceria and the samaria system was identical, the time needed for the foaming process to be completed was distinctly different. Whereas the foaming of the samaria ended within 5 minutes resulting in a rigid polyurethane (PU)-foam, in the case of the ceria system this process took longer than 1 hour. After drying for 24 h, the foams were cut into the desired shape and sintered (heated in air) to remove the polymeric foaming agent and sinter the samaria and ceria particles into a porous network.
Fig. 1A and B show photographs of samaria and ceria foams after sintering for 2 hours at 1300 and 1650 °C, respectively. Lower sintering temperatures resulted in non-stable structures, while higher temperatures led to shrinkage and densification of the foams. In the case of ceria, sub-mm pores are visible with the naked eye, while the samaria foams have distinctly smaller pores, suggesting that the difference in foaming time resulted in a different porosity.
 | ||
| Fig. 1 Photographs (A, B) and scanning electron micrographs (C–F) of the ceria (left) and the samaria (right) foam. In both cases the ligaments of the foams are porous. The ceria foam shows larger pores than the samaria sample. | ||
To check the phase composition of the foams, XRD patterns were collected. The data (see ESI†) reveal that ceria exhibited a cubic structure (space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m), whereas samaria was monoclinic (space group C2/m) after sintering.
m), whereas samaria was monoclinic (space group C2/m) after sintering.
Additional details of the foams microstructure were obtained using scanning electron microscopy (SEM). The SEM images in Fig. 1C–F reveal the different microstructures more clearly than the photographs. When describing the microstructure of foams obtained by a direct foaming method, it is important to distinguish two kinds of pores. Only the larger pores visible in the lower magnified micrographs in Fig. 1 are the result of the foaming itself. When comparing both materials on this scale, it turns out that the ceria foam shows a similar cell structure – although roughly a factor of five larger – than the samaria sample. The pore size in such foams is a function of foaming kinetics, aging and drying of the foams.3 In many cases, wet foams are subject to Oswald ripening so that the pore size increases with time. As described above, the kinetics of the foaming processes were quite different in the case of samaria and ceria. The smaller pore sizes in the former case are consistent with the much faster aging.
Considering the identical preparation process and similar grain sizes of the primary particles (2–3 μm determined by DLS (Dynamic Light Scattering)), this finding is surprising at first sight. An explanation can be found on the basis of the surface chemistry of the two oxides, differing in their hydrophilicity. Thermogravimetric data and supporting IR spectroscopic data (see ESI†) confirm this point. Whereas samaria exhibits a small but significant weight loss at 280 °C, ceria shows no relevant weight loss in the measured temperature range up to 700 °C. The weight loss at 280 °C can be assigned to H2O desorption due to decomposition of OH groups on the surface of samaria as is corroborated by the IR spectra revealing a loss of OH species by a negative band in the OH stretching frequency region between 260 and 300 °C.20 The samaria powder apparently contains more surface hydroxyl groups compared with ceria21 (even the formation of a bulk hydroxide is possible),20 and these hydroxyl groups can react with the isocyanate precursor for the PU-foam, which in turn accelerates the aging of the foam meaning that Oswald ripening occurs to a smaller extent.
Important for catalytic applications is the observation that the struts of the foams (surrounding the larger pores) are porous as well. Whereas foams with dense cell walls are usually desired to achieve maximum mechanical strength, the sintering times and temperatures were optimized in our study in such a way that such a bimodal pore size distribution was obtained while still a sufficient stability was warranted. This is very promising in view of using such foams as monolithic OCM catalysts, since the hierarchical porosity combines good mass transport through the large pores with sufficient surface area due to the finer pores. From the SEM images pore diameters in the range of <1 μm, 20 μm and 100 μm for samaria and of 1 μm, 100 μm and 500 μm in the case of ceria can be estimated.
To quantify the pore sizes in more detail, Hg porosimetry was performed with both foams. The results are presented in Fig. 2, showing the pore size distributions and the volume of intruded mercury. The measurement is limited to pores <200 μm in our case so that the data obtained by this experiment will not show all pores which are visible in the scanning electron micrographs (especially in the case of the ceria foam). For the samaria, we observe pore filling in the range of 140–90 μm, 30–10 μm and 400–300 nm; the latter value characterizing the fine pores in the cell walls. For the ceria foams, steps are found in the range of 150–60 μm, 3 μm and 1 μm, meaning that the fine pores are a factor of 2–3 larger than for the samaria foam. Notably, 80% of the ceria pore volume is represented by the pores larger than 60 μm (keeping in mind that not all pores are included). At this point for the samaria only roughly 10% of the pore volume are filled with mercury. Due to this difference, the surface area of the samaria foam, as calculated from the intrusion data, is about a factor of 2.5 larger than for ceria (0.2 m² g−1 compared to 0.48 m² g−1) and fits into the range of surface areas of honeycomb monoliths tested for OCM previously.
 | ||
| Fig. 2 The results of the mercury porosimetry for the samaria (upper) and the ceria (lower) foam. The pore volume (red areas) is shown below the intrusion curve in each case. The larger number of bigger pores in the case of the ceria sample leads to a higher amount of intruded mercury. However, the specific surface area of the samaria sample is higher, because it provides more smaller pores. | ||
One issue with using direct foaming methods is the possible formation of closed pores within the material. Especially for the use in catalysis this would result in a large pressure drop over the monolith. For the samaria foam, we determined a pressure drop of 0.33 mbar mm−1 while there was no detectable pressure drop over the ceria foam. Such macroscopic characterization does not provide any information regarding the question whether the whole inner surface of the foam is accessible or, in other words, all cells are open and connected. To address this question, we performed magnetic resonance imaging (MRI) on the ceria foams.
Fig. 3A depicts a photo of the sample positioned in a cylindrical pot made of Teflon, while Fig. 3B and C show 2D MR images with horizontal and vertical slice orientation, extracted from 3D MR images of the complete sample measured with an isotropic nominal spatial resolution of 143 μm.22,23 The porous sample was inserted into water filled into the pot. The two representative slices shown in Fig. 3B and C illustrate the uniform spatial distribution of water within the sample. This observation indicates that the foam does not contain larger regions that are inaccessible from the outside proving the open porosity of the foam and thus underpinning the potential of the direct foaming approach for the synthesis of catalytic monoliths.
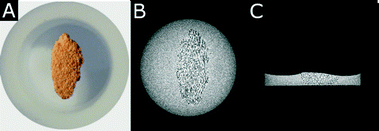 | ||
| Fig. 3 (A) Photo of the ceria foam in the sample holder, (B, C) 2D MR images with (B) horizontal and (C) vertical slice orientation. Both MR images were extracted from 3D water images measured with an isotropic spatial resolution of 143 μm. | ||
Catalytic activity measurements
The samaria foams were investigated in the oxidative coupling of methane (OCM) and directly compared to reactions over the micron-sized samaria powder which was used for the preparations of the foams. Experiments were carried out for different methane-to-oxygen ratios at temperatures of 740 °C and 800 °C applying virtually the same residence times in the catalyst beds for the foam and the powder. In Table 1, the CH4 conversion, the product distribution, the yield of C2 products (ethane and ethylene) and the space time yields per gram und specific surface area of the catalyst are shown. At both temperatures, no products are formed in the absence of catalyst. Only oxygen deficient conditions were tested as increasing the O2 concentration decreases the C2 selectivity. Under all conditions applied in the study, all oxygen was consumed.| Catalyst | CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 Feed ratio O2 Feed ratio |
Rxn temp./°C | CH4 conversion [%] | Product selectivitya [%] | C2 Yieldb [%] | C2 STYmc/sccm g−1 | C2 STYsd /sccm m−2 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| C2H4 | C2H6 | CO2 | CO | |||||||
| a Selectivity is defined as the number of moles of CH4 reacted to form the product over the total number of moles of CH4 reacted. b C2 yield, i.e. fraction of methane which has reacted to form C2 products (CH4 conversion · C2 selectivity). c STYm: space time yield referred to the mass of the catalyst (standard cubic centimeters per minute (sccm) of C2 product formed per gram catalyst). d STYs: space time yield referred to surface area of the catalyst (standard cubic centimeters per minute (sccm) of C2 product formed per m2 of the catalyst). | ||||||||||
| Foam | 9 | 740 | 10.7 | 27.3 | 24.4 | 30.6 | 17.2 | 5.5 | 7.5 | 15.7 |
| 7 | 18.8 | 29.2 | 20.5 | 33.2 | 17.0 | 9.3 | 8.7 | 18.1 | ||
| 4 | 26.6 | 27.8 | 12.8 | 42.1 | 17.2 | 10.8 | 11.3 | 23.6 | ||
| 9 | 800 | 16.5 | 35.5 | 17.3 | 30.4 | 16.6 | 8.7 | 8.9 | 18.7 | |
| 7 | 15.6 | 35.1 | 14.5 | 33.3 | 17.0 | 7.7 | 9.9 | 20.7 | ||
| 4 | 33.3 | 31.3 | 9.1 | 40.4 | 19.0 | 13.5 | 11.8 | 24.4 | ||
| Powder | 9 | 740 | 7.8 | 17.6 | 26.5 | 44.5 | 11.2 | 3.4 | 4.2 | 0.47 |
| 7 | 10.5 | 19.2 | 24.6 | 46.3 | 9.6 | 4.6 | 5.1 | 0.58 | ||
| 4 | 18.3 | 22.7 | 20.5 | 50.2 | 6.4 | 7.9 | 7.7 | 0.86 | ||
| 9 | 800 | 8.6 | 29.7 | 21.3 | 40.2 | 8.6 | 4.4 | 5.3 | 0.60 | |
| 7 | 12.7 | 29.8 | 17.3 | 44.8 | 8.0 | 6.0 | 6.6 | 0.74 | ||
| 4 | 18.8 | 28.3 | 12.9 | 51.1 | 7.6 | 7.7 | 7.3 | 0.82 | ||
Focussing first on similar trends observed for the foam and the powder, the conversion increases with decreasing CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 ratio (between 9
O2 ratio (between 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). At the same time, the selectivity for ethane remains fairly constant, while the selectivity for ethylene decreases and more COx, in particular more CO2, is produced. While the selectivity is lower at CH4
1). At the same time, the selectivity for ethane remains fairly constant, while the selectivity for ethylene decreases and more COx, in particular more CO2, is produced. While the selectivity is lower at CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 ratios of 4
O2 ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, it is evident in Table 1 that more C2 products are formed due to a higher CH4 conversion at this ratio compared with the higher ratios. The C2 yield also increases with the reaction temperature: at 800 °C the values are slightly higher than at 740 °C.
1, it is evident in Table 1 that more C2 products are formed due to a higher CH4 conversion at this ratio compared with the higher ratios. The C2 yield also increases with the reaction temperature: at 800 °C the values are slightly higher than at 740 °C.
Comparing foam and powder, very interesting conclusions can be drawn. As far as the C2 yields (in %) are concerned, the foam outperforms the powder bed by a factor of up to 2; the space time yield (STY) per mass is increased by a factor of 3–4, while the STY referenced to the specific surface area is even more increased (by a factor of 20–30). (Yet, as pointed out in the introduction, the surface area is not a good point of reference since increasing surface area not necessarily means improved C2 selectivity.) Another factor that differs between foam and powder is the ratio of the combustion products CO2 and CO. For the foam it is shifted towards CO, whereas for the powder only little CO is observed (i.e. the CO selectivity is comparatively smaller).
The findings clearly indicate that with respect to the C2 yields the foams show a distinctly better performance as compared to the powder, notably in contrast to previous work comparing particle beds and honeycomb monoliths.10 An obvious explanation is the different flow pattern in the foam as compared to the particle bed. Using CO oxidation as a test reaction, Kraushaar-Czarnetzki et al. investigated the influence of the different gas flows in packed beds of beads, honeycomb structures and foams and found for the latter an intermediate situation with respect to the mass transfer between the gas phase and the catalyst surface.7,24,25 In this context, it is important to know that a certain degree of mass transport limitation was shown to improve the C2 selectivity26 (because of a lower oxygen partial pressure at the surface27). Therefore, the smaller mass transfer is likely to be the reason for the better performance of the foam as compared to the particle bed, whereas the honeycomb structure is inferior because of the laminar flow pattern.10
Conclusion
Ceria and samaria foams were fabricated by a direct foaming process. In both cases mechanically stable, homogeneous open-cell foams were obtained as revealed by 3D magnetic resonance imaging, porosimetry, and SEM. Although the same synthesis parameters were used, distinctly smaller cells were observed for samaria, ranging between 100 μm and 10 μm. If the sintering conditions are chosen properly, porous cell walls can be obtained, which, in the case of samaria, exhibit pores in the range of 300–400 nm, whereas they were larger by a factor of ∼2 for ceria.The introduced foaming methodology leading to this unique bimodal pore size distribution represents a very promising process for the synthesis of monolithic OCM catalysts. Using the samaria foams, it was possible to achieve C2 space time yields that were significantly higher than those obtained for a fixed bed reactor packed with samaria particles pointing to a flow pattern with a more favorable mass transfer between the catalyst surface and the gas phase.
In contrast to the conclusions drawn for honeycomb structures (exhibiting similar surface areas): “that the unique channel structure of the monolith is not a positive factor in the primary reaction and that the C2 selectivities are significantly less”,10 the foams with their open cell porosity synthesized in this study showed the improved performance previously claimed for monolithic OCM catalysts in the literature.9,10 Although our setup was not optimized for maximum C2 (space time) yields, the maximum value of about 13.5% found in our study is already competitive to values for rare earth oxides reported in the literature.9,23 Further optimization of the catalytic material, such as doping of samaria with alkali, is of course possible to increase the (space time) yields even further and will be part of future work.
Experimental section
For the preparation of the ceria and samaria foams, we used commercially available isocyanate PAPI 27 (DOW Chemical). All syntheses were performed using 27.5 vol% of ceramic powder, 0.4 mL polyethylene glycol - 200, 0.1 mL Tween 80 and 0.8 mL of isocyanate. 1 mg of diazabicyclo[2.2.2]octane was added to start the foaming process. Catalytic activity measurements were carried out in a quartz tube reactor with an inner diameter of 10 mm. The quartz tube reactor was used in a previously reported reactor system which was slightly modified for the current application.28,29 For loading the catalyst, a monolith with a height of 3 mm and a weight of 0.225 g was pressed into a quartz reactor tube. The act of pressing the monolith into the tube effectively removed the excess from the edges and minimized the void space in between the edges of the monolith and the reactor tube's inner walls. Any visible void space was packed with quartz wool to prevent the reactant gas from bypassing the foam monolith. The foam catalyst was held in place inside the reactor using a piece of quartz wool on both sides. For the powder catalyst, a 3 mm catalyst bed (0.4 g) was supported in the reactor tube in the same manner. CH4, O2, and an internal standard (N2) were fed through the system at a rate of 120 standard cm3 per minute (sccm) (with N2 constant at 23.2 sccm) using three mass flow controllers (MFCs). Product analysis was performed by a custom-configured, on-line Agilent 6890N gas chromatograph. Further experimental details regarding the material's characterization, the magnetic resonance imaging and the catalytic activity test are included in the ESI.†Acknowledgements
The authors are thankful to Andrea Deranek for the collaboration during the initial ceria foaming attempts and Thorsten Gesing for XRD measurements. JCN would like to acknowledge the financial support by the U.S. National Science Foundation (DMR 0449710 grant) and the Hauck Foundation. HHW and TE also gratefully acknowledge NSF support (Chemistry 1026712). BN and MB are grateful for financial support from the DFG. BN is grateful for a fellowship of the Deutsche Telekom Stiftung.References
- A. Reitzmann, F. C. Patcas and B. Kraushaar-Czarnetzki, Chem. Ing. Tech., 2006, 78, 885 CrossRef CAS.
- G. Eigenberger and W. Ruppel, Ullmann's Encyclopedia of Industrial Chemistry, 2012, vol. b04, p. 199 Search PubMed.
- A. R. Studart, U. T. Gonzenbach, E. Tervoort and L. J. Gauckler, J. Am. Ceram. Soc., 2006, 89, 1771 CrossRef CAS.
- T. A. Nijhuis, A. E. W. Beers, T. Vergunst, I. Hoek, F. Kapteijn and J. A. Moulijn, Catal. Rev.: Sci. Eng., 2001, 43, 345 CAS.
- P. Avila, M. Montes and E. E. Miró, Chem. Eng. J. (Amsterdam, Neth.), 2005, 109, 11 CAS.
- M. V. Twigg and J. T. Richardson, Ind. Eng. Chem. Res., 2007, 46, 4166 CrossRef CAS.
- F. C. Patcas, G. I. Garrido and B. Kraushaar-Czarnetzki, Chem. Eng. Sci., 2007, 62, 3984 CrossRef CAS.
- G. Martin and C. Mirodatos, Surface chemistry in the oxidative coupling of methane, Fuel Process. Technol., 1995, 42, 179 CrossRef CAS.
- L. Mleczko and M. Baerns, Fuel Process. Technol., 1995, 42, 217 CrossRef CAS.
- J. M. Aigler and J. H. Lunsford, Appl. Catal., 1991, 70, 29 CrossRef CAS.
- W. Wang, S. Ji, D. Pan and C. Li, Fuel Process. Technol., 2011, 92, 541 CrossRef CAS.
- P. Witt and L. D. Schmid, J Catal., 1996, 163, 465 CrossRef CAS.
- K. Hohn, P. Witt, M. Davis and L. Schmidt, Catal. Lett., 1998, 54, 113 CrossRef CAS.
- M. Hartmann, T. Kaltschmitt and O. Deutschmann, Catal. Today, 2009, 147, S204 CrossRef CAS.
- K. Otsuka, K. Jinno and A. Morikawa, J. Catal., 1986, 100, 353 CrossRef CAS.
- K. D. Campbell, H. Zhang and J. H. Lunsford, J. Phys. Chem., 1988, 92, 750 CrossRef CAS.
- H. Liu, D. Yanga, R. Gaoa, L. Chen, S. Zhang and X. Wang, Catal. Commun., 2008, 9, 1302–1306 CrossRef CAS.
- M. P. Rosynek, Catal. Rev.: Sci. Eng., 1977, 16, 111 CAS.
- L. Wucherer, J. C. Nino, F. Basoli and E. Traversa, Int. J.Appl. Ceram. Technol., 2009, 6, 651 CrossRef CAS.
- S. Bernal, F. J. Botana, R. Garcia, J. Pintado and J. M. Rodriguez-Izquierdo, Mater. Res. Bull., 1987, 22, 131 CrossRef CAS.
- A. Kossoy, H. Cohen, T. Bendikov, E. Wachtel and I. Lubomirsky, Solid State Ionics, 2011, 194, 1 CrossRef CAS.
- I. L. L. D.P. Madio, Magn. Reson. Med., 1995, 34, 525 CrossRef.
- A. G. Dedov, A. S. Loktev, I. I. Moiseev, A. Aboukais, J.-F. Lamonier and I. N. Filimonov, Appl. Catal., A, 2003, 245, 209 CrossRef CAS.
- L. Giani, G. Groppi and E. Tronconi, Ind. Eng. Chem. Res., 2005, 44, 4993 CrossRef CAS.
- J. T. Richardson, D. Remue and J. K. Hung, Appl. Catal., A, 2003, 250, 319 CrossRef CAS.
- G. Follmer, L. Lehmann and M. Bearns, Catal. Today, 1989, 4, 323 CrossRef CAS.
- D. Wolf, M. Slinko, E. Kurkina and M. Baerns, Appl. Catal., A, 1998, 166, 47 CrossRef CAS.
- S. Jones, L. Neal and H. Hagelin-Weaver, Appl. Catal., B, 2008, 84, 631 CrossRef CAS.
- S. Jones, Activity and Characterization Studies in Methanol Reforming Catalysis: Cu and Cu–ZnO Catalysts and the Role of Nanomaterials, Dissertation, University of Florida, 2008 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2cy20458d |
| This journal is © The Royal Society of Chemistry 2013 |
