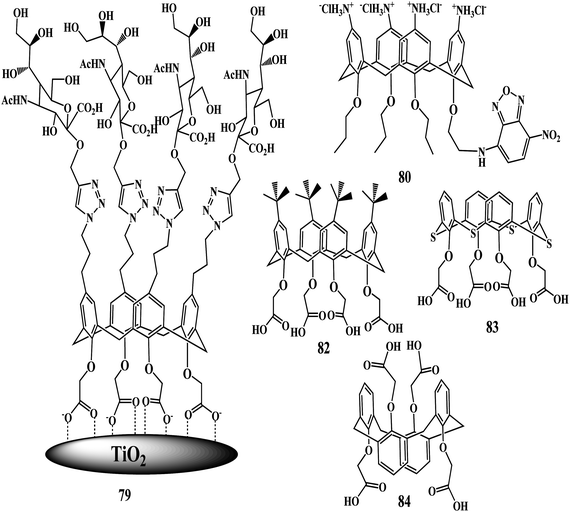
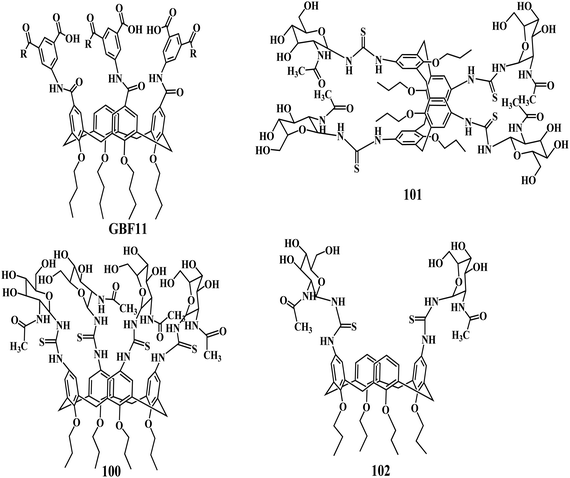
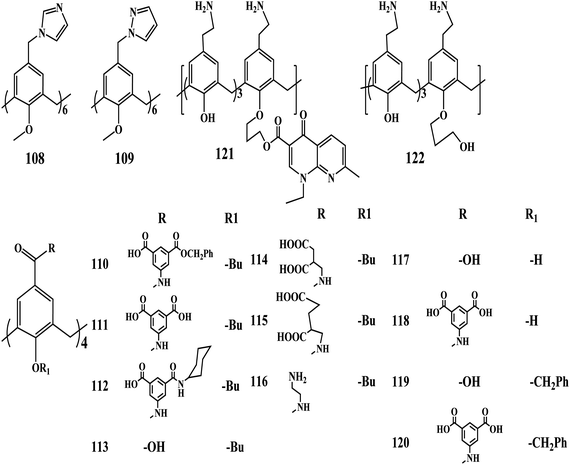
Biological applications of functionalized calixarenes
Satish Balasaheb Nimsea and Taisun Kim*b
aInstitute for Applied Chemistry and Department of Chemistry, Hallym University, Chuncheon, 200-702, Korea. Fax: +82-33-256-3421
bBiometrix Technology, Inc. 202 Bio Venture Plaza, Chuncheon, 200-161, Korea. E-mail: tskim@hallym.ac.kr; Fax: +82-33-256-3421
First published on 2nd October 2012
Abstract
The functionalized calixarene derivatives exhibit remarkable properties towards organic and bioorganic molecules. However, the ability of calixarene derivatives to form stable complexes with biomolecules allows them to be applied for the development of biosensors and in the field of biology, biotechnology, and drug discovery. The applications of the functionalized calixarenes are summarized in this review, and an outlook for the future developments is discussed. A brief survey (of the last 10 years) on their biological application in various fields is also considered (199 references).
 Satish Balasaheb Nimse | Satish Balasaheb Nimse earned a Bachelor of Pharmaceutical science from University of Pune, Maharashtra, India and a Master of Medicinal Chemistry from Biju Pattanaik University Technology, Orissa, India. He joined the group of Prof. Taisun Kim in 2007 and received a PhD in 2011 from the Department of Chemistry, Hallym University, South Korea. Soon after completion of his PhD he joined the faculty in the same department and is currently working with Prof. Kim. His research interests concern the medicinal Chemistry, bioorganic chemistry, supramolecular chemistry, and the applications of the supramolecules for the fabrication of the diagnostic DNA chips. |
 Taisun Kim | Taisun Kim received his PhD from the Department of Chemistry and Biochemistry at University of Texas at Austin. After a two-year postdoctoral fellowship at Texas A&M University, he joined the faculty at Hallym University in 1995. He founded the bioventure company, Biometrix Technology, Inc. in 2000, which is proficient in making 9G DNA Chips for research and diagnostic purposes. To date, his research records many peer-reviewed scientific publications and 23 domestic and international patents. |
1. Introduction
Calixarenes, with their unique three-dimensional surface, are one of the best known host molecules along with cyclodextrins, cucurbiturils, cryptands, and crown ethers. By their availability and easy functionalization at either the upper and/or lower rim of the molecular skeleton among potential building blocks, calixarenes have become important receptors in synthesis and applications as supramolecular platforms for molecular recognition, sensing and self-assembly, catalysis, nanotechnology, and drug discovery. They have several conformational isomers, and a large number of cavities of different sizes and shapes are involved in molecular recognition.1The recognition and formation of selective complexes with biological compounds are particularly interesting applications of the functionalized calixarenes. Likewise, protein surface recognition has been studied using various cyclic peptides attached to a calixarene core and establishing the importance of the functional group and complementary geometry in binding protein surfaces.2,3 The development of the water soluble calixarenes as receptors for biomolecules has become important in view of their potential medical applications.4–6
According to the data presented in the literature by renowned research groups, the binding properties of calixarenes and water-soluble calixarenes towards organic cationic substrates have been studied in solid state, gas phase, and in solution by different techniques. Calixarene complexes are significantly stronger than the corresponding inclusion complexes of cyclodextrins and crown ethers with amino acids. In spite of their small cavity, a calixarene scaffold with appropriate functionalization (sulfonate, phosphate, ammonium etc.) on the upper rim renders this class of receptors as a template for the design and construction of special receptors for cations, anions and neutral species recognition mimicking biological processes.7,8 As such, the investigation of the interactions between functionalized calix[4]arenes and different compounds is of fundamental interest to give new insights into molecular recognition, self-assembly processes, and a better understanding of the recognition processes running in biological systems.9,10 Due to their ability to form reversible complexes with both neutral and charged compounds, the calixarenes are also employed in separation chemistry. Exhaustive molecular dynamic studies on liquid–liquid interfaces in assisted ion extraction involving calixarenes pointed out the importance of solvation effects and of small amounts of water on the selectivity.11
Apart from being efficient receptors and forming interesting complexes by specific interactions with a large variety of chemical and biological compounds (e.g., ammonium ions, amines, amino acids, and peptides), a significant feature of functionalized calix[n]arenes is their ability to act as carriers through membranes or to be incorporated into channelling systems, with analytical and therapeutic applications.12–15 The aim of the present Review is to summarize the recent studies about calix[n]arenes (n = 4, 6, 8) and their derivatives with respect to their applications in the DNA chip technology/biosensing, biotechnology, biology, and drug discovery (Scheme 1), as well as advancing some hints on future areas of scientific research related to the above topics. Review articles on calixarenes have covered their molecular recognition properties for organic molecules16–18 and biomolecules.19–22 However, the pioneering work by Cornforth et al. on the antitubercular activity of the calixarene derivatives23 which eventually lead to the investigation of the biological applications of the calixarenes has not been followed. The present Review specifically addresses the biological applications of calixarenes.
![Various applications of calix[n]arenes (n = 4, 6, 8).](/image/article/2013/CS/c2cs35233h/c2cs35233h-s1.gif) | ||
| Scheme 1 Various applications of calix[n]arenes (n = 4, 6, 8). | ||
2. Applications of calixarenes
During the last 15 years, amphiphilic calix[n]arenes (n = 4, 6, 8) and especially water-soluble calixarenes have been the subject of growing interest in the biological domain. The different studies concerning the specific interactions of calixarenes with molecules such as organic and inorganic ions, peptides, polar or neutral organic molecules and biomolecules, including proteins and lipids have lead to the numerous applications of calixarenes in DNA chip technology/biosensor technology and biotechnology. The interactions have been studied in solution and in the solid and gaseous states using a wide range of methods such as NMR spectroscopy, RP-HPLC, micro-calorimetry, X-ray crystallography, atomic force microscopy, fluorescence spectroscopy, Langmuir balance, etc. Increasingly detailed knowledge is being obtained on the mechanism of the interactions of the calixarenes with biomolecules.Until this time, most of the calixarene derivatives showed low or no toxicity in the animal models, further increasing their interest in the field of biopharmaceutical applications such as drug discovery. However, it is important to notice that very few calixarene derivatives have been assessed for their toxicity or immunological responses.24 The ability of the amphiphilic calixarenes to cross the cell membranes also ensured their applications in biology.
2.1 Applications of calixarenes in DNA chip technology/biosensing technology
It is well known that the lone-pair electrons of the crown ethers in the calix[4]crown-5 derivatives can easily capture the cationic substrates, such as the metal cations and ammonium ions.25,26 The amino acids, as basic structural building blocks of proteins and other biomolecules, sport ammonium ions and hence are attractive targets for calix[4]crown-5 derivatives.27 A number of remarkable studies were addressed to this topic, including their molecular recognition by calixarenes.28Due to the presence of multiple amino functions in proteins, the possibilities of the complexation of proteins by calixarenes have led to a new class of applications: the protein microarray. One important factor in fabricating protein microarrays is to immobilize proteins, without losing their activity, on a solid phase. To keep them functional, it is necessary to immobilize proteins in a way that preserves their folded structural integrity. Recently, H. Kim et al. reported the detection of the alanine aminotransferase antigen with the detection limit of 0.05 ng mL−1.29 However, in 2003, Y. Lee and T. Kim developed a highly sensitive microarray protein chip coated with the calixcrown derivatives with a bifunctional coupling property that permits efficient immobilization of capture proteins on solid matrices and makes high-throughput analysis of protein–protein interactions possible.30,31
In fabricating the protein microarray, T. Kim et al. created a self-assembled monolayer (SAM) of the calix[4]crown-5 derivatives 3 or 4 on the amine modified the glass slides. In second step, the proteins were immobilized on the surface of a SAM modified glass slide, as shown in Scheme 2. The fabricated protein microarray showed excellent sensitivity of as low as 1–10 fg mL−1 of the tumor biomarker, PSA. There are some more reports on the application of the calix[4]crown-5 derivatives for the fabrication of protein microarrays.32–35 In 2007, T. Kim et al. developed the technique for the electrochemical immunosensing of glucose oxidase (GOx)-labeled C-reactive protein antigen (CRPAg) using the capture antibody monolayer immobilized on the calix[4]crown-5 3 as shown in Fig. 1.36
![Immobilization of proteins on the slide glass modified with the calix[4]crown-5 derivatives 3, 4 (left), microarray image, the array spot density is 4900 ea cm−2 (right). (Reproduced fromref. 30with permission from WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. Copyright © 2003.)](/image/article/2013/CS/c2cs35233h/c2cs35233h-s2.gif) | ||
| Scheme 2 Immobilization of proteins on the slide glass modified with the calix[4]crown-5 derivatives 3, 4 (left), microarray image, the array spot density is 4900 ea cm−2 (right). (Reproduced fromref. 30with permission from WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. Copyright © 2003.) | ||
![Schematic representation of the preparation of a competitive immunosensor using a CRP antibody monolayer (left): (a) calix[4]crown-5 SAMs on an Au electrode, (b) the CRP antibody immobilized with and without redox molecules (ferrocenecarboxylic acid) (c) GOx-CRPAg conjugates reacted and completely covering the CRP antibody monolayer. Typical CVs for the electrodes modified with CRP antibody monolayer interacted to CRP-GOx conjugates (right): (d) monolayer formed without ferrocene carboxylic acid, (e) monolayer formed with 0.3 mM ferrocene carboxylic acid. (f) Signal amplification by the effect of inserted redox molecules. (Reproduced fromref. 36with permission from Korean Chemical society.)](/image/article/2013/CS/c2cs35233h/c2cs35233h-f1.gif) | ||
| Fig. 1 Schematic representation of the preparation of a competitive immunosensor using a CRP antibody monolayer (left): (a) calix[4]crown-5 SAMs on an Au electrode, (b) the CRP antibody immobilized with and without redox molecules (ferrocenecarboxylic acid) (c) GOx-CRPAg conjugates reacted and completely covering the CRP antibody monolayer. Typical CVs for the electrodes modified with CRP antibody monolayer interacted to CRP-GOx conjugates (right): (d) monolayer formed without ferrocene carboxylic acid, (e) monolayer formed with 0.3 mM ferrocene carboxylic acid. (f) Signal amplification by the effect of inserted redox molecules. (Reproduced fromref. 36with permission from Korean Chemical society.) | ||
The calix[4]crown-5 SAMs provide an excellent platform for the immobilization of the proteins with reproducible density. However, to reduce the insulating effect on the gold electrode, the redox molecules (ferrocenecarboxylic acid) were inserted in between the proteins during the immobilization process. The use of the redox molecules significantly reduced the insulating effect which resulted in an improved detection limit. However, the complete elimination of the insulating effect was not achieved as the redox molecules were not immobilized on the electrode surface.
Therefore, the applications of the SAMs of calixarenes would be more important if the mixed self-assembled monolayers of two or more diverse substrates could be constructed.37,38
The ferrocenium/ferrocene redox couple shows a high binding affinity for the electron rich receptors such as the calix[4]crown-5 derivatives.39 In 2011, T. Kim et al. reported the characterization of the mixed self-assembled monolayer (M-SAM) at the molecular scale. The M-SAM was constructed by using ferrocenium/ferrocene redox couple 2 and calix[4]crown-5 derivative 1 as shown in the Scheme 3.40 The electrochemical characteristics of the mixed SAM modified gold electrodes were studied by cyclic voltammetry.
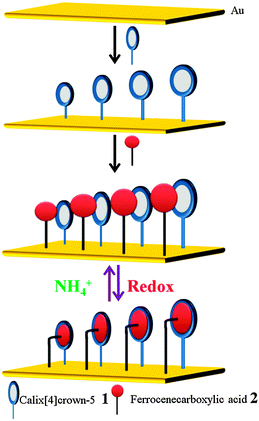 | ||
| Scheme 3 Construction of a well-defined mixed SAM and its characterization. (Reproduced fromref. 40with permission from The Royal Society of Chemistry.) | ||
Based on the CV data presented in Fig. 2a, the maximum surface coverage of the guest 2 on the gold surface is found to be 2.29 × 10−11 mol cm−2. Theoretically, the maximum surface coverage of host 1 would be 9.80 × 10−11 mol cm−2. The ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the surface coverage of the host 1 and guest 2 on the mixed SAM, respectively, indicates that molecules of the host 1 leave some spaces between each other during the formation of the SAM,41 which are large enough to introduce guest 2 to form a well-defined mixed SAM. Moreover, the addition of the ammonium ions to the above system has demonstrated that the complexed guest can be released with the input of the ammonium ions, as they have more affinity towards the host 1. The capture and release of the guest 2 in the absence and in the presence of ammonium ions demonstrated the application of the M-SAM as a molecular switch (Scheme 3, Fig. 2b).
1 for the surface coverage of the host 1 and guest 2 on the mixed SAM, respectively, indicates that molecules of the host 1 leave some spaces between each other during the formation of the SAM,41 which are large enough to introduce guest 2 to form a well-defined mixed SAM. Moreover, the addition of the ammonium ions to the above system has demonstrated that the complexed guest can be released with the input of the ammonium ions, as they have more affinity towards the host 1. The capture and release of the guest 2 in the absence and in the presence of ammonium ions demonstrated the application of the M-SAM as a molecular switch (Scheme 3, Fig. 2b).
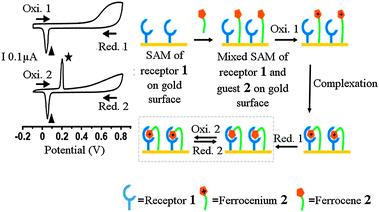 | ||
| Fig. 2 Interaction of the receptor 1 and guest 2 in the mixed SAM on the gold surface. Schematic diagram illustrating the molecular recognition of the ferrocene/ferrocenium (2/2+) by the receptor 1 in the mixed SAM on the gold surface based on cyclic voltammogram. The ★ and ▲ indicate the oxidation and reduction potential of the complexed ferrocene (2) and ferrocinium (2+), respectively. (Reproduced fromref. 40with permission from The Royal Society of Chemistry.) | ||
In 2009, and following years, Nimse et al. reported on the molecular recognition properties of the calix[4]arene derivatives 5–9 towards the structurally flat guest molecules 10–19 with binding constant (log K) in the range of 4.4 to 4.9 in the aqueous medium.42–45
Based on the knowledge of the mixed-self assembled monolayer and the molecular recognition properties of the imincalix[4]arene 5, and aminocalix[4]arnes 6–9, recently, T. Kim et al. introduced the phenomenon of molecular recognition to immobilize oligonucleotides on aminocalix[4]arene (AMCA) slides for the production of 9G DNAChips.46
The preparation of the 9G DNAChip and the hybridization thereafter is briefly explained in Scheme 4. The molecular recognition of the 9 consecutive guanine subunits in the oligonucleotide by the deep hydrophobic cavity of the aminocalix[4]arene was investigated by a competition experiment. To provide evidence for the immobilization of the probes based on molecular recognition, the immobilization of the oligonucleotide probe appended with 9 consecutive guanines was carried out in the presence of benzoic acid and 4-picoline and analyzed after hybridization. According to the earlier report, the structurally flat 4-picoline 12 showed strong binding (Ka = 3.9 × 104) with the host, while benzoic acid did not show any affinity.
 | ||
| Scheme 4 The preparation of the 9G DNAChip and hybridization with the Cy5-labeled target DNA (Cy5-T1). | ||
The 9G DNAChips shows more than 90% hybridization efficiency at 25 °C in 30 min. Moreover, the 9 consecutive guanines can be easily added to the oligonucleotide probes during their synthesis. The excellent properties shown by the 9G DNAChip enables it to be a powerful and promising tool for the DNA chip technology.
Based on the 9G DNAChip technology of using 9 consecutive guanines in the oligonucleotide for their immobilization on the AMCA slides, Nimse et al. reported the fabrication of HPV 9G DNAChips and their real time applications for the detection and discrimination of the human papillomavirus (HPV) in the clinical samples.47 The HPV 9G DNA chip test established its efficiency in terms of a signal-to-background ratio (SBR) of 200, which is 50 times superior to commercial HPV DNA chips, and 100% target-specific hybridization at 25 °C. The clinical sensitivities of HPV genotyping by the HPV 9G DNA chip test and a commercial HPV DNA chip test were 100% and 88%, respectively. However, the clinical specificities of HPV genotyping by the HPV 9G DNA chip test and the commercial HPV DNA chip test were 100% and 94%, respectively. The 100% clinical sensitivity and specificity of the HPV 9G DNA chip test make it a promising diagnostic tool for HPV genotyping.48–50
The 9G DNA chips are also used for the detection of the cancer biomarkers such as PSA (Prostate Specific Antigen) and CRP (C-Reactive Protein). The 9G DNA chips produced by using the aminocalix[4]arene derivative have several advantages over the conventional protein detection methods. The protein microarrays produced by the direct immobilization methods are known to suffer from drawbacks like the instability of the immobilized proteins, thus resulting in the low sensitivity.51–54 DNA-Directed Immobilization (DDI) was employed to improve the stability of the proteins by immobilizing them on the surface shortly before the detection of antigens.55,56 In such stepwise methods, first the proteins are immobilized on the surface and in the second step they are allowed to react with the target proteins. The disadvantage of this method is, once the proteins are immobilized on the surface they still have a chance to lose their activity over the period of time. Hence, the lower limit of detection of such methods is restricted to only sub-nanogram level.57,58
However, the DNA-guided detection (DAGON) method (Scheme 5) developed by using the 9G DNA chips can detect the biomarkers in sub-picogram level.59 The method can be explained in detail as follows. The biomolecular complex of the Cy5-labeled secondary antibody, the antibody-DNA conjugate and the target antigen formed in the solution is site-specifically guided to the predestined area on the chip surface and hybridized at the room temperature.
 | ||
| Scheme 5 DNA-Guided Detection (DAGON) method. (Reproduced fromref. 59with permission from The Royal Society of Chemistry.) | ||
The 9G DNAChips allowed the detection of biomarkers, C-reactive protein (CRP) and prostate specific antigens (PSA), at concentrations of 1 pg mL−1 and 0.1 pg mL−1 in the mixture of the proteins without any amplification technique. Moreover, the proteins with concentrations of 1 pg mL−1 to 10 pg mL−1 were easily differentiated in the buffer matrix.
The ability of the calixarene based molecules to form complexes with amino-acids has been the central topic of many studies. Hassen et al. studied the potential complex formation between the SAM of calix[4]arene based molecules on gold and some amino acids including arginine and lysine using faradaic electrochemical impedance spectroscopy (EIS).60 Šnejdárková et al. used self-assembled monolayers (SAMs) of 25,26,27,28-tetrakis(11-sulfanylundecyloxy)calix[4]arene 20 on the gold surface for the detection of dopamine with a limit of detection (LOD) of 50 pM.61
2.2 Applications of calixarenes in biotechnology
Highly specific recognition of the biological molecules by synthetic compounds is a fast-growing and challenging field both in academic and applied research.62 The use of calixarenes has attracted an increasing interest in the last two decades as a powerful tool for biochemical recognition and separation of the bioactive molecules such as amino acids, peptides, proteins, lectins, nucleotides, nucleosides, saccharides and steroids.63In 2003, Shahgaldian et al. reported the cryoprotective effect of four carbohydrates (glucose, fructose, mannose and maltose) on para-dodecanoyl-calixarene-based solid lipid nanoparticles. The photon correlation spectroscopy data indicate that the four carbohydrates act as good cryoprotectants, allowing reconstitution of the suspensions after the freeze-drying process.64 In 2005, Oshima et al. reported that a calix[6]arene carboxylic acid derivative exhibited a high affinity for cationic proteins such as cytochrome c (Cyt c) by promoting its extraction in organic media. The same group has also investigated several calix[4,6,8]arenes as protein extraction agents, which revealed that the large cavities of the carboxylic acid derivatives of p-tert-octylcalix[6]arene and p-tert-octylcalix[8]arene exhibit high extraction capacities for proteins compared with the p-tert-octylcalix[4]arene.65
Though the enzymatic reactions in organic media are reported to be difficult,66 they have several advantages over those in water due to the unique property and widespread utilities of the water-insoluble substrates.67,68 However, in 2007, Baba et al. reported the enzymatic polymerization of o-phenylenediamine with cytochrome c activated by a calixarene derivative in an organic medium.69 Yilmaz et al. encapsulated Candida rugosa lipase (CRL) within a chemically inert sol–gel support prepared by polycondensation with tetraethoxysilane (TEOS) and octyltriethoxysilane (OTES) in the presence of the N-methylglucamine-based calix[4]arene magnetic nanoparticles. The encapsulated lipase (calix-MN-Se-Lipase) showed high conversion and enantioselectivity for the S-Naproxen.70 Geraci et al. designed the calix[4]arene functionalized with the α-l-C-fucosyl as a new potential Pseudomonas aeruginosa biofilm inhibitor. The anti-biofilm activity of the synthesized compound 21 showed dose-dependent activity against the PAO1 strain.71
Preorganization of the functional elements onto macromolecular platforms has the potential to allow control of the self-assembling behavior of discrete architectures to produce nanometric objects that can be programmed to be complex, compact, deliver, and release plasmid DNA in a target cell.72
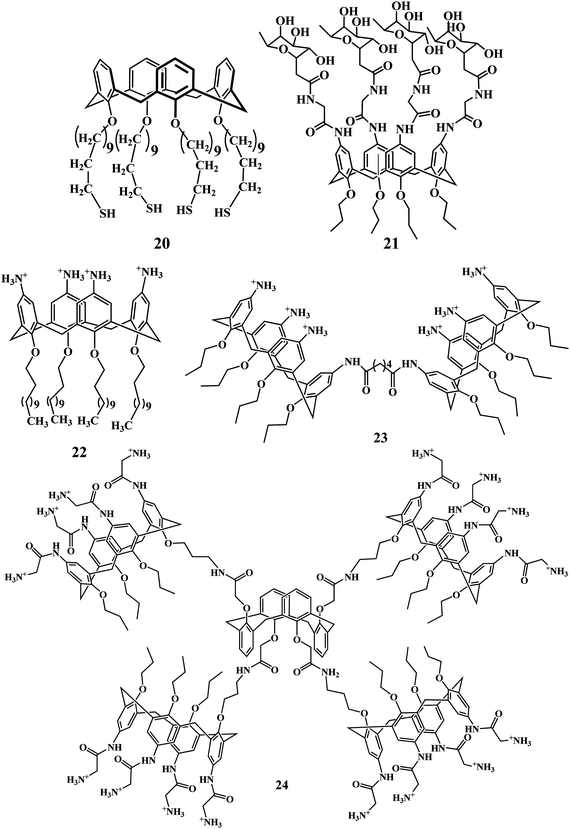
Compound 22 has been shown to self-assemble, with the absence of a co-surfactant, to form positively charged solid lipid nanoparticles (SLNs) that interact with double stranded DNA.73 The dimeric derivative 23, with a higher degree of preorganization, had been previously shown to interact with DNA, probably by targeting the major groove, though transfection abilities were not demonstrated. Matthews and co-workers proposed an alternative macromolecular design based on the installation of four amino-functionalized amphiphilic calixarene motifs onto rigid calix[4]arene scaffolds.74 Multicalixarene–pDNA binding turned out to be much stronger than that of their monomeric analogs, indicating the existence of cooperativity effects. Multicalixarenes blocked pDNA electrophoretic mobility, therefore efficiently condensing them, and are virtually nontoxic up to mM concentrations. Transfection experiments carried on Chinese hamster ovary (CHO) cells revealed that the presence of aliphatic amino groups, as in 24, was necessary to efficiently promote the GFP-encoding gene expression. pDNA complexes from multicalixarenes with arylamino groups were ineffective irrespective of the alternating or cone conformation of the central scaffold.
Recently, Rodik et al. reported a two-step hierarchical assembly of small DNA nanoparticles for gene delivery based on amphiphilic cone-shaped cationic calixarenes 25 (Scheme 6).75 Na et al. reported the possibilities of the luminescent quantum dots derived from calixarene derivatives as platforms for probing in vitro and in vivo biological processes.76 Schatz et al. in their report summarized the further applications of calixarenes in biotechnology as mimics of ion channels through artificial cell membranes, as mimics of metalloenzymes, and in the promising emerging field of calixarene based agents for medical imaging and radiotherapy.77
 | ||
| Scheme 6 Hierarchical assembly of small DNA nanoparticles for gene delivery based on amphiphilic cone-shaped cationic calixarenes 25. | ||
Ion transport across membranes is one of the most important processes in living cells. Davis et al. have done considerable work in the development of the artificial ion channels which bind to the lipid bilayer and transport the ions effectively.78–80 In 2002 they reported the ion transport activity of calix[4]arene tetrabutylamide 1,3-alt 26 in liposomes, planar lipid bilayers, and HEK-293 cells. These experiments, together with 1H NMR and X-ray crystallography data, indicate that calix[4]arene tetrabutylamide 26 (1) forms ion channels in bilayer membranes, (2) mediates ion transport across cell membranes at positive holding potential, (3) alters the pH inside liposomes experiencing a Cl− gradient, and (4) shows a significant Cl−/SO42− transport selectivity.
In 2006 Davis et al. developed calixarene derivatives 27 and 28 to control the transmembrane Cl− transport (Scheme 7). The key was that the calixarene derivative 27 enables the transmembrane Cl− transport. However, calixarene derivative 28 inhibits the transmembrane Cl− transport.81 They argued that the reason behind the control of the transmembrane Cl− transport is that the 28 and 27 form inactive heteroaggregates 27n·28m in the membrane, thus shifting the equilibrium away from active 27n structures. However, in 2007 Davis et al. reported on the transmembrane anion transport as mediated by calixarene 27 mimicking the natural ion transport mechanism across the cell membrane.82 The expertise on the applications of calixarene derivatives in the transmembrane Cl− transport allowed the group of Davis et al. to develop the small molecules that facilitate exchange of bicarbonate and chloride anions across liposomal membranes.83,84
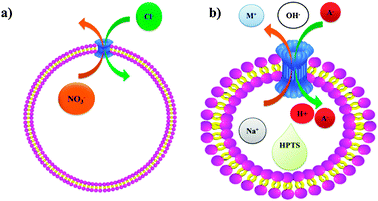 | ||
| Scheme 7 (a) Transmembrane anion transport as mediated by calixarene 27, (b) schematic representation of the transport of anions mediated by calixarene 27. A pH gradient results from addition of extravesicular NaOH solution. The charge caused by H+ efflux or OH− influx is compensated by cation influx or anion efflux, as mediated by the exogenous ligand such as 27. The increase in intravesicular pH, monitored by the entrapped pH-sensitive dye, HPTS, reflects the electrolyte exchange rate. | ||
2.3 Applications of calixarenes in biology
Calixarenes have been described as efficient multivalent platforms for biological targets, such as peptides85 and protein surfaces.86,87Ungaro and his group published several papers on the synthesis and application of calixarene derivatives for the recognition of neutral, cationic, and anionic guest molecules.88–92 In 2001, Ungaro et al. synthesized the upper rim modified C-Linked peptidocalix[4]arenes 29–32 of which 29 preferentially interacted with the anionic species.93 These C-linked peptidocalix[4]arene derivatives were further used to produce the nanotubes.94,95
In 2003, Ungaro et al. developed a new macrocyclic receptor 33 for carbohydrate recognition based on upper rim peptide bridged calix[4]arene.96,97 Receptor 33, in which a charged phosphate group cooperates with peptide hydrogen-bonding donor and acceptor groups in the binding process, is the most efficient and selective in the complexation of simple carbohydrate derivatives. The selectivity observed is toward β-glucoside 34, which is better bound (ΔG° = 19.6 kJ mol−1) compared to the corresponding α anomer 35 (ΔG° = 17.0 kJ mol−1) and to the β-galactoside 36 (ΔG° = 17.7 kJ mol−1) in CDCl3. A substantial drop in the stability constant is observed by esterification of the phosphate group in the host 33 or by alkylation of the OH groups in the 2 and 3 positions in the β-glucoside and β-galactoside derivatives.
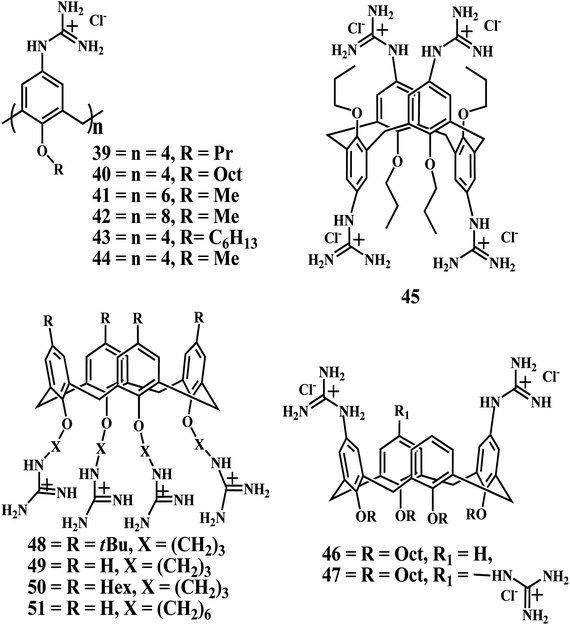
In 2004, Ungaro group reported the p-guanidiniumcalix[n]arenes 39–42, with good solubility in water except for the 40, which form a macroscopic gelatinous aggregate even at very low concentration. Moreover, these compounds were reported to bind to plasmid DNA efficiently without any cytotoxicity.98
In 2006, Ungaro et al. reported on calix[n]arenes 39–47 functionalized with guanidinium groups at the upper rim and alkyl chains at the lower rim could bind to DNA, condense it, and in some cases, promote cell transfection depending on their structure and lipophilicity.99 The atomic force microscopy (AFM) studies indicated that upon DNA binding the hydrophobic association of the lipophilic chains of cone guanidinium calix[4]arenes 39, 40, and 43 drives the formation of intramolecular DNA condensates, characterized by DNA loops emerging from a dense core. Furthermore, hexyl and octyl chains confer to these calixarenes cell transfection capabilities. Conversely, larger and conformationally mobile calix[6]- and calix[8]arene methoxy derivatives 41 and 42 form intermolecular aggregates characterized by “gorgonlike” structures composed of multiple plectomenes. These adducts, in which interstrand connections are dominated by electrostatic interactions, fail to promote cell transfection. Finally, calix[4]arene 45 in a 1,3-alternate conformation show an intermediate behavior because they condense DNA, but the process is driven by charge–charge interactions.
These results indicate that relatively small variations in the size, lipophilicity, and conformation of these water-soluble compounds, together with a subtle interplay between hydrophobic and electrostatic supramolecular interactions, significantly affect the outcome of DNA condensation and cell transfection. This study by the Ungaro and coworkers also contributes to a better understanding of the factors affecting DNA delivery at the molecular level and may help to design more efficient and safer vectors for gene therapy. However the only limitation for this approach is that these compounds are characterized by low transfection efficiency and high cytotoxicity especially at the vector concentration required for observing cell transfection (10–20 μM), even in the presence of the helper lipid DOPE (dioleoylphosphatidylethanolamine).100,101
Interestingly, in 2008 Ungaro et al. found that attaching guanidinium moieties at the phenolic OH groups (lower rim) instead of the aromatic moiety (upper rim) of the calix[4]arene through a three carbon atom spacer results in a new class of cytofectins 48–51. Compound 48, when formulated with DOPE, performs cell transfection quite efficiently and with very low toxicity, surpassing a commercial lipofectin widely used for gene delivery.102
In a more recent study,103 Ungaro et al. described the phenomenon of the cell transfection activity of the guanidinium calix[4]arene derivatives. The attachment of the guanidinium groups at the lower rim of calix[4]arenes disclosed the possibility to significantly enhance the cell transfection ability of the synthetic vectors based on this scaffold and reduce their toxicity to cells, when compared to the analogues 39, 40, and 43 with the charged groups directly linked to the aromatic nuclei (upper rim). In particular, the macrocycle 49 which was used in formulation with DOPE, was found to be better than the widely used, commercially available lipofectamine LTX in the transfection of RD-4 human Rhabdomyosarcoma.
The comparison of 49 with a series of analogues showed that, subtle variations in conformational freedom, distance of the charges from the cavity, and the nature of the cationic head groups decorating the apolar cavity cause drastic changes in the biological properties. Moreover, the ability for these calixarene based vectors to self-assemble in water and condense DNA, as detected by AFM experiments,104 is a necessary but not sufficient condition for getting significant transfection activity.
It is also interesting that, at least for lower rim derivatives in the cone conformation, the guanidinium is essential as a cationic group to observe transfection activity, and its replacement with other ammonium moieties is definitely detrimental, although some of these have been reported as effective when attached at the upper rim of calix[4]arene derivatives.105
The recognition of toxic protein surfaces by designed ligands has become an attractive approach in drug discovery. However, the variable nature and irregular behavior of protein surfaces defy this new area of research. The easy to understand “lock and key” model is far from being the ideal paradigm in biomolecular interactions and, hence, new findings on how proteins and ligands behave in recognition events pave steps of the way. Recently, Coleman et al. reported the enhanced detection of the pathogenic prion protein by its supramolecular association with para-sulfonato-calix[n]arene derivatives.106
The cholera toxin is a pentavalent sugar-binding protein belonging to the class of AB5 toxins. The doughnut-shaped B pentamer presents five identical sugar-binding sites on a single face and is responsible for cell-surface binding. The cell surface ligand of cholera toxin is ganglioside GM1 [Galβ1-3GalNAcβ1-4(NeuAcα2-3)Galβ1-4Glcβ1-1Cer] 53. The GM1 oligosaccharide interacts with the toxin via the terminal galactose and the sialic acid residues. However, in 2005, Ungaro et al. extended their research on calixarene carbohydrate interactions and developed a novel glycocalix[4]arene 52 which has a higher affinity for the cholera toxin than its natural cell surface ligand ganglioside GM1.107,108
Growing insights into the functionality of lectin–carbohydrate interactions are identifying attractive new targets for drug design.109,110 As glycan recognition is regulated by the structure of the sugar epitope and also by topological aspects of its presentation, a suitable arrangement of ligands in synthetic glycoclusters has the potential to enhance their avidity and selectivity. If adequately realized, such compounds might find medical applications. Ungaro et al. focused on lectins of clinical interest, acting either as a potent biohazard (a toxin from Viscum album L. akin to ricin)111–113 or as a factor in tumor progression (human galectins-1, -3, and -4).114–116 Using a set of 12 calix[n]arenes 54–65 (n = 4, 6, and 8) with thiourea linked galactose or lactose moieties, Ungaro et al. first ascertained the lectin-binding properties of the derivatized sugar head groups conjugated to the synthetic macrocycles.117 Despite their high degree of flexibility, the calix[6,8]arenes 62–65 proved especially effective for the plant AB-toxin. The bioactivity of the calix[n]arenes 54–65 was also proven for human galectins. Notably, selectivity for the tested galectin-4 among the three subgroups was determined at the level of solid-phase and cell assays, the large flexible macrocycles again figuring prominently as inhibitors. Alternate and cone versions of calix[4]arene 55, 57, 59, 61, and 64 with lactose units distinguished between galectins-1 and -4 versus galectin-3 in cell assays. The results thus revealed bioactivity of galactose-/lactose-presenting calix[n]arenes for medically relevant lectins and selectivity within the family of adhesion/growth-regulatory human galectins.
Recently, Ungaro et al. reported the new class of calixarene glycoclusters 66–69 based on 2/3′-substitutions in the N-acetyllactosamine (LacNAc) core.118,119 The overall results illustrate the benefit of combining core derivatisation with conjugation of the biomimetic product to create distinct scaffolds for multivalent display.120 Further tailoring the nature of the substituent to fully match the galectin's individual microenvironment in the contact site, comprising Arg144, His158, Asn160, Lys176 and Trp181, affords the possibility for iterative improvements.121,122 Using bivalent glycophanes with/without conformational flexibility the attained selectivity increases could then be exploited to affect galectin-3 and its proteolytically processed form differently.123 In general terms, the strategy presented by Ungaro and coworkers to amalgamate the carbohydrate and supramolecular chemistry can have relevance beyond the particular lectins tested in studies reported by them.
Most often, ligand candidates are designed as rigid molecules to minimize the entropic penalty associated with the loss of degrees of freedom upon binding proteins.124 However, there are many other erratic variables in the equation that can eventually lead to an unpredictable outcome in the macromolecular recognition event,125,126 and flexibility might not always be the penalizing factor.127,128 In 2001, Coleman et al. reported the complexation of Bovine Serum Albumin (BSA) with various derivatives of sulfonatocalix[n]arene in cone conformations. The effects on the structure of thin films formed by surface deposition of BSA show that the sulfonatocalix[n]arenes act to reticulate the films and produce essentially planar systems.129,130
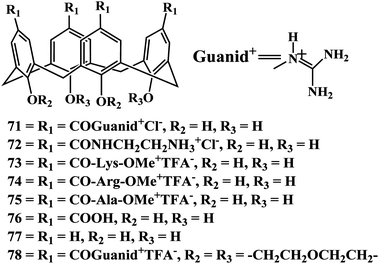
In 2008, Mendoza et al. explained how an increase in the flexibility of both protein and ligand can result in an increase in the stability of the macromolecular complex. The biophysical study of the interaction between a designed flexible tetraguanidinium-calix[4]arene13170 and the tetramerization domain of protein p53 (p53TD), and its natural mutant p53TD-R337H shows how the mutant domain interacts more tightly with the ligand than the well-packed wild-type protein.132 Moreover, the flexible calixarene ligand interacts with higher affinity to both wild-type and mutated protein domains than a conformationally rigid calixarene analog previously reported. These findings underscore the crucial role of flexibility in molecular recognition processes, for both small ligands and large biomolecular surfaces.133
Potassium channels are among the core functional elements of life because they underpin essential cellular functions, including excitability, homeostasis, and secretion. Mendoza et al. have reported a series of multivalent calix[4]arene ligands 71–78 that bind to the surface of voltage-dependent potassium channels in a reversible manner. The tetraacylguanidinium or tetraarginine calixarene derivatives with small lower rim O-substituents shows reversible inhibition, without noticeable damage of the oocytes.134
Based on these results, a new generation of ligands can be developed, taking advantage of the divergent synthetic strategy for upper-rim functionalization of OH-free, the rigidity of bridged calix[4]arenes, and a study of the footprints of toxins.135,136 Increasing the affinity of the channel-calix[4]arene interaction through further functionalization of 74 with suitable peptide chains attached to the upper rim is yet to be completed. Ultimately, the development of this class of compounds might lead to therapeutic agents against various ailments such as autoimmune disorders, diabetes, epilepsy, or cardiac diseases.137
The synthesis and biological applications of calixarene glycoconjugates represented a new way of considering calixarenes, in particular, with respect to their traditional role as ionic and molecular receptors.138 Preliminary experiments from Dondoni and Marra laboratories have shown that compound 79 acts as an inhibitor of the BK virus-induced hemagglutination.139
Ungaro and coworkers reported the interaction of Concanavalin A (ConA) and peanut lectin (PNA, Arachishypogaea) with the tetravalent glucocluster 54 by turbidimetric analysis.140 The thiourea group has been exploited to link two 54 or four 56 carbohydrate units at the upper rim of tetrapropoxycalix[4]arene derivatives in the cone conformation. The thiourea groups act not only as linkers but also as binding units for anionic substrates as evidenced by solution 1H NMR and ESI-MS experiments.
In 2008, Mueller et al. investigated the uptake and localization of fluorescent derivative calix[4]arene 80 in cellular organelles, which provided useful information for the development of calix[4]arenes as drugs and as drug-delivery systems.141 The nature of the cellular uptake of the derivative was reported to be a nonspecific process, not linked to either of the main endocytic pathways which results in accumulation of the probe 80 within the cell cytoplasm. Until the publication of the confocal microscopic images of the fluorescence-tagged calixarenes by Mueller et al., there were no concrete evidences about the localization of the calixarenes in cells.
Furthermore, the Mueller group proposed the applications of the vanadyl bearing calix[4]arene ligands for cell imaging and monitoring the cellular uptakes. Vanadyl bearing calix[4]arene ligands have undergone evaluation against several cell lines, and show varying degrees of toxicity. For vanadyl complexed to a sulfonylcalix[4]arene, monitoring of the strong blue fluorescence revealed slow uptake kinetics. The complex is exclusively found in the cytoplasm of the cells and uptake is not due to active endocytotic mechanisms. Interestingly in different cell lines the vanadyl calixarenes exhibit differences in toxicity, which is the starting point for creating complexes in the near future which have selective cell type toxicity and can therefore be used as anti-tumor therapies.142,143
Bezouška et al. recently identified a new class of high affinity ligands the carboxylated calixarenes 82–84, for a CD69 leukocyte membrane receptor. The thiacalix[4]arene 83 had the highest affinity for CD69 in direct binding assays, and proved to be the most specific inhibitor of CD69 identified so far in receptor precipitation and cellular activation experiments. Carboxylated calixarenes also proved effective at the protection of CD69high lymphocytes from apoptosis triggered by a multivalent ligand or antibody.144
In 2004, Cunsolo et al. reported calix[8]arene derivatives 85–90 that bind to the active region of the lung tryptase enzyme blocking the approach of the substrate resulting in a time-dependent competitive inhibition of the enzyme.145 Human tryptase was indirectly inhibited due to the antagonist effect of derivatives 87–90 on the proteoglycan heparin. At the same time, competitive inhibition of rHT was also observed supporting the effectiveness of these surface binding receptors that could outline a new approach to the design of artificial enzymatic inhibitors. Trypsin, another serine protease, showed no inhibition by derivative 88, underlining its selectivity for tryptase. Although the high molecular weight makes these derivatives fairly unattractive as drug candidates, they could have potential utility as novel tools to better understand how tryptase is involved in protein–protein interactions. The cluster of positive charges exposed by these basic derivatives makes them the first class of nonproteic and nonpolymeric derivative heparin binders. In 2007, Consoli et al. reported a water-soluble thymine nucleotide-calixarene conjugate 91 and the preliminary investigation of their in vitro DNA replication inhibitory activity.146
In 2008, Geraci et al. synthesized a novel fully synthetic cancer vaccine candidate in which a cluster of four Tn antigen glycomimetic units (S-Tn) is conjugated to an immunoadjuvant moiety, tripalmitoyl-S-glycerylcysteinylserine, through a calix[4]arene scaffold. The presence of a rigid platform of the calix[4]arene scaffold provided a tetravalent derivative 92 with a definite spatial preorganization of the antigenic moieties. Biological tests, performed in vivo by the immunization of mice, showed that the target construct possesses higher immunostimulating activity with respect to the corresponding monovalent analogues, emphasizing the presence of a cluster effect and the important role of the calixarene macrocycle.147 In the same year, Geraci et al. extended their study on the anticancer activity of calixarene derivatives by using a calix[8]arene derivative instead of calix[4]arenes. Glycocalix[8]arenes14893, 94 exposing multiple N-acetylglucosamine (GlcNAc) residues and urido functionalities inhibit the rat C6 glioma cell migration and proliferation in a scratch wound model.149,150 In 2000, similar anticancer activity of calixarene derivatives was reported by Blaskovich et al. up on peripheral administration of the modified calixarene scaffold.151
In 2004, Schrader et al. reported on the application of the calix[4]arene tetraphosphonate 95 as an artificial receptor152–154 embedded in the stearic acid monolayer on water which resembles the natural cell membrane.155,156 In this investigation they found that the addition of increasing concentrations of tetraphosphonate 95 to a stearic acid monolayer on water led to the incorporation of increasing numbers of receptor molecules in the monolayer. The artificial receptors embedded in a monolayer were demonstrated to be capable of “multipoint binding” of complementary charged proteins such as histone H1, Cytochrome C, proteinase K etc., similar to the natural receptors.
In 2005, Schrader et al. used the same calix[4]arene tetraphosphonate 95 receptor molecules and developed a new method for the nanomolar detection of the proteins by embedding the molecules of receptor 95 in the stearic acid monolayer.157 The acidic and basic proteins were selectively detected by this method. However, this method is not useful for the detection of proteins in the clinical samples as the biological matrix has a mixture of lot of different proteins.
In the following year, Schrader et al. achieved the “naked eye” color detection of proteins by embedding calixarene receptors 95, 98, 99 within vesicles comprising phospholipids and the chromatic polymer polydiacetylene.158 Dramatic visible absorbance changes were induced through electrostatic interactions between the protein surface and the vesicle incorporated host 95, however host 96 did not show expected results. The reason behind that is that the anionic and cationic functional groups on calixarene moieties in the receptor 95 and 96, respectively show strong binding with the proteins as compared to the neutral receptor 97. The colorimetric responses can be induced by micromolar protein concentrations, and furthermore, specific protein fingerprints could be obtained by incorporating different receptors within the vesicles.159
In 2006, Schrader et al. turned their attention to the recognition of DNA in the aqueous buffer, this time with the large calixarene dimers 98, 99. With the hexaaniline calixarene dimer 98, they introduced a large amphiphilic molecule that selectively binds ds DNA and RNA with submicromolar KD values in buffered aqueous solution; contrary to most other DNA binders, it most likely targets exclusively the major groove and causes no conformational change or destabilization in the DNA duplex.160–163
2.4 Applications of calixarenes in drug discovery
It is well known that the toxicity is a barrier to the discovery and development of potent drug molecules. However, most calixarene derivatives showed low or no toxicity in the animal models.164 Calixarenes have shown antiviral,165 antibacterial,166 antifungal, antitubercular,167 and anticancer activities.168,169 In 2009, Fátima et al. has reviewed the use of calixarenes as new chemical entities of distinct biological activities or as host for bioactive guest molecules.170Platelet-derived growth factor (PDGF) and its receptor PDGFR are required for tumor growth and angiogenesis, so disruption of the PDGF–PDGFR interaction should lead to starvation of tumors and reduction of tumor growth. Hamilton et al. discovered the potent PDGF antagonist through the synthesis of a series of calix[4]arene-based compounds GBF11 that are designed to bind to the three-loop region of PDGF.171 Three isophthalate arms turned out to be sufficient to obtain good binding affinity and also to give competitive cellular activity.
In 2007, Křen et al. has reported that the N-Acetyl-D-glucosamine substituted calix[4]arenes 100–102 stimulate the antitumor immune response mediated by NK (natural killer) cells.172 The binding affinity of GlcNAc-substituted calix[4]arenes carrying two to four sugar units were in good agreement with the inhibitory potencies of the linear chitooligomers (chitobiose to chitotetraose) reported previously by the same group.173,174 The influence of GlcNAc substitution of the calix[4]arene skeleton on binding affinity for the CD69 receptor was more profound and the GlcNAc-terasubstituted calix[4]arene 100 proved to be the best CD69 ligand. Lower GlcNAc substitution led to a substantial decrease of the binding activity. The immunostimulating activity results with the GlcNAc tetramers on calix[4]arene scaffolds exhibited stimulation of the natural cytotoxicity of human PBMC by compound 100 in the concentrations of 10−4 and 10−8 M.
Protein tyrosine phosphatases (PTPs) are known to be signal transduction enzymes that catalyze dephosphorylation of phosphotyrosine residues in proteins. The PTP termed YopH is a key outer membrane protein secreted by the pathogenic bacteria Yersinia. The species of these bacteria cause several diseases ranging from gastrointestinal syndromes to bubonic plague.175 Recently, Kukhar et al. reported the inhibition of Yersinia protein tyrosine phosphatase by calix[4]arene176,177 mono-, bis-, and tetrakis(methylenebisphosphonic) acids 103–105 as well as calix[4]arene178106 and thiacalix[4]arene179107 tetrakis(methylphosphonic) acids.180 This study indicates that the calix[4]arenes are promising scaffolds for the design of inhibitors of the PTPs. The phosphorylated calix[4]arenes investigated in this paper exhibited strong competitive inhibition of Yersinia PTP. The phosphonate groups as well as macrocyclic structure are essential for the activities of these compounds. Model docking studies revealed a similar binding mode for calixarene inhibitors covering the active site, with the location of the phosphonate residues being around the entry of the phosphotyrosine binding cavity. This provides a molecular basis for the understanding of the mechanism of the enzyme–inhibitor interaction and can be useful for the development of calixarene inhibitors of YopH.
In another study, the Kukhar group found that the thiacalix[4]arene tetrakis(methylphosphonic) acid 107 is a stronger inhibitor of the alkaline phosphatase than the calix[4]arene tetrakis(methylenebisphosphonic) 106.181 Alkaline phosphatase is a known metal-dependent enzyme, which catalyzes the hydrolysis and transphosphorylation of the monoalkylphosphates and regulates the functions of many biological systems.182 Enhanced activity of the enzyme leads to the calcification of soft tissues,183 development of inflammation184 and other diseases.185 Fišerová et al. studied the anticancer activity of the N-acetyl-D-glucosamine-substituted glycoconjugates of calix[4]arene, which acts as a ligand for the NKR-P1 molecule and induces NK cell-mediated cytotoxicity in vitro. The in vivo effect of these glycoconjugates of calix[4]arene on the mouse melanoma model demonstrated the significant reduction in the tumor growth in 14 days.186
Sakaguchi et al. found that the derivatives of calix[6]arene 108, 109 can enhance the transcriptional activity of mutant p53 tumor suppressor protein.187 Calix[6]arene derivative 108 with six imidazole groups could stabilize the p53 tetrameric structure under physiological conditions. On the contrary Guanidinium-calix[4]arene 70, which has four guanidinium groups, does not show the stabilization of the mutant p53 under the same conditions. Furthermore, the imidazole-calixarene 108 could enhance the transcriptional activity of p53 Arg337His in cells. The further optimization of this calix[6]arene derivative might lead to the development of a candidate for cancer therapy as the derivative showed promising results in the stabilization of the p53 tetrameric structure. Coleman and coworkers have reported the calix[4]arene derivatives for the extraction and stabilization of the functional membrane proteins.188 However, the comparison of the results between the calix[4]arene and calix[6]arene derivatives with similar functional groups is very important to establish the structure–activity relationship.
Coinfection with both human immunodeficiency virus (HIV) and hepatitis C virus (HCV) has become a public health challenge, afflicting more than 10 million people worldwide.189,190 Recently, Hamilton et al. reported the potent compounds 110–120 based on a tetrabutoxy-calix[4]arene scaffold that possesses dual inhibition for both HIV and HCV. The potency of the compounds is due to the aromatic isophthalate spacers and diacid groups at the upper rim for anti-HIV activities and anti-HCV effects, respectively.191 Among the seven compounds with a butyl chain 110–116 at the bottom rim, compound 111 exhibited the most potent anti-HIV activity. When the isophthalic derivative was replaced with aspartic acid in compound 114, anti-HIV activity decreases about 10-fold but anti-HCV activity is slightly improved, while cytotoxicity remained the same. This suggested that aromatic substitutions are superior to aliphatic ones at the upper rim for HIV inhibition, while anti-HCV activity appears not to be as sensitive to this change. Compounds 110 and 120 contained benzyl ester and cyclohexylamide derivatives of the isophthalate linkers, respectively, suggesting that substituting both acid groups are required for potent antiviral activity. Overall, these observations suggest that four charges on the top rim are important for anti-HIV and anti-HCV activities. The lack of antiviral activity in positively charged 116 confirms the importance of the negative charges on the projected periphery of the compounds. These results demonstrate remarkable anti-HIV and anti-HCV activities for a series of compounds based on the tetrabutyl-calix[4]arene scaffold. It can be noticed from the results that the cone conformation of the scaffold is important for antiviral activity. In addition, aromatic isophthalate spacers at the upper rim are essential for anti-HIV activities and the diacid groups are also necessary for the observed anti-HCV effects. The molecular targets and mechanisms for anti-HIV and anti-HCV activities with these calix[4]arene compounds can provide valuable insight for future attempts to improve potency.
Regnouf-de-Vains et al. reported a calixarene-based prodrug, the conjugation of the nalidixic acid to the lower rim of the calixarene. The prodrug 121 showed good antibacterial activity against gram-positive and gram-negative bacterial strains.192 Calixarene 121 displays MIC values of 35 μg mL−1 for E. coli, 70 μg mL−1 for the two S. aureus strains, and 140 μg mL−1 for E. faecalis and P. aeruginosa, while the mono-alcohol 122 needs from 2 (E. faecalis, P. aeruginosa) to 4 (E. coli, S. aureus) times higher concentrations, in ranges considered as non-active. The compound 121, designed as a nalidixic acid prodrug, effectively displays this behavior, that is, the release of quinolone in biological medium. This observation calls for much deeper investigation, related to its proper antibacterial behavior, as well as to additive and/or multiplicative effects between the two released by-products or between the title compound and nalidixic acid.
A folate receptor (FR) is a highly selective cancer cell and activated macrophage marker,193 and folic acid vitamin (FA), which binds to FR with high affinity (Kd = 0.1 nM), and behaves as a “Trojan Horse”194 that can promote the specific delivery of imaging and therapeutic agents into FR-positive cells.195 Recently, Consoli et al. reported the multivalent folate conjugate 123 in which four folate units are clustered by means of a calix[4]arene platform.196
The presence of multiple folate homing moieties and a good water-solubility at physiological pH, in addition to the capability to increase indomethacin water solubility are prerequisites that make 123 potentially appealing for targeted drug delivery. The ability of the compound 123 to bind with the folate receptor gives new dimensions to the future work as it can show cellular cell uptake thus having potential application in the targeted drug delivery of the anticancer agents.
3. Concluding remarks
Although much work is currently devoted to the complexation of biomolecules by calixarenes that provides the chemists with numerous data on the thermodynamics of complexation, very little is known on the specific interactions maintaining the complexes. Important applications of the functionalized calixarenes in biosensor technology and biology investigated by different techniques have been presented in this Review. The similar properties of various calix[n]arene derivatives may open up new future usages in medications after determining their toxicity, an issue which has been in progress for a while.197–199 Likewise, microchip-based calixarenes for the development of novel immunosensors with applications in clinical diagnostics have been studied. However, the protein chips based on direct immobilization of the proteins on calixarene-modified slides lacks the current demands in biomarker detection for diagnostic purposes. Innovations in the applications of calixarenes in diagnostics are needed for the highly efficient detection of marker proteins. The potential applications of tetrakis- (dihydroxy-phosphorylmethyl) derivatives of calix[4]arene and thiacalix[4]arene displaying inhibition properties towards alkaline phosphatases from bovine intestine mucosa and shrimp and human placenta would indeed encourage calixarene chemists to broaden their research areas. Moreover, the involvement of calixarenes as synthetic ionophores in the challenging field of transmembrane ion transport has broadened their area of applications in biotechnology. Apart from their binding ability to many organic and bioorganic molecules, the functionalized calixarenes also act as the antibacterial, antiviral, and anticancer agents demonstrating their potential applications in the pharmaceutical industry. However, a more elaborated study on the modified calixarene derivatives is necessary for their real time induction in the drug development and drug discovery.References
- M. W. Peczuh and A. D. Hamilton, Chem. Rev., 2000, 100, 2479–2494 CrossRef CAS.
- H. Yin and A. D. Hamilton, Angew. Chem., Int. Ed., 2005, 44, 4130–4163 CrossRef CAS.
- A. W. Coleman, F. Perret, A. Mousa, M. Dupin, Y. Guo and H. Perron, Top. Curr. Chem., 2007, 277, 31–88 CrossRef CAS.
- F. Perret, A. N. Lazar and A. W. Coleman, Chem. Commun., 2006, 2425–2438 RSC.
- F. Sansone, S. Barboso, A. Casnati, D. Sciotto and R. Ungaro, Tetrahedron Lett., 1999, 40, 4741–4744 CrossRef CAS.
- D.-D. Guo, K. Wang and Y. Liu, J. Inclusion Phenom. Macrocyclic Chem., 2008, 62, 1–21 CrossRef CAS.
- S. B. Nimse, J. Kim, V. Ta, H. Kim, K. Song, C. Jung, V. Nguyen and T. Kim, Tetrahedron Lett., 2009, 50, 7346–7350 CrossRef CAS.
- S. B. Nimse, V. Nguyen, J. Kim, H. Kim, K. Song, W. Eoum, C. Jung, V. Ta, S. R. Seelam and T. Kim, Tetrahedron Lett., 2010, 51, 2840–2845 CrossRef CAS.
- A. Mulder, J. Huskens and D. N. Reinhoudt, Org. Biomol. Chem., 2004, 2, 3409–3424 CAS.
- M. Mammen, S.-K. Choi and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 2754–2794 CrossRef.
- N. Sieffert, A. Chaumont and G. Wipff, J. Phys. Chem. A, 2009, 113, 10610–10622 CrossRef CAS.
- N. Maulucci, F. De Riccardis, C. B. Botta, A. Casapullo, E. Cressina, M. Fregonese, P. Tecilla and I. Izzo, Chem. Commun., 2005, 1354–1356 RSC.
- P. J. Cragg and K. S. J. Iqbal, Dalton Trans., 2007, 26–32 Search PubMed.
- L. Mutihac, Anal. Lett., 2010, 43, 1355–1366 CrossRef CAS.
- F. Perreta and A. W. Coleman, Chem. Commun., 2011, 47, 7303–7319 RSC.
- P. A. Gale, Chem. Soc. Rev., 2010, 39, 3746–3771 RSC.
- H. N. Kim, W. X. Ren, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2012, 41, 3210–3244 RSC.
- H. J. Kim, M. H. Lee, L. Mutihac, J. Vicens and J. S. Kim, Chem. Soc. Rev., 2012, 41, 1173–1190 RSC.
- L. Mutihac, J. H. Lee, J. S. Kim and J. Vicens, Chem. Soc. Rev., 2011, 40, 2777–2796 RSC.
- H. C. Visser, D. N. Reinhoudt and F. de Jong, Chem. Soc. Rev., 1994, 23, 75–81 RSC.
- H. Schneider and A. K. Yatsimirsky, Chem. Soc. Rev., 2008, 37, 263–277 RSC.
- L. Baldini, A. Casnati, F. Sansone and R. Ungaro, Chem. Soc. Rev., 2007, 36, 254–266 RSC.
- J. W. Cornforth, P. D. Hart, G. A. Nicholls, R. J. W. Rees and J. A. Stock, Br. J. Pharmacol., 1955, 10, 73–86 CrossRef CAS.
- E. Da Silva, A. N. Lazar and A. W. Coleman, J. Drug Delivery Sci. Technol., 2004, 14, 3–20 CAS.
- H. Yamamoto and S. Shinkai, Chem. Lett., 1994, 23, 1115–1118 CrossRef.
- J. S. Li, Y. Y. Chen and X. R. Lu, Eur. J. Org. Chem., 2000, 485–490 CrossRef CAS.
- J. L. Atwood, A. J. Dalgarno, M. J. Hardie and C. L. Raston, Chem. Commun., 2005, 337–339 RSC.
- R. Zadmard and T. Schrader, in Calixarene in the Nanoworld, ed. J. Vicens and J. Harrowfield, Springer, Dordrecht, 2007, pp. 287–309 Search PubMed.
- A. Amiri, E. Y. Choi and H. J. Kim, J. Inclusion Phenom. Macrocyclic Chem., 2010, 66, 185–194 CrossRef CAS.
- Y. Lee, E. K. Lee, Y. W. Cho, T. Matsui, I.-C. Kang, T. Kim and M. H. Han, Proteomics, 2003, 3, 2289–2304 CrossRef CAS.
- S. W. Oh, J. D. Moon, H. J. Lim, S. Y. Park, T. Kim, J. B. Park, M. H. Han, M. Snyder and E. Y. Choi, FASEB J., 2005, 19, 1335–1340 CAS.
- H. Chen, M. Lee, S. Choi, J. Kim, H. Choi, S. Kim, J. Lee and K. Koh, Sensors, 2007, 7, 1091–1107 CrossRef CAS.
- H. Chen, Y. S. Kim, J. Lee, S. J. Yoon, D. S. Lim, H. J. Choi and K. Koh, Sensors, 2007, 7, 2263–2272 CrossRef CAS.
- F. Caruso, E. Rodda and D. N. Furlong, J. Colloid Interface Sci., 1996, 178, 104–115 CrossRef CAS.
- H. Chen, L. Gu, Y. Yin, K. Koh and J. Lee, Int. J. Mol. Sci., 2011, 12, 2315–2324 CrossRef CAS.
- H. Jung, K. Song and T. Kim, Bull. Korean Chem. Soc., 2007, 28, 1792–1796 CrossRef CAS.
- P. Blanchard, O. Alévêque, S. Boisard, C. Gautier, A. El-Ghayoury, F. Le Derf, T. Breton and E. Levillain, Phys. Chem. Chem. Phys., 2011, 13, 2118–2120 RSC.
- Y. Yakota, K. Fukui, T. Enoki and M. Hara, J. Am. Chem. Soc., 2007, 129, 6571–6573 CrossRef.
- Y. Wang, J. Alvarez and A. E. Kaifer, Chem. Commun., 1998, 1457–1458 RSC.
- V. Ta, S. B. Nimse, K. Song, J. Kim, D. R. Sayyed, V. Nguyen and T. Kim, Chem. Commun., 2011, 47, 11261–11263 RSC.
- L. M. Meskamp, B. Lüssem, S. Karthäuser, M. Homberger, U. Simon and R. Waser, J. Phys. Conf. Ser., 2007, 61, 852–855 CrossRef.
- S. B. Nimse, K. Song, C. Jung, W. Eoum and T. Kim, Bull. Korean Chem. Soc., 2009, 30, 1247–1251 CrossRef CAS.
- S. B. Nimse, K. Song, J. Kim, H. Kim, V. Nguyen, W. Eoum, C. Jung, V. Ta and T. Kim, Tetrahedron Lett., 2010, 51, 6156–6160 CrossRef CAS.
- S. B. Nimse, J. Kim, K. Song, J. Kim, J. T. Lee, V. Nguyen, V. Ta and T. Kim, Tetrahedron Lett., 2011, 52, 3751–3755 CrossRef CAS.
- S. B. Nimse, J. Kim, J. T. Lee, K. Song, J. Kim, V. Ta, V. Nguyen and T. Kim, Bull. Korean Chem. Soc., 2011, 32, 1143–1145 CrossRef CAS.
- K. Song, S. B. Nimse, J. Kim, J. Kim, V. Nguyen, V. Ta and T. Kim, Chem. Commun., 2011, 47, 7101–7103 RSC.
- S. B. Nimse, K. Song, J. Kim, V. Ta, V. Nguyen and T. Kim, Chem. Commun., 2011, 47, 12444–12446 RSC.
- H. An, K. Song, S. B. Nimse, J. Kim, V. Nguyen, V. Ta, D. R. Sayyed and T. Kim, J. Clin. Microbiol., 2012, 50, 562–568 CrossRef CAS.
- K. Song, S. B. Nimse, J. Kim, H. Kim, V. Ta, V. Nguyen and T. Kim, J. Virol. Methods, 2012, 183, 132–138 CrossRef CAS.
- V. Nguyen, S. B. Nimse, K. Song, J. Kim, J. Kim, V. Ta and T. Kim, Chem. Commun., 2012, 48, 4582–4584 RSC.
- E. Phizicky, P. I. H. Bastiaens, H. Zhu, M. Snyder and S. Fields, Nature, 2003, 422, 208–212 CrossRef CAS.
- D. Kambhampati, in Protein Microarray Tachnology, Wiely-VCH, Verlag GmbH & Co. KGaA, 2003 Search PubMed.
- J. L. Richens, E. A. M. Lunt, D. Sanger, G. McKenzie and P. O'Shea, J. Proteome Res., 2009, 8, 5103–5108 CrossRef CAS.
- H.-Y. Hsu and Y.-Y. Huang, Biosens. Bioelectron., 2004, 20, 123–127 CrossRef CAS.
- C. M. Niemeyer, T. Sano, C. L. Smith and C. R. Cantor, Nucleic Acids Res., 1994, 22, 5530–5536 CrossRef CAS.
- R. Wacker, H. Schröeder and C. M. Niemeyer, Anal. Biochem., 2004, 330, 281–285 CrossRef CAS.
- R. C. Bailey, G. A. Kwong, C. G. Radu, O. N. Witte and J. R. Heath, J. Am. Chem. Soc., 2007, 129, 1959–1961 CrossRef CAS.
- H. Zhu and M. Snyder, Curr. Opin. Chem. Biol., 2003, 7, 55–62 CrossRef CAS.
- K. Song, S. B. Nimse, J. Kim, J. Kim, V. Ta, V. Nguyen and T. Kim, Chem. Commun., 2011, 47, 7716–7718 RSC.
- W. M. Hassen, C. Martelet, F. Davis, S. P. J. Higson, A. Abdelghani, S. Helali and N. Jaffrezic-Renault, Sens. Actuators, B, 2007, 124, 38–45 CrossRef.
- M. Šnejdárková, A. Poturnayová, P. Rybár, P. Lhoták, M. Himl, K. Flídrová and T. Hianik, Bioelectrochemistry, 2010, 80, 55–61 CrossRef.
- R. Ludwig, Microchim. Acta, 2005, 152, 1–19 CrossRef CAS.
- K. Shimojo, T. Oshima, H. Naganawa and M. Goto, Biomacromolecules, 2007, 8, 3061–3066 CrossRef CAS.
- P. Shahgaldian, J. Gualbert, K. Aissa and A. W. Coleman, Eur. J. Pharm. Biopharm., 2003, 55, 181–184 CrossRef CAS.
- T. Oshima, H. Higuchi, K. Ohto, K. Inoue and M. Goto, Langmuir, 2005, 21, 7280–7284 CrossRef CAS.
- M. Goto, H. Sumura, K. Abe and F. Nakashio, Biotechnol. Tech., 1995, 9, 101–104 CAS.
- M. J. Pires, M. R. Aires-Barros and J. M. S. Cabral, Biotechnol. Prog., 1996, 12, 290–301 CrossRef CAS.
- T. Oshima, M. Goto and S. Furusaki, Biomacromolecules, 2002, 3, 438–444 CrossRef CAS.
- T. Oshima, M. Sato, Y. Shikaze, K. Ohto, K. Inoue and Y. Baba, Biochem. Eng. J., 2007, 35, 66–70 CrossRef CAS.
- S. Sayin, E. Yilmaz and M. Yilmaz, Org. Biomol. Chem., 2011, 9, 4021–4024 CAS.
- G. M. L. Consoli, G. Granata, V. Cafiso, S. Stefani and C. Geraci, Tetrahedron Lett., 2011, 52, 5831–5834 CrossRef CAS.
- C. O. Mellet, J. M. Benito and J. M. G. Fernánde, Chem.–Eur. J., 2010, 6, 6728–6742 CrossRef.
- P. Shahgaldian, M. A. Sciotti and U. Pieles, Langmuir, 2008, 24, 8522–8526 CrossRef CAS.
- R. Lalor, J. L. DiGesso, A. Mueller and S. E. Matthews, Chem. Commun., 2007, 4907–4909 RSC.
- R. V. Rodik, A. S. Klymchenko, N. Jain, S. I. Miroshnichenko, L. Richert, V. I. Kalchenko and Y. Mély, Chem.–Eur. J., 2011, 17, 5526–5538 CrossRef CAS.
- H. Mattoussi, G. Palui and H. B. Na, Adv. Drug Delivery Rev., 2012, 64, 138–166 CrossRef CAS.
- D. T. Schuhle, J. A. Peters and J. Schatz, Coord. Chem. Rev., 2011, 255, 2727–2745 CrossRef.
- V. Sidorov, F. W. Kotch, G. Abdrakhmanova, R. Mizani, J. C. Fettinger and J. T. Davis, J. Am. Chem. Soc., 2002, 124, 2267–2278 CrossRef CAS.
- J. L. Seganish, J. C. Fettinger and J. T. Davis, Supramol. Chem., 2006, 18, 257–264 CrossRef CAS.
- F. W. Kotch, V. Sidorov, Y.-F. Lam, K. J. Kayser, H. Li, M. S. Kaucher and J. T. Davis, J. Am. Chem. Soc., 2003, 125, 15140–15150 CrossRef CAS.
- J. L. Seganish, P. V. Santacroce, K. J. Salimian, J. C. Fettinger, P. Zavalij and J. T. Davis, Angew. Chem., Int. Ed., 2006, 45, 3334–3338 CrossRef CAS.
- O. A. Okunola, J. L. Seganish, K. J. Salimian, P. Y. Zavalij and J. T. Davis, Tetrahedron, 2007, 63, 10743–10750 CrossRef CAS.
- J. T. Davis, P. A. Gale, O. A. Okunola, P. Prados, J. C. Iglesias-Sánchez, T. Torroba and R. Quesada, Nat. Chem., 2009, 1, 138–144 CrossRef CAS.
- J. T. Davis, O. Okunola and R. Quesada, Chem. Soc. Rev., 2010, 39, 3843–3862 RSC.
- L. Baldini, A. Casnati, F. Sansone and R. Ungaro, Chem. Soc. Rev., 2007, 36, 254–266 RSC.
- M. Torvinen, R. Neitola, F. Sansone, L. Baldini, R. Ungaro, A. Casnati, P. Vainiotalo and E. Kalenius, Org. Biomol. Chem., 2010, 8, 906–915 CAS.
- V. Martos, P. Castreño, J. Valero and J. de Mendoza, Curr. Opin. Chem. Biol., 2008, 12, 698–706 CrossRef CAS.
- A. Casnati, M. Fochi, P. Minari, A. Pochini, M. Reggiani and R. Ungaro, Gazz. Chim. Ital., 1996, 126, 99–106 CAS.
- A. Dondoni, A. Marra, M.-C. Scherrmann, A. Casnati, F. Sansone and R. Ungaro, Chem.–Eur. J., 1997, 3, 1774–1782 CrossRef CAS.
- F. Sansone, S. Barboso, A. Casnati, M. Fabbi, A. Pochini, F. Ugozzoli and R. Ungaro, Eur. J. Org. Chem., 1998, 897–905 CrossRef CAS.
- F. Sansone, S. Barboso, A. Casnati, D. Sciotto and R. Ungaro, Tetrahedron Lett., 1999, 40, 4741–4744 CrossRef CAS.
- L. Frish, F. Sansone, A. Casnati, R. Ungaro and Y. Cohen, J. Org. Chem., 2000, 65, 5026–5030 CrossRef CAS.
- M. Lazzarotto, F. Sansone, L. Baldini, A. Casnati, P. Cozzini and R. Ungaro, Eur. J. Org. Chem., 2001, 595–602 CrossRef CAS.
- L. Baldini, F. Sansone, A. Casnati, F. Ugozzoli and R. Ungaro, J. Supramol. Chem., 2002, 2, 219–226 CrossRef CAS.
- L. Baldini, F. Sansone, F. Scaravelli, C. Massera, A. Casnati and R. Ungaro, Tetrahedron Lett., 2009, 50, 3450–3453 CrossRef CAS.
- M. Segura, B. Bricoli, A. Casnati, E. M. Munoz, F. Sansone, R. Ungaro and C. Vincent, J. Org. Chem., 2003, 68, 6296–6303 CrossRef CAS.
- A. Casnati, F. Sansone and R. Ungaro, Acc. Chem. Res., 2003, 36, 246–254 CrossRef CAS.
- M. Dudic, A. Colombo, F. Sansone, A. Casnati, G. Donofrio and R. Ungaro, Tetrahedron, 2004, 60, 11613–11618 CrossRef CAS.
- F. Sansone, M. Dudić, G. Donofrio, C. Rivetti, L. Baldini, A. Casnati, S. Cellai and R. Ungaro, J. Am. Chem. Soc., 2006, 128, 14528–14536 CrossRef CAS.
- L. Wasungu and D. Hoekstra, J. Controlled Release, 2006, 116, 255–264 CrossRef CAS.
- H. Farhood, N. Serbina and L. Huang, Biochim. Biophys. Acta, Biomembr., 1995, 1235, 289–295 CrossRef.
- V. Bagnacani, F. Sansone, G. Donofrio, L. Baldini, A. Casnati and R. Ungaro, Org. Lett., 2008, 10, 3953–3956 CrossRef CAS.
- V. Bagnacani, V. Franceschi, L. Fantuzzi, A. Casnati, G. Donofrio, F. Sansone and R. Ungaro, Bioconjugate Chem., 2012, 23, 993–1002 CrossRef CAS.
- H. G. Hansma, R. Golan, W. Hsieh, C. P. Lollo, P. Mullen-Ley and D. Kwoh, Nucleic Acids Res., 1998, 26, 2481–2487 CrossRef CAS.
- K. Helttunen and P. Shahgaldian, New J. Chem., 2010, 34, 2704–2714 RSC.
- A. W. Coleman, F. Perret, S. Cecillon, A. Moussa, A. Martin, M. Dupin and H. Perron, New J. Chem., 2007, 31, 711–717 RSC.
- D. Arosio, M. Fontanella, L. Baldini, L. Mauri, A. Bernardi, A. Casnati, F. Sansone and R. Ungaro, J. Am. Chem. Soc., 2005, 127, 3660–3661 CrossRef CAS.
- F. Sansone, L. Baldini, A. Casnati and R. Ungaro, Supramol. Chem., 2008, 20, 161–168 CrossRef CAS.
- H.-J. Gabius, H.-C. Siebert, S. André, J. Jiménez-Barbero and H. Rüdiger, ChemBioChem, 2004, 5, 740–764 CrossRef CAS.
- A. Villalobo, A. Nogales-Gonzáles and H.-J. Gabius, Trends Glycosci. Glycotechnol., 2006, 18, 1–37 CrossRef CAS.
- K. Kayser, D. Hoeft, P. Hufnagl, J. Caselitz, Y. Zick, S. André, H. Kaltner and H.-J. Gabius, Histol. Histopathol., 2003, 18, 771–779 CAS.
- N. Nagy, H. Legendre, O. Engels, S. André, H. Kaltner, K. Wasano, Y. Zick, J.-C. Pector, C. Decaestecker, H.-J. Gabius, I. Salmon and R. Kiss, Cancer, 2003, 97, 1849–1858 CrossRef.
- S. Langbein, J. Brade, J. K. Badawi, M. Hatzinger, H. Kaltner, M. Lensch, K. Specht, S. André, U. Brinck, P. Alken and H.-J. Gabius, Histopathology, 2007, 51, 681–690 CrossRef CAS.
- A. M. Wu, J. H. Wu, J.-H. Liu, T. Singh, S. André, H. Kaltner and H.-J. Gabius, Biochimie, 2004, 86, 317–326 CrossRef CAS.
- S. André, T. Koźár, R. Schuberth, C. Unverzagt, S. Kojima and H.-J. Gabius, Biochemistry, 2007, 46, 6984–6995 CrossRef.
- A. M. Wu, T. Singh, J.-H. Liu, M. Krzeminski, R. Russwurm, H.-C. Siebert, A. M. J. J. Bonvin, S. André and H.-J. Gabius, Glycobiology, 2007, 17, 165–184 CrossRef CAS.
- S. André, F. Sansone, H. Kaltner, A. Casnati, J. Kopitz, H.-J. Gabius and R. Ungaro, ChemBioChem, 2008, 9, 1649–1661 CrossRef.
- S. André, C. Grandjean, F.-M. Gautier, S. Bernardi, F. Sansone, H.-J. Gabiusa and R. Ungaro, Chem. Commun., 2011, 47, 6126–6128 RSC.
- F. Sansone, L. Baldini, A. Casnati and R. Ungaro, New J. Chem., 2010, 34, 2715–2728 RSC.
- H. Sanchez-Ruderisch, C. Fischer, K. M. Detjen, M. Welzel, A. Wimmel, J. C. Manning, S. André and H.-J. Gabius, FEBS J., 2010, 277, 3552–3556 CrossRef CAS.
- P. Sörme, P. Arnoux, B. Kahl-Knutsson, H. Leffler, J. M. Rini and U. J. Nilsson, J. Am. Chem. Soc., 2005, 127, 1737–1739 CrossRef.
- M. Krzeminski, T. Singh, S. André, M. Lensch, A. M. Wu, A. M. J. J. Bonvin and H.-J. Gabius, Biochim. Biophys. Acta, Gen. Subj., 2011, 1810, 150–155 CrossRef CAS.
- R. Leyden, T. Velasco-Torrijos, S. André, S. Gouin, H.-J. Gabius and P. V. Murphy, J. Org. Chem., 2009, 74, 9010–9013 CrossRef CAS.
- C. E. Chang, W. Chen and M. K. Gilson, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 1534–1539 CrossRef CAS.
- A. Cooper, Biophys. Chem., 2005, 115, 89–97 CrossRef CAS.
- C. Clarke, R. J. Woods, J. Gluska, A. Cooper, M. A. Nutley and G. J. Boons, J. Am. Chem. Soc., 2001, 123, 12238–12247 CrossRef CAS.
- Z. Li and T. Lazaridis, J. Phys. Chem. A, 2005, 109, 662–670 CrossRef CAS.
- F. Yin, R. Cao, A. Goddard, Y. Zhang and E. Oldfield, J. Am. Chem. Soc., 2006, 128, 3524–3525 CrossRef CAS.
- L. Memmi, A. Lazar, A. Brioude, V. Ball and A. W. Coleman, Chem. Commun., 2001, 2474–2475 RSC.
- Y. Tauran, M. Grosso, A. Brioude, R. Kassabb and A. W. Coleman, Chem. Commun., 2011, 47, 10013–10015 RSC.
- S. Gordo, V. Martos, E. Santos, M. Menendez, C. Bo, E. Giralt and J. de Mendoza, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 16426–16431 CrossRef CAS.
- J. S. Marvin and H. W. Hellinga, Nat. Struct. Biol., 2001, 8, 795–798 CrossRef CAS.
- S. Gordo, V. Martos, M. Vilaseca, M. Menéndez, J. de Mendoza and E. Giralt, Chem.–Asian J., 2011, 6, 1463–1469 CrossRef CAS.
- V. Martos, S. C. Bell, E. Santos, E. Isacoff, D. Trauner and J. de Mendoza, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10482–10486 CrossRef CAS.
- A. Gross and R. McKinnon, Neuron, 1996, 16, 399–406 CrossRef CAS.
- J. Imredy, C. Chen and R. McKinnon, Biochemistry, 1998, 37, 14867–14874 CrossRef CAS.
- M. J. Coghlan, W. A. Carroll and M. Gopalakrishnan, J. Med. Chem., 2001, 44, 1627–1653 CrossRef CAS.
- D. A. Fulton and J. F. Stoddart, Bioconjugate Chem., 2001, 12, 655–672 CrossRef CAS.
- A. Dondoni and A. Marra, Chem. Rev., 2010, 110, 4949–4977 CrossRef CAS.
- F. Sansone, E. Chierici, A. Casnati and R. Ungaro, Org. Biomol. Chem., 2003, 1, 1802–1809 CAS.
- R. Lalor, H. Baillie-Johnson, C. Redshaw, S. E. Matthews and A. Mueller, J. Am. Chem. Soc., 2008, 130, 2892–2893 CrossRef CAS.
- C. Redshaw, M. R. J. Elsegood, J. A. Wright, H. Baillie-Johnson, T. Yamato, S. De Giovannie and A. Mueller, Chem. Commun., 2012, 48, 1129–1131 RSC.
- A. Mueller, R. Lalor, C. M. Cardaba and S. E. Matthews, Cytometry, Part A, 2011, 79A, 126–136 CrossRef CAS.
- K. Bezouška, R. Šnajdrova, K. Křenek, M. Vančurová, A. Kádek, D. Adámek, P. Lhoták, D. Kavan, K. Hofbauerova, P. Mana, P. Bojarová and V. Křen, Bioorg. Med. Chem., 2010, 18, 1434–1440 CrossRef.
- T. Mecca, G. M. L. Consoli, C. Geraci and F. Cunsolo, Bioorg. Med. Chem., 2004, 12, 5057–5062 CrossRef CAS.
- G. M. L. Consoli, G. Granata, E. Galante, I. Di Silvestro, L. Salafia and C. Geraci, Tetrahedron Lett., 2007, 63, 10758–10763 CAS.
- C. Geraci, G. M. L. Consoli, E. Galante, E. Bousquet, M. Pappalardo and A. Spadaro, Bioconjugate Chem., 2008, 19, 751–758 CrossRef CAS.
- G. M. L. Consoli, F. Cunsolo, C. Geraci, T. Mecca and P. Neri, Tetrahedron Lett., 2003, 44, 7467–7470 CrossRef CAS.
- S. Viola, G. M. L. Consoli, S. Merlo, F. Drago, M. A. Sortino and C. Geraci, J. Neurochem., 2008, 107, 1047–1055 CAS.
- S. Viola, S. Merlo, G. M. L. Consoli, F. Drago, C. Geraci and M. A. Sortino, Pharmacology, 2010, 86, 182–188 CrossRef CAS.
- M. A. Blaskovich, Q. Lin, F. L. Delarue, J. Sun, H. S. Park, D. Coppola, A. D. Hamilton and S. M. Sebti, Nat. Biotechnol., 2000, 18, 1065–1070 CrossRef CAS.
- R. Zadmard, M. Arendt and T. Schrader, J. Am. Chem. Soc., 2004, 126, 7752–7753 CrossRef CAS.
- R. Zadmard, T. Schrader, T. Grawe and A. Kraft, Org. Lett., 2002, 4, 1687–1690 CrossRef CAS.
- T. Schrader, Chem.–Eur. J., 1997, 3, 1537–1540 CrossRef CAS.
- S. Rensing, A. Springer, T. Grawe and T. Schrader, J. Org. Chem., 2001, 66, 5814–5821 CrossRef CAS.
- S. Rensing and T. Schrader, Org. Lett., 2002, 4, 2161–2164 CrossRef CAS.
- R. Zadmard and T. Schrader, J. Am. Chem. Soc., 2005, 127, 904–915 CrossRef CAS.
- S. Kolusheva, R. Zadmard, T. Schrader and R. Jelinek, J. Am. Chem. Soc., 2006, 128, 13592–13598 CrossRef CAS.
- S. Dutt, C. Wilch and T. Schrader, Chem. Commun., 2011, 47, 5376–5383 RSC.
- R. Zadmard and T. Schrader, Angew. Chem., 2006, 118, 2769–2772 CrossRef.
- R. Zadmard, S. Taghvaei-Ganjali, B. Gorji and T. Schrader, Chem.–Asian J., 2009, 4, 1458–1464 CrossRef CAS.
- R. Zadmard, M. Junkers, T. Schrader, T. Grawe and A. Kraft, J. Org. Chem., 2003, 68, 6511–6521 CrossRef CAS.
- W. Hu, C. Blecking, M. Kralj, L. Šuman, I. Piantanida and T. Schrader, Chem.–Eur. J., 2012, 18, 3589–3597 CrossRef CAS.
- R. V. Rodik, V. I. Boyko and V. I. Kalchenko, Curr. Med. Chem., 2009, 16, 1630–1655 CrossRef CAS.
- K. M. Hwang, Y. M. Qi and S.-Y. Liu, Patent, US5312837, 1994 Search PubMed.
- A. Casnati, M. Fabbi, N. Pelizzi, A. Pochini, S. Francgesco, R. Ungaro, E. di Modugno and G. Tarzia, Bioorg. Med. Chem. Lett., 1996, 6, 2699–2704 CrossRef CAS.
- P. D. Hart, J. A. Armstrong and E. Brodaty, Infect. Immun., 1996, 64, 1491–1493 CAS.
- K. Fujimoto, T. Miyata and Y. Aoyama, J. Am. Chem. Soc., 2000, 122, 3558–3559 CrossRef CAS.
- F. M. Menger, J. Bian, E. Sizova, D. E. Martinson and V. A. Seredyuk, Org. Lett., 2004, 6, 261–264 CrossRef CAS.
- Â. de Fátima, S. A. Fernandes and A. A. Sabino, Curr. Drug Discovery Technol., 2009, 6, 151–170 CrossRef.
- H. Zhou, D. Wang, L. Baldini, E. Ennis, R. Jain, A. Carie, S. M. Sebti and A. D. Hamilton, Org. Biomol. Chem., 2006, 4, 2376–2386 CAS.
- K. Křenek, M. Kuldová, K. Hulíková, I. Stibor, P. Lhoták, M. Dudič, J. Budka, H. Pelantová, K. Bezouška, A. Fišerovaá and V. Křen, Carbohydr. Res., 2007, 342, 1781–1792 CrossRef.
- P. Krist, E. Herkommenová-Rajnochová, J. Rauvolfová, T. Semeňuká, P. Vavrušková, J. Pavlǐček, K. Bezouška, L. Petruš and V. Křen, Biochem. Biophys. Res. Commun., 2001, 287, 11–20 CrossRef CAS.
- K. Bezouška, J. Sklenář, J. Dvořáková, V. Havlǐček, M. Pospǐšil, J. Thiem and V. Křen, Biochem. Biophys. Res. Commun., 1997, 238, 149–153 CrossRef.
- G. R. Cornelis, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 8778–8783 CrossRef CAS.
- A. Vovk, V. Kalchenko, S. Cherenok, V. Kukhar, O. Muzychka and M. Lozynsky, Org. Biomol. Chem., 2004, 2, 3162–3167 CAS.
- S. Cherenok, A. Vovk, I. Muravyova, A. Shivanyuk, V. Kukhar, J. Lipkowski and V. Kalchenko, Org. Lett., 2006, 8, 549–554 CrossRef CAS.
- O. Kasyan, D. Swierczynski, A. Drapailo, K. Suwinska, J. Lipkowski and V. Kalchenko, Tetrahedron Lett., 2003, 44, 7167–7172 CrossRef CAS.
- A. I. Vovk, L. A. Kononets, V. Y. Tanchuk, A. B. Drapailo, V. I. Kalchenko and V. P. Kukhar, J. Inclusion Phenom. Macrocyclic Chem., 2009, 45, 921–927 Search PubMed.
- A. I. Vovk, L. A. Kononets, V. Y. Tanchuk, S. O. Cherenok, A. B. Drapailo, V. I. Kalchenko and V. P. Kukhar, Bioorg. Med. Chem. Lett., 2010, 20, 483–487 CrossRef CAS.
- A. I. Vovk, L. A. Kononets, V. Y. Tanchuk, A. B. Drapailo, V. I. Kalchenko and V. P. Kukhar, J. Inclusion Phenom. Macrocyclic Chem., 2010, 66, 271–277 CrossRef CAS.
- M. H. L. Du and J. L. Millan, J. Biol. Chem., 2002, 277, 49808–49814 CrossRef.
- P. Mathieu, P. Voisine, A. Pepin, R. Shetty, N. Savard and F. Dagenais, J. Heart Valve Dis., 2005, 14, 353–357 Search PubMed.
- F. Sanchez de Medina, O. Martinez-Augustin, R. Gonzalez, I. Ballester, A. Nieto, J. Galvez and A. Zarzuelo, Biochem. Pharmacol., 2004, 68, 2317–2326 CrossRef CAS.
- C. B. Tung, C. F. Tung, D. Y. Yang, W. H. Hu, D. Z. Hung, Y. C. Peng and C. S. Chang, Hepatogastroenterol., 2005, 52, 1347–1350 CAS.
- K. Hulíková, V. Grobárová, R. Křivohlavá and A. Fišerová, Folia Microbiol., 2010, 55, 528–532 CrossRef.
- R. Kamada, W. Yoshino, T. Nomura, Y. Chuman, T. Imagawa, T. Suzuki and K. Sakaguchi, Bioorg. Med. Chem. Lett., 2010, 20, 4412–4415 CrossRef CAS.
- R. Matar-Merheb, M. Rhimi, A. Leydier, F. Huché, C. Galián, E. Desuzinges-Mandon, D. Ficheux, D. Flot, N. Aghajari, R. Kahn, A. D. Pietro, J.-M. Jault, A. W. Coleman and P. Falson, PLoS One, 2011, 6, e18036 CAS.
- A. Y. Kim and R. T. Chung, BMC Gastroenterol., 2009, 137, 795–814 CAS.
- A. K. Singal and B. S. Anand, World J. Gastroenterol., 2009, 15, 3713–3724 CrossRef.
- L. K. Tsou, G. E. Dutschman, E. A. Gullen, M. Telpoukhovskaia, Y.-C. Cheng and A. D. Hamilton, Bioorg. Med. Chem. Lett., 2010, 20, 2137–2139 CrossRef CAS.
- H. M. Dibama, I. Clarot, S. Fontanay, A. B. Salem, M. Mourer, C. Finance, R. E. Duval and J. Regnouf-de-Vains, Bioorg. Med. Chem. Lett., 2009, 19, 2679–2682 CrossRef CAS.
- H. Elnakat and M. Ratnam, Adv. Drug Delivery Rev., 2004, 56, 1067–1084 CrossRef CAS.
- C. M. Paulos, J. A. Reddy, C. P. Leamon, M. J. Turk and P. S. Low, Mol. Pharmacol., 2004, 66, 1406–1414 CrossRef CAS.
- P. S. Low, W. Henne and D. D. Doorneweerd, Acc. Chem. Res., 2008, 41, 120–129 CrossRef CAS.
- G. M. L. Consoli, G. Granata and C. Geraci, Org. Biomol. Chem., 2011, 9, 6491–6495 CAS.
- F. Perret, A. N. Lazar and A. W. Coleman, Chem. Commun., 2006, 2425–2438 RSC.
- J. L. Atwood, R. J. Bridges, R. K. Juneja and A. K. Singh, US Patent, 5 489 612, 1996 Search PubMed.
- A. W. Coleman, S. Jebors, S. Cecillon, P. Perret, D. Garin, D. Marti-Battle and M. Moulin, New J. Chem., 2008, 32, 780–782 RSC.
| This journal is © The Royal Society of Chemistry 2013 |