The intriguing reaction of aromatic sulfonyl phthalimides with gold surfaces†
Kallum M.
Koczkur
,
Emad M.
Hamed
,
Colin R.
Hesp
and
Abdelaziz
Houmam
*
University of Guelph, Department of Chemistry, Electrochemical Technology Centre, 50 Stone Road East, Guelph, Ontario N1G 2W1, Canada. E-mail: ahoumam@uoguelph.ca; Fax: +1 519 766 1499; Tel: +1 519 824 4120
First published on 24th October 2012
Abstract
We report on a series of arene sulfonyl phthalimides which were prepared and used to modify polycrystalline gold and Au(111) gold surfaces. Three investigated compounds are the p-iodo-, the p-methoxy-, and the p-fluoro-benzenesulfonyl phthalimides. X-ray photoelectron spectroscopy (XPS), cyclic voltammetry (CV), and scanning tunneling microscopy (STM) studies were used to characterize the modified surfaces. The XPS data show that all three investigated compounds decompose on gold surfaces. The decomposition leads to the adsorption of sulfur and ejection of the other groups except for the p-iodo compound, which also leads to the deposition of iodine. The cyclic voltammetry data confirm these results and show that high coverage values of deposited sulfur are obtained. High-resolution STM imaging showed a dynamic behaviour of sulfur on gold for all compounds. Movement of sulfur species on the Au(111) surface is observed. Various phases including a new ‘zig-zag’ pattern and a new 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 line pattern are presented. Sequential STM imaging also showed movement of one area of sulfur while another remains static. These results are important because (i) they provide direct experimental evidence that these hexavalent sulfur compounds react with gold surfaces breaking all sulfur chemical bonds, (ii) they show that sulfonyl phthalimides can be used as efficient precursors for the deposition of sulfur on gold, and (iii) very importantly they show the adlayer nature of the sulfur modified gold surface which has been a heavily debated question.
1 line pattern are presented. Sequential STM imaging also showed movement of one area of sulfur while another remains static. These results are important because (i) they provide direct experimental evidence that these hexavalent sulfur compounds react with gold surfaces breaking all sulfur chemical bonds, (ii) they show that sulfonyl phthalimides can be used as efficient precursors for the deposition of sulfur on gold, and (iii) very importantly they show the adlayer nature of the sulfur modified gold surface which has been a heavily debated question.
Introduction
Over the past three decades, and continuing to this day, ultra-thin films on solid surfaces have seen numerous research efforts devoted to their study.1–3 A common example of this is self-assembled monolayers (SAMs) which modify the surfaces for important applications. These include studies directed towards optoelectronics,4 template self-assembly with DNA,5 wettability,6 lithography,7–9and nanomaterials.10 One area in particular which has received a lot of attention is that of the sulfur–gold interface, either with organosulfur compounds or sulfur attached to the gold surface. The first reported self-assembly of a sulfur compound on gold was that of bifunctional organic disulfides on gold surfaces.11 Since that time, different organosulfur compounds, with numerous derivatives, have been used to form SAMs including alkane/aryl thiols,12,13 thiocyanates14,15 and sulfenyl chlorides.16,17 Gold is an attractive surface for binding of organosulfur based SAMs because of a strong interaction between the two groups and due to gold not readily forming oxides. High quality single crystalline surfaces used in imaging can be prepared relatively easily in the form of gold beads,18 gold on chromium on glass systems19 and gold on mica.20 The two most common phases observed by STM for the adsorption of aliphatic thiols on Au(111) are c(4 × 2) and (√3 × √3)R30°.21 They can also exist in multiple striped structures due to differences in surface coverage. During the adsorption process, as the surface coverage increases, a series of p × √3 striped phases (where p denotes the period) are formed before the final close-packed structure of the (√3 × √3)R30° phase.22 Continued interest in this area, specifically the thiolate–gold interface, stems from several unsolved mysteries about the lifting of herringbone reconstruction, incorporation of gold adatoms into the monolayers, adsorption site determination and the initial binding mechanism.23The behaviour of the shortest thiolate chain, sulfur, on both polycrystalline gold and Au(111) has also attracted a lot of attention in the literature. Sources of sulfur that have been previously used to study S/Au interactions include S2 and SO2 from the gas phase,24,25 Na2S from electrochemical reduction,26,27 and more recently hexamethyldisilathiane28 and dithiobisphthalimide29 from solution deposition. Sulfur is initially chemisorbed on Au(111) at step edges.30 Low-energy electron diffraction (LEED) and scanning tunneling microscopy (STM) experiments have shown that as sulfur continues to deposit on a gold surface, a (5 × 5) phase is formed at coverages of ∼0.25 ML.31 Increasing coverages a little further results in a (√3 × √3)R30° structure (0.33 ML) with interatomic distances corresponding to ∼0.5 nm with adsorption at fcc and hcp hollow sites, similar to what has been reported for selenium.32 When increasing the coverage slightly to greater than 0.40 ML, S2 was found to be the more stable species on Au(111) compared to just S according to high-resolution photoemission studies.24 At high coverages, polysulfide species form on the gold surface. STM imaging has indicated that several Sx species can be observed including S3, S4, and S8. The interatomic distances of sulfur in this rectangular structure have been previously measured at ∼0.30 nm30 which is significantly larger than the interatomic spacing for bulk S8 which is ∼0.20 nm.33 This indicates a strong interaction between sulfur and the gold substrate. At higher coverages, an important question has arisen in the literature that has led to divided opinions to the nature of the usually observed rectangular structures. On one side, the formation of a complex 2D AuS phase has been suggested for the rectangular structures.25,34–37 A model for this phase has been proposed on the basis of STM, Auger electron spectroscopy and density functional theory calculations.38 On the other side, the behaviour of the rectangles has been attributed to chemisorbed sulfur adlayers.27,30 These findings are supported by XPS, surface enhanced Raman spectroscopy and high-resolution STM data which have shown the movement of the squares as individual rectangles which means that the rectangular structure corresponds to sulfur adlayers on gold.27–30
We have recently reported the first example showing the complete decomposition of a hexavalent sulfur compound upon interaction with gold.39 Here we provide more insight into this interaction through a more detailed study and through extension of the investigation to new aromatic sulfonyl phthalimides. Gold electrodes and Au(111) substrates modified using 4-iodobenzenesulfonyl phthalimide, 4-methoxybenzenesulfonyl phthalimide, and 4-fluorobenzenesulfonyl phthalimide (Chart 1) were characterized using XPS, electrochemistry and cyclic voltammetry. The results show the generality of the reaction of aromatic sulfonyl phthalimides with gold and deposition of sulfur on the surface is observed in each case. High-resolution STM imaging has indicated a number of important features. Sulfur may appear as “sponge-like” structures on both the inside and outside of etch-pits. Rectangular structures of sulfur may move independently of one another within a small area or have movement all across large areas. Two new phases of sulfur on gold have been observed including a zigzag structure and a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 line pattern. The presently demonstrated complete decomposition of the sulfonyl phthalimides on gold leading to the deposition of sulfur is intriguing. Sulfonamides are stable compounds and have never been shown before to lead to the formation of sulfur or to act as sulfur transfer agents. They yield the corresponding desulfonation products under elevated temperatures.40,41
1 line pattern. The presently demonstrated complete decomposition of the sulfonyl phthalimides on gold leading to the deposition of sulfur is intriguing. Sulfonamides are stable compounds and have never been shown before to lead to the formation of sulfur or to act as sulfur transfer agents. They yield the corresponding desulfonation products under elevated temperatures.40,41
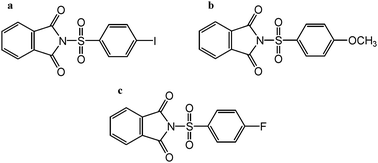 | ||
| Chart 1 (a) p-Iodo, (b), p-methoxy and (c) p-fluoro benzenesulfonyl phthalimide. | ||
Experimental part
Chemicals
Potassium phthalimide (Sigma-Aldrich), p-iodobenzenesulfonyl chloride (Sigma-Aldrich), p-methoxybenzenesulfonyl chloride (Sigma-Aldrich) and p-fluorobenzenesulfonyl chloride (Sigma-Aldrich) were used as received. Acetonitrile (HPLC grade, Caledon Labs) was dried with calcium hydride (Sigma-Aldrich) and distilled under argon just prior to use. Tetrahydrofuran (THF), (HPLC grade, Caledon Labs) was dried with sodium metal (Sigma-Aldrich) and benzophenone (Sigma-Aldrich, indicator) and distilled under argon just prior to use. Methanol (HPLC grade, Caledon Labs) was used as received.The synthesis of the sulfonyl phthalimide compounds was done according to known literature methods. In an air atmosphere, 4-X-benzenesulfonyl phthalimide (X = I, H3CO, F) was made from adding 20–30 mL of freshly distilled acetonitrile (CH3CN) to equimolar amounts of 4-X-benzenesulfonyl chloride and potassium phthalimide in a round bottom flask with continuous stirring. The resulting mixture was refluxed for 48 hours and then the product was precipitated by the addition of 40–60 mL of Milli-Q water. After filtering and washing thoroughly with water, the product(s) were left to dry in air. N-(p-Iodobenzenesulfonyl phthalimide) was obtained in a 73.0% yield, N-(p-methoxybenzenesulfonyl phthalimide) a 72.4% yield and N-(p-fluorobenzenesulfonyl phthalimide) a 93% yield.
Melting points were determined in capillary tubes from an electrothermal MEL-TEMP® melting point apparatus. Compressed pellet KBr IR experiments were performed on a Nicolet 4700 FT-IR instrument. 1H-NMR and 13C-NMR experiments were conducted on a Bruker 400 MHz NMR spectrometer.
N-(p-Iodobenzenesulfonyl phthalimide): mp 282–284 °C, IR (KBr, cm−1): 1795.0, 1750.9, 1389.2, 1260.3, 1194.6, 1083.6 and 1029.1; 1H-NMR (CDCl3) δ (ppm): 7.79 (2H, dd, J = 5.3, 3.0 Hz), 7.90 (2H, d, J = 7.8 Hz), 7.87 (2H, d, J = 7.8 Hz), 7.89 (2H, dd, J = 5.4, 3.0 Hz); 13C-NMR (CDCl3) δ (ppm): 103.36, 124.95, 129.81, 131.01, 135.90, 138.15, 138.92 and 163.00.
N-(p-Methoxybenzenesulfonyl phthalimide): mp 216–219 °C, IR (KBr, cm−1): 1789.6, 1748.5, 1592.5, 1575.9, 1496.7, 1466.0, 1385.7, 1258.7, 1190.6, 1170.3, 1142.6, 1090.0, 1037.1, 1024.0. 1H-NMR (CDCl3) δ (ppm): 3.84 (3H, s), 6.98 (2H, d, J = 9.08 Hz), 7.75–7.79 (2H, m), 7.86–7.90 (2H, m), 8.12 (2H, d, J = 9.08 Hz); 13C-NMR (CDCl3) δ (ppm): 55.78, 114.49, 124.52, 129.73, 130.85, 130.95, 135.42, 163.11 and 164.58.
N-(p-Fluorobenzenesulfonyl phthalimide): mp 220–221 °C. IR (KBr, cm−1): 1801.7, 1759.4, 1588.2, 1491.1, 1383.1, 1263.0 1224.5, 1185.9, 1154.9, 1142.2, 1088.5, 1033.8. 1H-NMR (CDCl3) δ (ppm): 7.34 (2H, t, J = 8.8 Hz), 7.84–7.90 (4H, m), 8.16 (2H, dd, J = 5.04, 3.96 Hz). 13C-NMR (CDCl3) δ (ppm) 116.64, 116.86, 124.69, 130.83, 131.45, 131.55, 135.63, 162.84, 165.10 and 167.66.
Preparation of Au(111) substrates
A gold wire (0.762 mm dia., Premion®, 99.999%, Alfa Aesar) was evaporated onto freshly cleaved mica (V1 Grade, Ted Pella) in a custom built evaporation system, consisting of a Kurt J. Lesker bell jar, Varian turbo pump, and operating at a pressure of 1 × 10−7 Torr. The base plate holding the mica was heated at 600 K for 12 hours prior to depositing the gold and was kept at 600 K for an additional 3 hours after the gold was deposited to help ensure the formation of high quality Au (111) surfaces. Upon removal, the gold samples were cleaned with chromic sulfuric acid, (Chromerge®, Bel-Art), anhydrous ethanol (Commercial Alcohols), and ultra-pure water (Millipore, 18.2 MΩ) and then dried under a nitrogen stream and stored in a dessicator until used.XPS
X-ray photoelectron spectroscopy studies were conducted in an ultrahigh vacuum (UHV) system (Omicron) operating at a base pressure of 5 × 10−11 Torr. X-rays were generated from an Al Kα source (1486.6 eV). The system contains a hemispherical sector analyzer coupled to a multichannel electron detector. The analyzer was operated in constant analyzer energy (CAE) mode with a pass energy of 20 eV. A takeoff angle of 35° was used for all samples. All XPS spectra presented in this work are referenced to the Au 4f7/2 peak at a binding energy of 84.0 eV, and the sulfur peaks were fitted using XPSPEAK 4.1 software.Cyclic voltammetry
Electrochemical measurements were conducted in a three electrode glass cell thermostated at 25 °C under dry nitrogen. A 2 mm diameter solid gold electrode (Ω Metrohm) was used. The electrode was carefully polished and ultrasonically rinsed with ethanol then electrochemically cleaned in a 0.5 M H2SO4 aqueous solution. The reference electrode was a SCE. The counter electrode was a platinum wire. The electrochemical instrument used was an Autolab PGSTAT30 (Eco Chemie).STM
A 5500 scanning probe microscope system (Agilent Technologies) was used for STM imaging. Images were obtained in air using a tungsten tip (0.25 mm dia., 99.95%, Alfa Aesar) electrochemically etched in 3 M NaOH (99.99%, Semiconductor Grade, Sigma-Aldrich).Results and discussion
Fig. 1 shows XPS results of polycrystalline gold modified for 3 days with p-iodobenzenesulfonyl phthalimide, p-methoxybenzenesulfonyl phthalimide and p-fluorobenzenesulfonyl phthalimide. In the sulfur 2p region for the p-iodo modified slide, Fig. 1(a), there are two unresolved doublets. The first, with the S 2p3/2 component at a binding energy of ∼161.2 eV, corresponds to monoatomic sulfur adsorbed on the gold surface.24 In the second doublet, the S 2p3/2 is located at ∼162.0 eV which suggests the formation of polymeric sulfur species,30 but also corresponds to the known position for a thiolate–gold bond.15 With the absence of a signal at ∼168 eV, and the lack of an observed signal in the N 1s region, it is likely that at least 5 of the original 6 chemical bonds to sulfur have been broken. The overall signal strength is weak compared to gold modified from the other compounds, Fig. 1c and e, and this is likely due to sulfur competing for adsorption sites on gold with iodine. In the iodine 3d region, Fig. 1b, there is a clear signal. The peaks show an expected 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 branching ratio and are positioned at ∼618.8 eV for the 3d5/2 component and ∼630.4 eV for the 3d3/2 component. This could be the result of the dissociation of iodine from the aromatic fragment of the molecule, which is known to happen for iodobenzene deposited on gold surfaces.42 On polycrystalline gold, iodobenzene dissociates between 200 and 250 K,43 which is well below the room temperature used in this study.
2 branching ratio and are positioned at ∼618.8 eV for the 3d5/2 component and ∼630.4 eV for the 3d3/2 component. This could be the result of the dissociation of iodine from the aromatic fragment of the molecule, which is known to happen for iodobenzene deposited on gold surfaces.42 On polycrystalline gold, iodobenzene dissociates between 200 and 250 K,43 which is well below the room temperature used in this study.
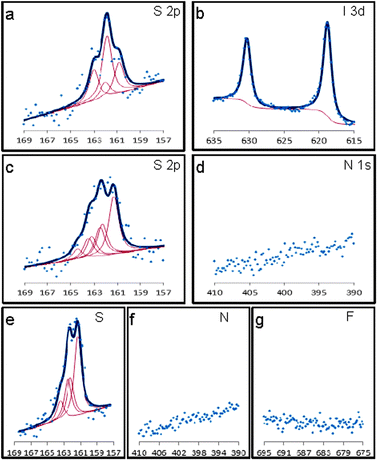 | ||
| Fig. 1 XPS data for polycrystalline gold on glass slides modified for 3 days with: p-iodobenzenesulfonyl phthalimide showing the S 2p region (a) and I 3d region (b), p-methoxybenzenesulfonyl phthalimide showing the S 2p (c) and N 1s region (d), and p-fluorobenzenesulfonyl phthalimide showing the S 2p (e), N 1s (f) and F 1s regions (g). | ||
XPS studies were also carried out on a gold on glass slide that was modified with p-methoxybenzenesulfonyl phthalimide and the results from scanning in the S 2p region are shown in Fig. 1(c). The curve can be fitted with three unresolved doublets, with the third doublet, S 2p3/2 163.2 eV, being attributed to weakly bound sulfur multilayers. The branching ratio is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the doublets (2p3/2, 2p1/2) and the spin–orbit splitting was fixed at a constant 1.2 eV.44 Scanning in the nitrogen 1s region, Fig. 1(d), suggests the cleavage of the phthalimide group during the parent molecules interaction with gold. The carbon 1s and oxygen 1s signals were minimal and did not show anything indicating the presence of the phthalimide moiety or aromatic moiety.
1 for the doublets (2p3/2, 2p1/2) and the spin–orbit splitting was fixed at a constant 1.2 eV.44 Scanning in the nitrogen 1s region, Fig. 1(d), suggests the cleavage of the phthalimide group during the parent molecules interaction with gold. The carbon 1s and oxygen 1s signals were minimal and did not show anything indicating the presence of the phthalimide moiety or aromatic moiety.
Similar XPS studies were conducted with p-fluorobenzenesulfonyl phthalimide. The signals obtained for a modified Au surface are shown in Fig. 1(e–g). A clear signal for sulfur was seen when scanning in the S 2p region, Fig. 1(e), after a 3 day modification. Scanning in the N 1s region did not yield any signal for nitrogen which suggests that the phthalimide–sulfur bond is cleaved at some point during the modification process. The expected peak for fluorine, located at ∼685 eV, was not observed, which indicates that the aromatic benzene ring separates itself from the sulfur.
Additional studies of freshly modified polycrystalline gold surfaces from p-iodobenzenesulfonyl phthalimide, p-methoxybenzenesulfonyl phthalimide and p-fluorobenzenesulfonyl phthalimide were done by way of cyclic voltammetry. A solid gold electrode was modified for 3 days in 1 mM solutions of p-iodobenzenesulfonyl phthalimide in THF, p-methoxybenzenesulfonyl phthalimide in acetonitrile, and p-fluorobenzenesulfonyl phthalimide in THF/MeOH (50/50). Fig. 2 shows oxidative stripping scans of a bare gold and modified gold electrodes in 0.5 M H2SO4. Similar stripping cyclic voltammograms are obtained for the p-fluorobenzenesulfonyl phthalimide and p-methoxybenzenesulfonyl phthalimide. The voltammograms are characteristics of the oxidative desorption of deposited sulfur on the polycrystalline gold electrode as previously reported.28,29 When a second scan is run for both electrodes a cyclic voltammogram similar to the one observed for a bare gold electrode is obtained. This indicates that the deposited sulfur during modification is stripped off the electrode during the first oxidative scan. An interesting note for the p-iodobenzenesulfonyl phthalimide curve is that the onset of oxidation begins about 0.1 V more positive than the other two compounds, the peak is narrower, and the peak oxidation current is about twice as large as the others. This indicates that the modification from this compound is significantly different compared to others, confirming the XPS data. Up to 5 additional scans are required to completely remove the film from the modified electrode. Additional evidence for iodine adsorption on gold was observed in the form of a small reductive peak at about 0.375 V vs. SCE, which has been previously assigned to the reduction of iodate.45
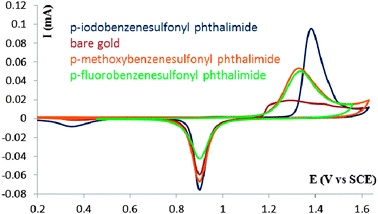 | ||
| Fig. 2 Cyclic voltammograms, scan rate of 0.2 V s−1, of a polycrystalline gold electrode in 0.5 M H2SO4 after modification in a 1 mM solution of p-iodobenzenesulfonyl phthalimide, p-methoxybenzenesulfonyl phthalimide and p-fluorobenzenesulfonyl phthalimide. | ||
Using the oxidative stripping cyclic voltammograms, values for the S coverage on the Au electrode were deduced. This is done by considering the oxidation peak in the first scan as corresponding to the oxidation of both the Au surface and the deposited sulfur structures (see ESI†). The deduced coverage values are ∼0.38 ML and ∼0.44 ML for the p-methoxybenzenesulfonyl phthalimide and p-fluorobenzenesulfonyl phthalimide, respectively. These values show the efficient deposition of sulfur on the gold surface using these sulfonyl phthalimides.
Subsequent characterization of the modified surfaces was done by STM. Samples were prepared in a similar manner to XPS, but using a freshly prepared piece of Au(111) on mica as the substrate. Imaging was done in air, using bias voltages and tunneling currents of 0.100 V and 0.400 nA for the p-iodobenzenesulfonyl phthalimide samples, 0.100 V and 0.300 nA for the p-methoxybenzenesulfonyl phthalimide samples and 0.150 V and 0.175 nA for the p-fluorobenzenesulfonyl phthalimide samples. The tips were freshly prepared pieces of tungsten wire, electrochemically etched in 3 M NaOH.
Fig. 3(a) shows a large scale STM image. Relatively large flat areas can be observed along with larger sized etch-pits in the left hand side of the image, and small etch-pits on the right hand side of the image. These etch-pits are similar to what has been previously observed for thiol modified gold.46 This is also similar to what has been reported for sulfur on gold systems where it was suggested that the formation of the “vacancy islands” could be due to Ostwald ripening.25 This process is initiated through dissolution of surface particles which are energetically less stable than core particles.47
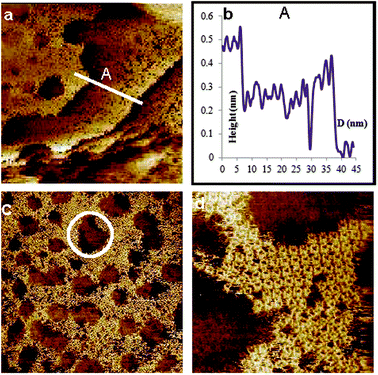 | ||
| Fig. 3 STM images, unfiltered, of Au(111) modified with p-iodobenzenesulfonyl phthalimide at a scan size of (a) 100 × 100 nm2, (b) the line profile of A showing 3 gold steps, (c) 60 × 60 nm2 and (d) 14.4 × 14.4 nm2 showing features consistent with sulfur deposition. Imaging conditions: I = 0.4 nA and V(bias) = 0.1 V. | ||
A line profile taken across three layers shows heights consistent with gold steps, Fig. 3(b). This shows that the modification takes place on a wide range. Looking at a slightly smaller area of 60 × 60 nm2, Fig. 3(c), additional features can be observed. The area outside of the etch-pits, about 50%, is seen to be completely modified, albeit in a random fashion. A very interesting note is that inside several of the pits more details can be made out. Looking at the circled area near to the p-middle image, formations which may represent sulfur rectangles can be observed. Zooming in closer to the area around an etch-pit, Fig. 3(d) 14.4 × 14.4 nm2, confirmation of sulfur rectangles on the outside of an etch-pit is seen. They are random in distribution and give the surface a kind of “sponge-like” appearance. In addition, the observation of sulfur rectangles was made on both the outside and inside of an etch-pit (see ESI†). This is important because it further highlights a controversial point in SAMs which is the production of etch pits. At one time they were thought to be caused by the etching of the gold surface by the thiol compounds as residual traces of gold were detected in the ethanol solution after modification had occurred.46 This was questioned however, when STM observations were made in which thiolates were seen on both the inside and outside of the pits,48 very similar to the present case (see ESI†) with sulfur.
Another interesting feature that was observed by STM is a clear zig-zag pattern which is presented in Fig. 4(a) and this was seen multiple times (see ESI†). This is important as it represents another phase of adsorbed sulfur on gold. The periodicities of the individual atoms along line A, Fig. 4(a) and (b), were measured at ∼0.44 nm (±0.03 nm) and the periodicities of the “zig” were at ∼0.86 nm (±0.05 nm). In the direction of line B, Fig. 4(a) and (c), the periodicities of the atoms were measured at ∼0.73 nm (±0.04 nm). A proposed structural model, based on the periodicities, is shown in Fig. 4(d), with the sulfur atoms positioned at bridge and hollow sites. The appearance of the zig-zag pattern may arise from the movement of sulfur atoms on the gold, which is known.49
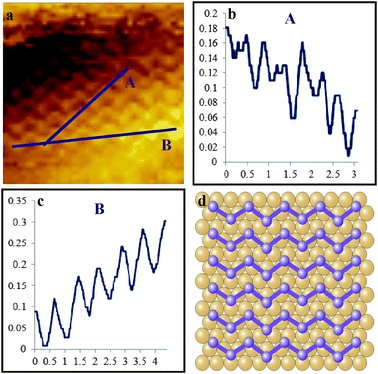 | ||
| Fig. 4 Unfiltered STM image, 5 × 5 nm2, of a zig-zag pattern of Au(111) modified with p-iodobenzenesulfonyl phthalimide (a) 5 × 5 nm2, line profiles (b) and (c), and a proposed structural model (d). Imaging conditions: I = 0.4 nA and V(bias) = 0.1 V. | ||
STM studies were also carried out on an Au(111) on the mica substrate modified with p-methoxybenzenesulfonyl phthalimide in order to see what structures may be present on the surface. Fig. 5 illustrates some very interesting results which clearly demonstrate the dynamic nature of the surface. In Fig. 5(a), two areas have been marked α and β, which will serve as reference points for the changes on the surface. In area α, there are two clear rows of sulfur rectangles, as indicated by the arrows, which are parallel in both directions, and will remain relatively unchanged over the next 8 scans. Area β is aligned but will be seen to undergo many changes before arriving at rectangular structures that are well aligned with area α. The circle contains a lone rectangle which will shift positions to another area in the next frame, Fig. 5(b). Along the path of the arrow where the lone rectangle was located, a gap can be seen to be forming. Moving forward one scan, Fig. 5(c), a gap approximately 3–4 rectangles long is clearly visible, indicating a shift in position of several rectangles. Also important in this frame is the alignment of the rectangles in area β as shown by the arrow. This is clearly at a different alignment than the rectangles in area α as indicated by two arrows. The area in and around where the circled gap was present becomes blurry in Fig. 5(d), but one frame later, Fig. 5(e), the sulfur rectangles have reappeared, and their orientation has changed from their previous position a few frames earlier. They are now starting to align with the structures in area α. Moving ahead to the next frame, Fig. 5(f), three rectangles in the top left corner are now orientated staggered relative to one another indicating that the sulfur rectangles can occupy different binding sites on the Au(111) surface. This becomes more evident in the same area in the following scan, Fig. 5(g). The circled area here contains 4 sulfur rectangles, of which three shift positions in the subsequent frame. Another important note in Fig. 5(h) is that the rectangles in the top left part of the image are no longer staggered but now aligned parallel with the rest of the structures in area β. There is a very noisy part present here as well as indicated by the ellipsoid. In the final frame, Fig. 5(i), however, the bottom row of rectangles in area β has been resolved, and is now well aligned with the rest of the frame. The constant shifting of sulfur rectangles provides clear evidence of their dynamic nature on gold, and the final formation gives an indication of the efficient packing of the structures and good adsorbate/adsorbate interactions. Periodicities of the rectangles were measured at ∼0.84 nm (±0.05 nm) × ∼0.90 nm (±0.05 nm) which is in good agreement with previously published results of sulfur deposition on Au(111).25
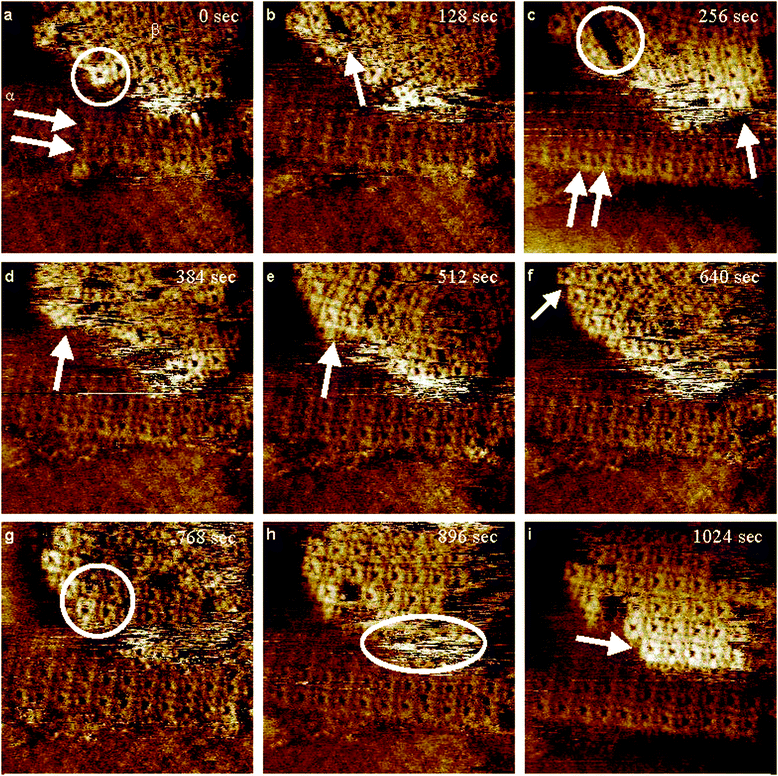 | ||
| Fig. 5 Consecutive STM images, unfiltered, (a–i), at a scan size of 10 × 10 nm2 of a piece of Au(111) on mica modified with p-methoxybenzenesulfonyl phthalimide. Imaging conditions: I = 0.3 nA and V(bias) = 0.1 V. | ||
An important issue of long standing discussion in the literature has been the debate about the nature of the rectangular structures.25,27–30,34–37Fig. 6 presents consecutive, unfiltered STM images at a scan size of 30 × 30 nm2, illustrating the dynamic nature of the modified surface using p-fluorobenzenesulfonyl phthalimide. In Fig. 6(a), there are 5 separate areas (α, β, γ, δ, and ε) which are labeled and these will serve as reference points for changes in subsequent images. Looking at Fig. 6(a) in general a number of features can be seen. There are multiple forms of sulfur present, including a 3 line structure, dense sulfur formations and the usual S rectangles, Fig. 7(a). The filled rectangular structures that we previously reported are also observed.39 Considering the area immediately below the α symbol, a nearly complete step edge is present. Going to the corresponding area in Fig. 6(b), the edge is now complete. However in Fig. 6(c) in this area, a single rectangle wide gap is present showing a missing rectangle along the right hand side. The whole row of filled rectangles has shifted in the next frame, Fig. 6(d), making a gap on the left hand side of the edge. When considering Fig. 6(e), area α has become slightly noisy, and the filled rectangles along the edge have dissociated completely and do not re-associate over the remainder of the 7 scans. Above area α, β, and γ in Fig. 6(a), randomly aligned S rectangles are present. They remain this way for a brief while until Fig. 6(d) where they are beginning to form parallel rows in both directions. Also of interest is the area in the top right corner, where a section of rectangles are aligned amongst themselves, but are at an angle relative to the rectangles to their left, and those to their bottom right. Continuing along to the top part of the image in Fig. 6(f), the dissociation of the rectangles above the β area can clearly be seen, but by the next frame, Fig. 6(g), they can be seen to be re-associating once more. Fig. 6(h) indicates that the rectangles are now well aligned in two sections separated by an uneven gap in the middle. Looking at the top right corner in Fig. 6(i) shows that most of the rectangles which were tilted with respect to their neighbours in Fig. 6(d) are now lined up very well with the rest of their neighbours. This is important because it provides clear evidence of reversible dissociation/association, good adsorbate/adsorbate interaction, efficient packing of the S rectangular structures, and mobility of S adsorbates on Au surfaces.
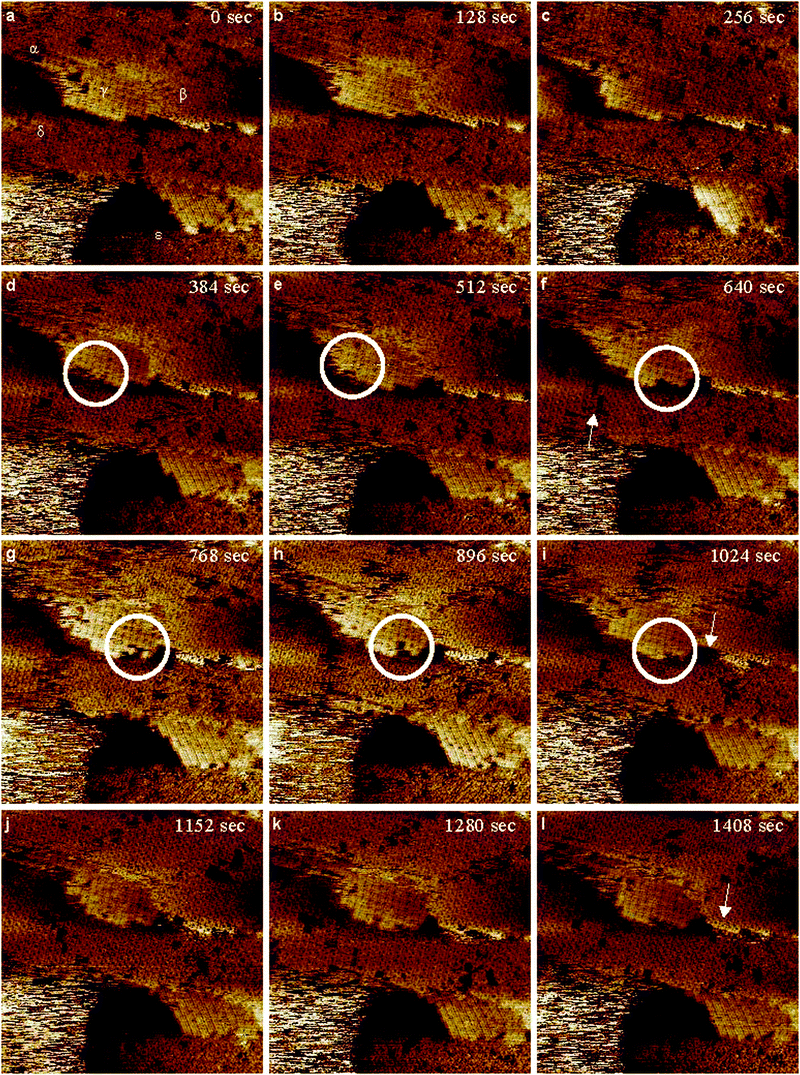 | ||
| Fig. 6 Consecutive, (a–l), high-resolution STM images, unfiltered, 30 × 30 nm2, after a 3 day modification of Au(111) on mica from p-fluorobenzenesulfonyl phthalimide. Imaging conditions: I = 0.175 nA and V(bias) = 0.150 V. | ||
 | ||
| Fig. 7 Proposed models for (a) well aligned regular rectangular structures of Fig. 6, (b) mis-matched area from Fig. 6(f) as indicated by the arrow, and (c) very randomly oriented sulfur rectangles representing Fig. 6, area ε. | ||
Area β in Fig. 6(a) shows a section of well aligned S rectangles. Of note in this area is the bottom edge where one can see 9 rectangles in the lowermost row. In Fig. 6(c), the two on the left are now gone, and in Fig. 6(d), 4 of the S rectangles have shifted creating two single gaps in the row. One frame later, two of the rectangles have dissociated and the remaining two have shifted to the right resulting in a row of 5. In Fig. 6(g), one more rectangle is gone and the row is now 4. The area directly beneath the arrow in Fig. 6(i) is empty, but over the next frame, the rectangles start shifting to the left. In Fig. 6(k), the row is clearly seen to be of 3 rectangles, but in Fig. 6(l), two more rectangles have re-associated with the others and there is a row of 5. A similar dissociation/association behaviour is observed along the edge of the filled S rectangles in area γ. This is highlighted by the circled areas in Fig. 6(d)–(i).
The area surrounding δ in Fig. 6(a) shows several uneven gaps in the rectangular structure. Following this area from one frame to the next, the surface can be seen to be very dynamic as the gaps are constantly shifting in position and size. In the area around the arrow in Fig. 6(f), the S structures are well aligned. What is interesting here is that directly above the arrow, there are two gaps, which have resulted from an opposite side alignment of the S rectangles, Fig. 7(b). This behaviour suggests that the bonding sites for the rectangular S8 structures are not fixed, they can change. The rectangles have reconfigured themselves by the next frame resulting in a wide gap, but soon start to associate once again and end up in a well ordered formation with several single gaps as can be seen in Fig. 6(l).
Very randomly oriented features are observed in area ε, in Fig. 6(a). A few frames pass and this area resolves into rectangles, in Fig. 6(c) for example and illustrated by the model in Fig. 7(c). These structures shift again by Fig. 6(g) where most of the rectangles are gone, but one frame later, there is a small well aligned area inside the pit. This association/dissociation behaviour is important because it shows that randomly aligned S rectangles can behave in a similar manner to their well-ordered counter parts.
In addition to the rectangular structures observed on the surface, a new adsorption pattern was also observed. Fig. 8 presents high-resolution STM images, unfiltered, of a Au(111) surface modified using p-fluorobenzenesulfonyl phthalimide, clearly illustrating a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 line pattern. A large scale image at 60 × 60 nm2, Fig. 8(a), shows a well-aligned surface with different domains. Modification is seen to take place both on the area outside and on the inside of the etch pits, for example in the top left corner. Fig. 8(b) shows a zoomed in area, 10 × 10 nm2, illustrating clear 2 parallel lines to a single line pattern. The unit cell for this structure was calculated at approximately 0.86 nm (±0.05 nm) × 0.89 nm (±0.05 nm). A small scan size, 5 × 5 nm2, is shown in Fig. 8(c). Line profiles taken across this image show the periodicities in both directions. These dimensions are very close to those of the rectangular structures. This indicates that the 2
1 line pattern. A large scale image at 60 × 60 nm2, Fig. 8(a), shows a well-aligned surface with different domains. Modification is seen to take place both on the area outside and on the inside of the etch pits, for example in the top left corner. Fig. 8(b) shows a zoomed in area, 10 × 10 nm2, illustrating clear 2 parallel lines to a single line pattern. The unit cell for this structure was calculated at approximately 0.86 nm (±0.05 nm) × 0.89 nm (±0.05 nm). A small scan size, 5 × 5 nm2, is shown in Fig. 8(c). Line profiles taken across this image show the periodicities in both directions. These dimensions are very close to those of the rectangular structures. This indicates that the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 line pattern may result from a shift in position of two atoms within a sulfur rectangle to one side, such that the rectangle appears to have been separated into a 2
1 line pattern may result from a shift in position of two atoms within a sulfur rectangle to one side, such that the rectangle appears to have been separated into a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 line pattern (see ESI†). A model illustrating the proposed shifting of sulfur atoms is presented in Fig. 8(d).
1 line pattern (see ESI†). A model illustrating the proposed shifting of sulfur atoms is presented in Fig. 8(d).
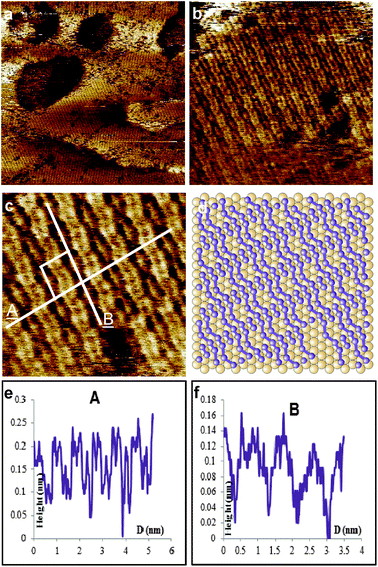 | ||
Fig. 8 High resolution, unfiltered, STM images of an area of the modified surface, using p-fluorobenzenesulfonyl phthalimide, at sizes of (a) 60 × 60 nm2, (b) 10 × 10 nm2, and (c) 5 × 5 nm2 showing a clear 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adsorption pattern for sulfur and (d) a corresponding proposed model of the sample surface. Imaging conditions: I = 0.175 nA and V(bias) = 0.150 V. 1 adsorption pattern for sulfur and (d) a corresponding proposed model of the sample surface. Imaging conditions: I = 0.175 nA and V(bias) = 0.150 V. | ||
Evidence has clearly been established for the deposition of S on the Au surface using p-iodobenzenesulfonyl phthalimide, p-methoxybenzenesulfonyl phthalimide as new precursors as well as new data from p-fluorobenzenesulfonyl phthalimide modified gold. Differences in the resultant signals from XPS experiments and shape of the oxidation peaks for cyclic voltammograms suggest a limited dependence in behaviour of the three compounds on the nature of the substituent. With the fluoro and methoxy substituted compounds, very similar XPS and electrochemical data are obtained. The S coverage values for these two compounds are also comparable (∼0.38 ML and ∼0.44 ML). These results indicate that the S deposition from substituted arene sulfonyl phthalimides does not depend on the electronic properties of the substituent. With the p-iodobenzenesulfonyl phthalimide, a weaker S signal is observed by XPS due to a competition with the adsorption of iodine on the Au surface. This is also confirmed by the electrochemical data. This indicates that the ability of the substituent to interact with the Au surface does affect the S adsorption. The size of the substituent may also have an impact on the efficiency of the S deposition. The observation of known sulfur structures by STM was seen for each sample. Given the optimized structures of the investigated compounds (Fig. 9), interaction of the hexavalent sulfur atoms with the gold surfaces is not completely hindered by the phthalimide and aromatic fragments of the parent molecules. The optimized structures obtained using Gaussian 03 at the B3LYP level50 show that the SO2 can efficiently interact with the gold surfaces. Deposition of S using SO2 is well established.25 The interaction of the investigated arene sulfonyl phthalimides probably involves an initial adsorption followed by ejection of both the aryl and phthalimide groups leaving the SO2 moiety on the Au surface which then leads to the deposition of S alone. Additional investigations are necessary to elucidate the mechanism of sulfur deposition on gold from these compounds.
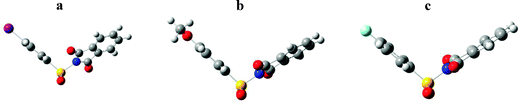 | ||
| Fig. 9 Optimized structures for (a) p-iodobenzenesulfonyl phthalimide, (b) p-methoxybenzenesulfonyl phthalimide and (c) p-fluorobenzenesulfonyl phthalimide. | ||
Conclusions
In conclusion, experimental observations of the interaction of a series of aromatic sulfonyl phthalimides on gold surfaces have been studied. Given enough time, this leads to the deposition of sulfur, the ejection of sulfonyl oxygens, the cleavage of the phthalimide group and the removal of the aromatic ring. XPS data from p-iodobenzenesulfonyl phthalimide modified gold have indicated that iodine is present on the gold surface and may be competing with sulfur for adsorption sites. However, p-methoxybenzenesulfonyl phthalimide modified gold and p-fluorobenzenesulfonyl phthalimide modified gold gave clear signals for sulfur, but none for the other components of their parent compounds. STM imaging has indicated a number of key features. Sponge-like formations similar to those observed with SAMs were observed. Two new phases for sulfur on gold have been observed including a new 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 line pattern, which is closely related to the rectangular structures, and a new zig-zag structure. The sulfur structures on the Au(111) surface are very dynamic. This is very important because it further supports that sulfur rectangles tend to behave as sulfur adlayers.
1 line pattern, which is closely related to the rectangular structures, and a new zig-zag structure. The sulfur structures on the Au(111) surface are very dynamic. This is very important because it further supports that sulfur rectangles tend to behave as sulfur adlayers.
Acknowledgements
The authors gratefully acknowledge the Natural Sciences and Engineering Research Council (NSERC), the Ontario Graduate Scholarship (OGS), the Canada Foundation for Innovation (CFI), the Ontario Innovation Trust (OIT) and the University of Guelph for funding. We are also very grateful to Dr Paul Rowntree for the use of the evaporator system.Notes and references
- A. Ulman, Chem. Rev., 1996, 96, 1533 CrossRef CAS.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103 CrossRef CAS.
- E. Pensa, E. Cortés, G. Corthey, P. Carro, C. Vericat, M. H. Fonticelli, G. Benítez, A. A. Rubert and R. C. Salvarezza, Acc. Chem. Res., 2012, 45, 1183 CrossRef CAS.
- G. Salassa, M. J. J. Coenen, S. J. Wezenberg, B. L. M. Hendriksen, S. Speller, J. A. A. W. Elemans and A. W. Kleij, J. Am. Chem. Soc., 2012, 134, 7186 CrossRef CAS.
- G. Wang, H. Tanaka, L. Hong, Y. Matsuo, K. Niikura, M. Abe, K. Matsumoto, T. Ogawa and K. Ijiro, J. Mater. Chem., 2012, 22, 13691 RSC.
- H. Bayat, D. Tranchida, B. Song, W. Walczyk, E. Sperotto and H. Schönherr, Langmuir, 2011, 27, 1353 CrossRef CAS.
- (a) W. Geyer, V. Stadler, W. Eck, M. Zharnikov, A. Gölzhäuser and M. Grunze, Appl. Phys. Lett., 1999, 75, 2401 CrossRef CAS; (b) T. Felgenhauer, C. Yan, W. Geyer, H. T. Rong, A. Gölzhäuser and M. Buck, Appl. Phys. Lett., 2001, 79, 3323 CrossRef CAS.
- (a) W. Eck, V. Stadler, W. Geyer, M. Zharnikov, A. Gölzhäuser and M. Grunze, Adv. Mater., 2000, 12, 805 CrossRef CAS; (b) A. Gölzhäuser, W. Eck, W. Geyer, V. Stadler, T. Weimann, P. Hinze and M. Grunze, Adv. Mater., 2001, 13, 806 CrossRef; (c) M. Zharnikov and M. Grunze, J. Vac. Sci. Technol., B, 2002, 20, 1793 CrossRef CAS.
- (a) I. Thom and M. Buck, Surf. Sci., 2005, 581, 33 CrossRef CAS; (b) R. K. Smith, P. A. Lewis and P. S. Weiss, Prog. Surf. Sci., 2004, 75, 1 CrossRef CAS.
- A. Turchanin, D. Weber, M. Buenfeld, C. Kisielowski, M. V. Fistul, K. B. Efetov, T. Weimann, R. Stosch, J. Mayer and A. Golzhauser, ACS Nano, 2011, 5, 3896 CrossRef CAS.
- R. G. Nuzzo and D. L. Allara, J. Am. Chem. Soc., 1983, 105, 4481 CrossRef CAS.
- C. Vericat, M. E. Vela and R. C. Salvarezza, Phys. Chem. Chem. Phys., 2005, 7, 3258 RSC.
- D. Käfer, A. Bashir and G. Witte, J. Phys. Chem. C, 2007, 111, 10546 Search PubMed.
- J. W. Ciszek, M. P. Stewart and T. M. Tour, J. Am. Chem. Soc., 2004, 126, 13172 CrossRef CAS.
- C. Shen, M. Buck, J. D. E. T. Wilton-Ely, T. Weidner and M. Zharnikov, Langmuir, 2008, 24, 6609 CrossRef CAS.
- A. Houmam, H. Muhammad, M. Chahma, K. Koczkur and D. F. Thomas, Chem. Commun., 2011, 47, 7095 RSC.
- A. Houmam, H. Muhammad and K. M. Koczkur, Langmuir, 2011, 27, 13544 CrossRef CAS.
- S. Sek, M. Chen, C. L. Brosseau and J. Lipkowski, Langmuir, 2007, 23, 12529 CrossRef CAS.
- U. Jung, S. Kuhn, U. Cornelissen, F. Tuczek, T. Strunskus, V. Zaporojtchenko, J. Kubitschke, R. Herges and O. Magnussen, Langmuir, 2011, 27, 5899 CrossRef CAS.
- J. Kang and P. Rowntree, Langmuir, 2007, 23, 509 CrossRef CAS.
- F. Li, L. Tang, W. Zhou and Q. Guo, Langmuir, 2010, 26, 9484 CrossRef CAS.
- N. Camillone III, T. Y. B. Leung, P. Schwartz, P. Eisenberger and G. Scoles, Langmuir, 1996, 12, 2737 CrossRef.
- P. Maksymovych, O. Voznyy, D. B. Dougherty, D. C. Sorescu and J. T. Yates Jr., Prog. Surf. Sci., 2010, 85, 206 CrossRef CAS.
- J. A. Rodriguez, J. Dvorak, T. Jirsak, G. Liu, J. Hrbek, Y. Aray and C. J. González, J. Am. Chem. Soc., 2003, 125, 276 CrossRef CAS.
- M. M. Biener, J. Biener and C. M. Friend, Langmuir, 2005, 21, 1668 CrossRef CAS.
- X. Gao, Y. Zhang and M. J. Weaver, J. Phys. Chem., 1992, 96, 4156 CrossRef CAS.
- C. Vericat, M. E. Vela, G. Andreasen, R. C. Salvarezza, L. Vázquez and J. A. Martín-Gago, Langmuir, 2001, 17, 4919 CrossRef CAS.
- K. M. Koczkur, E. M. Hamed and A. Houmam, Langmuir, 2011, 27, 12270 CrossRef CAS.
- A. Houmam, K. M. Koczkur, Md. G. Moula and E. M. Hamed, ChemPhysChem, 2012, 13, 1240 CrossRef CAS.
- P. G. Lustemberg, C. Vericat, G. A. Benítez, M. E. Vela, N. Tognalli, A. Fainstein, M. L. Martiarena and R. C. Salvarezza, J. Phys. Chem. C, 2008, 112, 11394 CAS.
- M. Yu, H. Ascolani, G. Zampieri, D. P. Woodruff, C. J. Satterly, R. G. Jones and V. R. Dhanak, J. Phys. Chem. C, 2007, 111, 10904 CAS.
- T. E. Lister and J. L. Stickney, J. Phys. Chem., 1996, 100, 19658 CrossRef.
- B. Meyer, Chem. Rev., 1976, 76, 367 CrossRef CAS.
- M. M. Biener, J. Biener and C. M. Friend, Surf. Sci., 2007, 601, 1659 CrossRef CAS.
- M. Yu, H. Ascolani, G. Zampieri, D. P. Woodruff, C. J. Satterly, R. G. Jones and V. R. Dhanak, J. Phys. Chem. C, 2007, 111, 10904 CAS.
- C. Schlaup, C. D. Friebel, P. Broekmann and K. Wandelt, Surf. Sci., 2008, 602, 864 CrossRef CAS.
- S. Y. Quek, M. M. Biener, J. Biener, J. Bhattacharjee, C. M. Friend, U. V. Waghmare and E. Kaxiras, J. Chem. Phys., 2007, 127, 104704 CrossRef (1–8).
- S. Y. Quek, M. M. Biener, J. Biener, J. Bhattacharjee, C. M. Friend, U. V. Waghmare and E. Kaxiras, J. Phys. Chem. B, 2006, 110, 15663 CrossRef CAS.
- K. M. Koczkur, E. M. Hamed, C. R. Hesp and A. Houmam, Chem. Commun., 2011, 47, 12128 RSC.
- M. Voronkov and E. Deryagina, Sulfur Rep., 2002, 23, 321 CAS.
- N. Hu, P. Liu, K. Jiang, Y. Zhou and Y. Pan, Rapid Commun. Mass Spectrom., 2008, 22, 2715 CrossRef CAS.
- V. K. Kanuru, G. Kyriakou, S. K. Beaumont, A. C. Papageorgiou, D. J. Watson and R. M. Lambert, J. Am. Chem. Soc., 2010, 132, 8081 CrossRef CAS.
- D. Syomin and B. E. Koel, Surf. Sci., 2001, 490, 265 CrossRef CAS.
- R. G. Cavell and K. H. Tan, Chem. Phys. Lett., 1992, 197, 161 CrossRef CAS.
- N. Batina, T. Yamada and K. Itaya, Langmuir, 1995, 11, 4568 CrossRef CAS.
- K. Edinger, A. Gölzhäuser, K. Demota, Ch. Wöll and M. Grunze, Langmuir, 1993, 9, 4 CrossRef CAS.
- R. Boistelle and J. P. Astier, J. Cryst. Growth, 1988, 90, 14 CrossRef CAS.
- G. E. Poirier, Langmuir, 1997, 13, 2019 CrossRef CAS.
- M. D. Lay, K. Varazo and J. L. Stickney, Langmuir, 2003, 19, 8416 CrossRef CAS.
- M. J. Frisch, et al., Gaussian 03, Revision B.03, Gaussian, Inc., Pittsburgh, PA, 2003 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional STM images from Au(111) modified with p-iodobenzenesulfonyl phthalimide and optimized structures of p-iodobenzenesulfonyl phthalimide, p-methoxybenzenesulfonyl phthalimide and p-fluorobenzenesulfonyl phthalimide. See DOI: 10.1039/c2cp42943h |
| This journal is © the Owner Societies 2013 |
