Constructing porous MOF based on the assembly of layer framework and p-sulfonatocalix[4]arene nanocapsule with proton-conductive property†
Guo-Li
Zheng
b,
Guo-Cheng
Yang
c,
Shu-Yan
Song
a,
Xue-Zhi
Song
a and
Hong-Jie
Zhang
*a
aState Key Laboratory of Application of Rare Earth Resources Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, PR China. E-mail: hongjie@ciac.jl.cn; Fax: +86 431 85698041; Tel: +86 431 85262127
bKey Laboratory of Catalysis and Materials Science of the State Ethnic Affairs Commission & Ministry of Education, Hubei Province, South-Central University for Nationalities, Wuhan, 430074, PR China
cSchool of Chemistry and Life Science, Changchun University of Technology, Changchun 130012, PR China
First published on 21st October 2013
Abstract
A novel porous MOF based on the layer framework and p-sulfonatocalix[4]arene nanocapsule has been established, which is assembled by molecule recognition. The channel structure of compound 1 shows the proton-conductive property with 5.42 × 10−7 S cm−1 at 298 K and 95% relative humidity.
1 Introduction
Metal–organic frameworks (MOFs) have attracted much attention due to their aesthetic architectures and applications in material science. To date, MOF chemistry focuses almost exclusively on gas sorption, catalysis, magnetism, and electrical conductivity, etc.1 However, very few studies focusing on the proton conductivities of MOFs have been reported.2–4 Recently, a new proton-conductive system based on the layer structures of the oxalate-bridged complexes has been reported by the Kitagawa group.2d–f Proton conductors based on sulfonate and phosphonate MOFs have also been investigated by the Shimizu group.3 Proton conductivities in crystallinity MOFs not only have potential applications in fuel cells, electrochemical devices, but also can provide insight into conduction mechanisms due to the long-range order. In comparison with other porous materials such as activated carbon and zeolites, the main advantage of porous MOFs is their highly designable nature, which enables us to control not only the size and shape but also the physical properties. So the design and synthesis of new proton conductors based on MOFs are becoming particularly interesting area of research.Recently calixarenes as building blocks have been widely used to construct various MOFs due to their interesting biological behaviors and potential applications in materials science.5,6 The self-assembly of calixarenes with metal ions and organic molecules, can afford various supramolecular wonderful structures, such as porous structures,7 molecular capsules,8 bilayers,9 2D and 3D coordination polymers.10 Proton conductivities in MOFs materials need proton carries such as NH4+, H3O+ or H+ given by acid or OH groups, and hydrogen-bond networks for proton conductions. The p-sulfonatocalix[n]arenes (n = 4, 5, 6, 8) possess many sulfonate groups and OH groups, which not only ligate the metal ions into multidimensional frameworks possible, but also may be available to further act as proton carries. A survey of the Cambridge Structural Database shows that most of MOFs based on the p-sulfonatocalix[n]arenes possess plenty of water molecules and OH groups inside the frameworks. In addition, many macromolecular sulfonic acid polymers as proton conductors have been established.11 Recently, a MOF (the trisodium salt of 2,4,6-trihydroxy-1,3,5-benzenetrisulfonate) that conducts protons with sulfonate groups had been reported.3a All above results indicate MOFs based on p-sulfonatocalix[4]arenes are appealing candidates for proton conductions.
Our group has been pursuing supramolecular porous structures built from p-sulfonatocalix[n]arene.12 The cavity of p-sulfonatocalix[n]arene structure suggests that the design of new porous MOFs based on p-sulfonatocalix[n]arenes can rely on molecular recognition. When suitable guests, such as tetraphenylphosphonium cations,13a phenanthroline,13b pyridine N-oxide,13c [Cu(bpdo)3]2+ (2,2′-bipyridine-N,N′-dioxide, bpdo),12aetc., are employed in these systems, porous materials can be expected. In this paper, a new supramolecular MOF which contains continuous water molecules inside the pores has been prepared by molecular recognition; and the proton conductivity of porous material has also been investigated.
2 Experimental
2.1 Materials
All reagents were commercial products of high purity and were not further purified except for Na3[p-sulfonatocalix[4]arene], which was prepared via the NaOH reaction with p-sulfonatocalix[4]arenes.2.2 Preparation of single crystals (Fig. 1)
A mixture of CuCl2 (0.0172 g, 0.1 mmol), Na3[p-sulfonatocalix-[4]arene] (0.0406 g, 0.05 mmol), L (4,4′-bipyridine-N,N′-dioxide, 0.0286 g, 0.15 mmol) and bpdo (2,2′-bipyridine-N,N′-dioxide, 0.0094 g, 0.05 mmol) with the ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (pH = 3.5) was dissolved in 20 ml hot water. Red crystals of compound 1 were obtained after volatilizing at room temperature for a few days (0.0446 g, yield: 57%, based on p-sulfonato-calix[4]arenes). Microanalysis (CHN) was not feasible due to the highly hydrated nature of the complexes
2 (pH = 3.5) was dissolved in 20 ml hot water. Red crystals of compound 1 were obtained after volatilizing at room temperature for a few days (0.0446 g, yield: 57%, based on p-sulfonato-calix[4]arenes). Microanalysis (CHN) was not feasible due to the highly hydrated nature of the complexes
2.2 Measurements
The single-crystal X-ray data was collected on a Bruker SMART 1000 CCD diffractometer with Mo Ka radiation (λ = 0.071073 nm) using the w-scan mode. Data were corrected for absorption using the SADABS program, and solution and refinement of the structure were performed using the SHELX-97 software package. Crystal/refinement details for 1: C116H134Cu3N12O66S8, M = 3199.02, compound 1 crystallized in the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , triclinic, a = 17.0111(11) Å, b = 17.6763(12) Å, c = 20.7596(15) Å, α = 70.1290(10)°, β = 70.3930(10)°, γ = 89.5450(10)°,V = 5490.4(6) Å3, Z = 2, T = 193 K, λ = 0.71073 Å, Rint = 0.0341. A total of 39
, triclinic, a = 17.0111(11) Å, b = 17.6763(12) Å, c = 20.7596(15) Å, α = 70.1290(10)°, β = 70.3930(10)°, γ = 89.5450(10)°,V = 5490.4(6) Å3, Z = 2, T = 193 K, λ = 0.71073 Å, Rint = 0.0341. A total of 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 319 reflections were collected in the range 1.25 < θ < 25, of which 19
319 reflections were collected in the range 1.25 < θ < 25, of which 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 243 were unique. GOF = 0.961, R1 = 0.1306, wR2 (all data) = 0.2774. One of the sulfonate groups (at Fig. S4†) was refined by disorder. Non-hydrogen atoms were refined anisotropically. The free water molecules were removed before the cited solvent accessible volume was calculated by Platon.14 Large water inside the channels and weak diffraction quality of compound 1 are responsible for the relatively high value of agreement factor R1. CCDC 885465 contains the supplementary crystallographic data for this paper.
243 were unique. GOF = 0.961, R1 = 0.1306, wR2 (all data) = 0.2774. One of the sulfonate groups (at Fig. S4†) was refined by disorder. Non-hydrogen atoms were refined anisotropically. The free water molecules were removed before the cited solvent accessible volume was calculated by Platon.14 Large water inside the channels and weak diffraction quality of compound 1 are responsible for the relatively high value of agreement factor R1. CCDC 885465 contains the supplementary crystallographic data for this paper.
The powder X-ray diffraction (XRD) of compound 1 was examined on a Rigaku-Dmax 2500 diffractometer using Cu Kα radiation (λ = 0.15405 nm). Fourier transform infrared (FTIR) spectra were measured within the 4000–400 cm wavenumber range using a Perkin-Elmer model 580B IR spectrophotometer with the KBr pellet technique. Thermogravimetric analysis (TGA) was performed on a SDT2960 analyzer (Shimadzu, Japan) up to 800 °C at a heating rate of 10 °C min−1 under N2.
The powders were prepared by grinding the sample into a homogeneous powder with a mortar and pestle. The powders were then added to a standard 11 mm die, sandwiched between two stainless steel electrodes and pressed at 5000 kg for 2 min; the pellet was 11 mm in diameter and 1.16 mm in thickness. Measurements were carried out using an impedance and gain-phase analyzer (PARSTAT 2273, Ametek, USA), 1 Hz–1 MHz, with a two-probe electrochemical cell and an applied ac voltage of 10–100 mV (unless otherwise stated). Measurements were taken in the temperature range of 25–85 °C with 95% relative humidity. Conductivity was calculated using the following equation:
3 Results and discussion
The crystal structure of 1 was determined by single-crystal X-ray diffraction at 193 K. For 1, it forms a typical three-dimensional porous framework, in which each layer is bridged by supramolecular nanocapsules (Fig. 2 and Fig. S1†). The nanocapsule is composed of two p-sulfonatocalix[4]arenes and [Cu(L)3]2+ (2,2′-bipyridine-N,N′-dioxide, bpdo), taking an overall charge of −4 with one of the sulfonate groups being protonated (Fig. 3 and Fig. S2†). The [Cu(bpdo)2·2H2O]2+ bridged the two cones through the charge-assisted C–H⋯π, and C–H⋯O interactions. The included aryl rings of the bpdo ligands have no obvious π⋯π interactions to the phenyl rings of the host calixarenes with the centroid⋯centroid distances of 4.111 Å. The neighboring aryl hydrogens from the included bpdo ligands are directed towards the aromatic rings of the calixarenes with CH⋯π interactions (CH⋯centroid distances = 2.571 Å). In addition, one hydrogen of the included bpdo ligand interacts through C–H⋯O hydrogen bond to the neighboring sulfonic group of p-sulfonatocalix[4]arene with the distance 2.301 Å. Although hydrogen atoms of two coordinated water molecules of [Cu(bpdo)2·2H2O]2+ were not located, hydrogen bonds with the neighbouring sulfonic groups of p-sulfonatocalix[4]arenes are implied by the short O⋯O contacts (O⋯O = 2.975 Å). | ||
| Fig. 2 The packing diagram of compound 1 down the a axis, showing the channel structure, the dissociative water molecules are omitted for clarity. | ||
The Cu and L (4,4′-bipyridine-N,N′-dioxide) form a layered structure based on (4, 4) topology which is composed of bowl shape cavities (Fig. 4 and Fig. S3†). The four rigid modules of L make up the bowl frameworks, these L modules are bridged by four copper centers. All the copper centers in the layer structure are octahedrally coordinated, which are ligated by two water molecules and four oxygen atoms of L. The bowl can be likened to a calix[4]arene with a cone conformation, in which each copper center is related to a methylene group on the backbone of a calix[4]arene (Fig. S3†). In the metallacalix[4]arenes 1, the four copper atoms build up an almost equilateral square with Cu⋯Cu distances of 12.378, 12.276, 12.365 and 12.274 Å, which make the layer as a suitable host for the supramolecular capsules (Fig. 4). The host layer and the guest capsules are held together through charge-assisted π⋯π interactions with the centroid⋯centroid distances of 3.762, 3.500, 3.612 and 3.507 Å (Fig. S4† and Fig. 5). In addition, the hydrogens of the L interact through C–H⋯O hydrogen bonds to the neighboring sulfonic groups of p-sulfonatocalix[4]arenes with the distances from 2.692 to 2.999 Å.
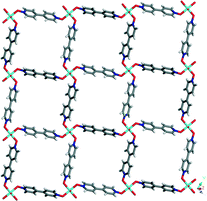 | ||
| Fig. 4 A layered structure consisting of the L and the copper ions with (4, 4) topology as host for p-sulfonatocalix[4]arene nanocapsules. | ||
All the cavities of the layers are occupied by the p-sulfonatocalix[4]arenes of supramolecular capsules (Fig. 6). The up-rim of neighbouring bowl shape cavities are oriented in opposite directions, which template the up-rim neighboring calixarenes are also oriented in opposite directions. The noteworthy aspect of the title complex is that when the structure is viewed along the a and b axis, the whole framework forms pores constructed by the capsules and metal–organic cation framework (MOCF), which is clearly shown in Fig. 2 and Fig. S1.† The interlayer separations of 1 is about 18 Å. Different to traditional porous materials, the porous material is sustained exclusively by weak interactions. The solvent-accessible volumes of the unit cells of 1 was estimated, (PLATON program)11 to be 2836.2 Å3, which is approximately 51.7% of the unit-cell volume (5490.4 Å3).
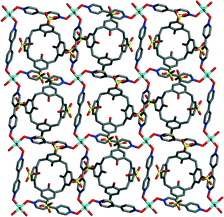 | ||
| Fig. 6 All the cavities of the layers occupied by the p-sulfonatocalix[4]arenes, all the hydrogen atoms are omitted for clarity. | ||
Recently, p-sulfonatocalix[4]arene templated by Ag-hexamethylenetetramine coordination polymer forming a porous structure was reported by our group.12a Different to the above mentioned structure, this MOCF not only acts as template, but also act as hosts for calixarene capsules. The surface of the pores are lined exclusively with oxygen atoms of sulfonate groups and OH groups. Significantly, these groups serve to anchor free water molecules into pores forming a potential hydrogen-bond transfer pathway.
We are interested in the proton conductivity of metal–organic frameworks based on calixarenes. The conductivity of 1 was carried out and determined from the semicircle in the Nyquist plot, as shown in Fig. S6.† The proton conductivity of 1 was 5.42 × 10−7 S cm−1 at 298 K and 95% relative humidity (RH) (Table S1†). The temperature dependence of the proton conductivity of 1 at 95% RH was shown in Fig. 7. Although the proton conductivity of compound 1 is lower compared with other known MOF material (8 × 10−3 S cm−1 at 298 K and 98% RH),2e our study reveals that the use of hydrophilic calixarenes with metal ions appears promising for producing proton conductive materials. The proton conductivity of compound 1 increased with increasing temperature. The activation energy was 0.39 eV, as determined from least-square fit of the slope. Therefore, proton conduction in compound 1 could be classified mainly as the Grotthuss mechanism. Hydrogen-bonded networks are formed along three-dimensional channels with the sulfonate groups, OH groups and water molecules. The contribution to the proton conductivity in compound 1 is thought to be these hydrogen-bonded networks.
4 Conclusions
In this report, a channel supramolecular framework based on p-sulfonatocalix[4]arene nanocapsule has been demonstrated, which is assembled by molecule recognition. The structure provides new opportunities for MOCFs as hosts to be applied in the field of supramolecular chemistry. All the results suggest the porosities in complexes can also be obtained under similar conditions with other guests such as a crown ether or aza macrocycle. In addition, the proton conductivity based on p-sulfonatocalix[4]arene has also been established. The results afford us numerous possibilities that using metal, p-sulfonatocalix-[n]arene or p-sulfonatothiacalix[n]arene (n = 4, 5, 6, 8) and guests for supramolecular frameworks with a view to accessing more porous and layered proton conductivity materials. All these studies are underway in our lab and will be reported in the future.Acknowledgements
The authors are grateful to the financial aid received from the National Natural Science Foundation of China (grant no. 21071140, 21201051, 21005008 and 91122030), ‘863’-National High Technology Research and Development Program of China (grant no. 2011AA03A407) and National Natural Science Foundation for Creative Research Group (grant no. 20921002).Notes and references
- (a) S. R. Batten and R. Robson, Angew. Chem., Int. Ed., 1998, 37, 1460–1494 CrossRef; (b) S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed; (c) B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629–1658 CrossRef CAS PubMed.
- (a) H. Kitagawa, Y. Nagao, M. Fujishima, R. Ikeda and S. Kanda, Inorg. Chem. Commun., 2003, 6, 346–348 CrossRef CAS; (b) Y. Nagao, M. Fujishima, R. Ikeda, S. Kanda and H. Kitagawa, Synth. Met., 2003, 133–134, 431–432 CrossRef CAS; (c) Y. Nagao, R. Ikeda, K. Iijima, T. Kubo, K. Nakasuji and H. Kitagawa, Synth. Met., 2003, 135–136, 283–284 CrossRef CAS; (d) T. Yamada, M. Sadakiyo and H. Kitagawa, J. Am. Chem. Soc., 2009, 131, 3144–3145 CrossRef CAS PubMed; (e) M. Sadakiyo, T. Yamada and H. J. Kitagawa, J. Am. Chem. Soc., 2009, 131, 9906–9907 CrossRef CAS PubMed; (f) H. Okawa, A. Shigematsu, M. Sadakiyo, T. Miyagawa, K. Yoneda, M. Ohba and H. Kitagawa, J. Am. Chem. Soc., 2009, 131, 13516–13522 CrossRef CAS PubMed; (g) A. Shigematsu, T. Yamada and H. Kitagawa, J. Am. Chem. Soc., 2011, 133, 2034–2036 CrossRef CAS PubMed.
- (a) J. A. Hurd, R. Vaidhyanathan, V. Thangadurai, C. I. Ratcliffe, I. L. Moudrakovski and G. K. H. Shimizu, Nat. Chem., 2009, 1, 705–710 CrossRef CAS PubMed; (b) J. M. Taylor, R. K. Mah, I. L. Moudrakovski, C. I. Ratcliffe, R. Vaidhyanathan and G. K. H. Shimizu, J. Am. Chem. Soc., 2010, 132, 14055–14057 CrossRef CAS PubMed.
- (a) S. C. Sahoo, T. Kundu and R. Banerjee, J. Am. Chem. Soc., 2011, 133, 17950–17958 CrossRef CAS PubMed; (b) E. Pardo, C. Train, G. Gontard, K. Boubekeur, O. Fabelo, H. Liu, B. Dkhil, F. Lloret, K. Nakagawa, H. Tokoro, S. Ohkoshi and M. Verdaguer, J. Am. Chem. Soc., 2011, 133, 15328–15331 CrossRef CAS PubMed.
- (a) P. D. Harvey, Coord. Chem. Rev., 2001, 233–234, 289–309 Search PubMed; (b) S. M. Biros and J. Rebek, Chem. Soc. Rev., 2007, 36, 93 RSC; (c) C. Wieser, C. B. Dieleman and D. Matt, Coord. Chem. Rev., 1997, 165, 93 CAS; (d) C. Redshaw, Coord. Chem. Rev., 2003, 244, 45 CrossRef CAS.
- (a) J. L. Atwood, L. J. Barbour, M. J. Hardie and C. L. Raston, Coord. Chem. Rev., 2001, 222, 3–32 CrossRef CAS; (b) S. J. Dalgarno, P. K. Thallapally, L. J. Barbour and J. L. Atwood, Chem. Soc. Rev., 2007, 36, 236–245 RSC; (c) F. Perret, A. N. Lazar and A. W. Coleman, Chem. Commun., 2006, 2425–2438 RSC.
- (a) C. Gaeta, C. Tedesco and P. Neri, in Calixarenes in the Nano world; ed. J. Vicens and J. Harrowfield, Springer, 2007, ch. 16, pp. 335–354 Search PubMed; (b) R. G. Harrison, N. K. Dalley and A. Y. Nazarenko, Chem. Commun., 2000, 1387–1388 RSC; (c) R. G. Harrison, O. D. Fox, M. O. Meng, N. K. Dalley and L. J. Barbour, Inorg. Chem., 2002, 41, 838–843 CrossRef CAS PubMed; (d) C. Tedesco, I. Immediata, L. Gregoli, L. Vitagliano, A. Immirzi and P. Neri, CrystEngComm, 2005, 7, 449–453 RSC; (e) Y. Ishii, Y. Takenaka and K. Konishi, Angew. Chem., Int. Ed., 2004, 43, 2702–2705 CrossRef CAS; (f) M. J. Hardie, M. Makha and C. L. Raston, Chem. Commun., 1999, 2409–2410 RSC; (g) S. J. Dalgarno and C. L. Raston, Chem. Commun., 2002, 2216–2217 RSC; (h) S. Airey, A. Drljaca, M. J. Hardie and C. L. Raston, Chem. Commun., 1999, 1137–1138 RSC.
- (a) S. J. Dalgarno, J. L. Atwood and C. L. Raston, Chem. Commun., 2006, 4567–4574 RSC; (b) M. J. Hardie and C. L. Raston, J. Chem. Soc., Dalton Trans., 2000, 2483–2492 RSC.
- (a) P. J. Nichols and C. L. Raston, Dalton Trans., 2003, 2923–2927 RSCJ. L. Atwood, T. Ness, P. J. Nichol and C. L. Raston, Cryst. Growth Des., 2002, 2, 171 CAS.
- (a) S. J. Dalgarno, M. J. Hardie, J. L. Atwood and C. L. Raston, Inorg. Chem., 2004, 43, 6351–6356 CrossRef CAS PubMed; (b) S. J. Dalgarno, M. J. Hardie, J. L. Atwood, J. E. Warren and C. L. Raston, New J. Chem., 2005, 29, 649–652 RSC; (c) G. L. Zheng, Y. Y. Li, R. P. Deng, S. Y. Song and H. J. Zhang, CrystEngComm, 2008, 10, 658–660 RSC.
- (a) K. A. Mauritz and R. B. Moore, Chem. Rev., 2004, 104, 4535–4586 CrossRef CAS; (b) K. D. Kreuer, S. J. Paddison, E. Spohr and M. Schuster, Chem. Rev., 2004, 104, 4637–4678 CrossRef CAS.
- (a) G. L. Zheng, Y. Y. Li, H. D. Guo, S. Y. Song and H. J. Zhang, Chem. Commun., 2008, 4918–4920 RSC; (b) G. L. Zheng, H. J. Zhang, S. Y. Song, Y. Y. Li and H. D. Guo, Eur. J. Inorg. Chem., 2008, 1756–1759 CrossRef CAS; (c) G. L. Zheng, W. Q. Fan, S. Y. Song, H. D. Guo and H. J. Zhang, J. Solid State Chem., 2010, 183, 1457–1463 CrossRef CAS PubMed.
- (a) M. Makha, A. N. Sobolev and C. L. Raston, Chem. Commun., 2006, 511–513 RSC; (b) Y. Liu, D. Guo, H. Zhang, F. Ding, K. Chen and H. Song, Chem.–Eur. J., 2007, 13, 466–472 CrossRef CAS PubMed; (c) G. W. Orr, L. J. Barbour and J. L. Atwood, Science, 1999, 285, 1049–1052 CrossRef CAS.
- A. L. Spek, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, C34 Search PubMed . The free water molecules were removed before the cited solvent accessible volume was calculated by PLATON.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of compounds 1 and 2, crystallographic information, supplementary figures, XRD and TGA of the compounds. CCDC 885465. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ce26075a |
| This journal is © The Royal Society of Chemistry 2014 |





