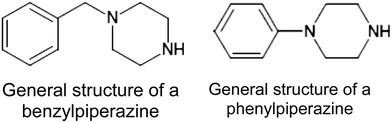
An optimised gas chromatographic-mass spectrometric method for the chemical characterisation of benzylpiperazine and 1-arylpiperazine based drugs
Chipo
Kuleya
,
Sarah
Hall
,
Lata
Gautam
and
Michael D.
Cole
*
Department of Life Sciences, Anglia Ruskin University, Cambridge, UK. E-mail: michael.cole@anglia.ac.uk
First published on 14th November 2013
Abstract
This study presents a method for the simultaneous detection of piperazines and congeners in street samples of amphetamine type stimulants. The method is based on simple solvent extraction of the drugs from the samples followed by GC-MS analysis. The analytical method separates 19 of the most common drugs found in piperazine samples and achieves for the first time the GC-MS separation of (i) 2-FPP, 3-FPP and 4-FPP and (ii) 2-TFMPP, 3-TFMPP and 4-TFMPP at the same time. This method provides operational laboratories with a more effective method for the chemical characterisation of street samples of piperazines.
Introduction
It is widely accepted that the use of new psychoactive substances on many continents including Australia, Europe, Asia and the USA is widespread. These compounds appear in the illicit drug market (i) as users experiment with the effects associated with, and caused by, the different chemistries and chemical effects associated with a drug class and (ii) to avoid prosecution under the different legislative systems which currently operate to control drugs of abuse.Recently appeared in the clandestine drug market are the benzylpiperazines and 1-arylpiperazines collectively known as the piperazines. These drugs have similar effects to amphetamines and ring substituted amphetamines which are now subject to some, but incomplete, legislative control both at national and international level. An example of national control arises in the U.K. where since December 2009 BZP and a number of piperazines have been controlled under the Misuse of Drugs Act 1971 as Class C drugs and are placed in Schedule 1 of the 2001 regulations since they have no medicinal use. The exceptions are mCPP and CPCPP which are used in the manufacture of antidepressants and these are therefore placed in Schedule 4. Other countries do not have such detailed regulations. Currently there are no global controls for any piperazine based drugs of abuse although they have now been highlighted by the United Nations Office of Drug Control.1 None of the piperazines are listed in the United Nations 1971 Convention on Psychotropic Substances.2,3 However several, including 1-benzylpiperazine (BZP), (trifluoromethylphenyl)piperazine (TFMPP) and 1-(3-chlorophenyl)piperazine (3-CPP), were pre-reviewed by the WHO Expert Committee on Drug Dependence in 2012. This might in the future lead to stricter controls.
Historically, in 1943 the Wellcome Research Laboratories introduced BZP as an anti-helminthic agent.4,5 This was followed by further research into it's pharmacological use as an anti-depressant in humans. During the 1970's it became clear that BZP induced psychoactive and addictive behaviours similar to that caused by amphetamines.5 This lead to the discontinuation of the trials on BZP owing to it's potential as a drug of abuse.6 The 1990's saw the use of BZP for recreational purposes as a ‘rave drug’ marketed extensively over the internet. According to Arbo and Bastos7 the first documented abuse of BZP was in 1996 in the USA. In 2002, BZP was marketed as a legal alternative to methamphetamine in New Zealand8 and it's abuse became widespread. The early 2000's saw the advent of the piperazines.9,10 Currently the most common of these are 3-CPP and 1-(3-trifluoromethylphenyl)piperazine (3-TFMPP). The piperazine drugs most frequently encountered in forensic analysis at the present time are given in Table 1.
| Common name | Abbreviation |
|---|---|
| 1-Benzylpiperazine | BZP |
| 1-Benzyl-4-methylpiperazine | MBZP |
| 1-(4-Bromo-2,5-dimethoxybenzyl)piperazine | 2C-B BZP |
| 1-Phenylpiperazine | N/A |
| 1-(3-Chlorophenyl)piperazine | 3-CPP |
| 1-(4-Chlorophenyl)piperazine | 4-CPP |
| 1-(3-Chlorophenyl)-4-(3-chloropropyl)piperazine | 3-CPCPP |
| 1-(4-Fluorophenyl)piperazine | 4-FPP |
| 4-Methylphenylpiperazine | 4-MePP |
| 1-(4-Methoxyphenyl)piperazine | 4-MeOPP |
| 1-(3-Trifluoromethylphenyl)piperazine | 3-TFMPP |
These drugs exist as a free base or salt and are sold as a powder, liquid, or tablet.11 Most commonly they are found as tablets and capsules.12–14 Piperazine based drugs are commonly found in drug cocktails. It is rare to find a single active ingredient in street samples of this group of drugs.12,14,15 The most frequent mixture encountered at the present time is that of BZP with 3-TFMPP sold under the name ‘Exotic’.14 Other more complex mixtures have been reported, for example (i) 1-(4-fluorophenyl)piperazine (4-FPP) with 1-(4-methylphenyl)piperazine (4-MPP) and nicotinamide and (ii) BZP with 3-TFMPP, 3,4-methylenedioxymethamphetamine (MDMA), procaine and caffeine.12 Generally these drugs are sold online and in herbal shops marketed under names such as ‘Exotic’, ‘A2’, ‘Rapture’, ‘Jump’, ‘Frenzy’ and ‘Nemesis’.7,12–14 Doses in these mixtures typically range between 50 and 200 mg for BZP, 5–75 mg for 3-TFMPP and 90–110 mg for 3-CPP.12 The use of piperazines as recreational drugs arise from their chemical properties. These drugs bind to serotonin receptors in the human nervous system.6,12,16 Piperazines act as 5-HT agonists and raise cellular levels of dopamine and serotonin thereby creating stimulating feelings of euphoria, alertness and social activeness, hence giving them status as ‘party pills’.5,16
Currently police within the European Union seize more piperazine type drugs than MDMA.17 In order to prosecute such cases, and indeed to relate such cases to each other, there is a need for a validated method for the analysis of this group of drugs. This process must include (i) identification of the drug(s) in the sample to determine whether or not an offence has occurred, (ii) quantification of the drug especially if certain charges, including drug supply, are applied and (iii) chemical characterisation of the drug, that is determination of the adulterants (pharmacologically active) and diluents (pharmacologically inactive) in the drug. This latter is required if drug samples are to be compared. Chemical characterisation is made more complicated by the drugs with which the piperazines are mixed. Examples of these drugs include amphetamine, methamphetamine, cocaine, diazepam, dapoxetine, dextromethorphan, caffeine and nicotinamide. This means that choice of extraction solvent is important to avoid, for example, hydrolysis of the drug (associated with methanol), reaction of amino groups with the solvent (associated with acetone) or acid hydrolysis (associated with chloroform). Additionally, it is also imperative that the analytical conditions are such that the integrity of the sample is not compromised.
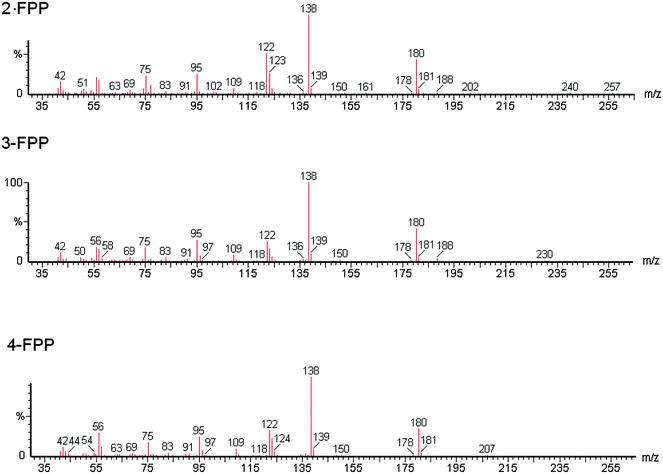
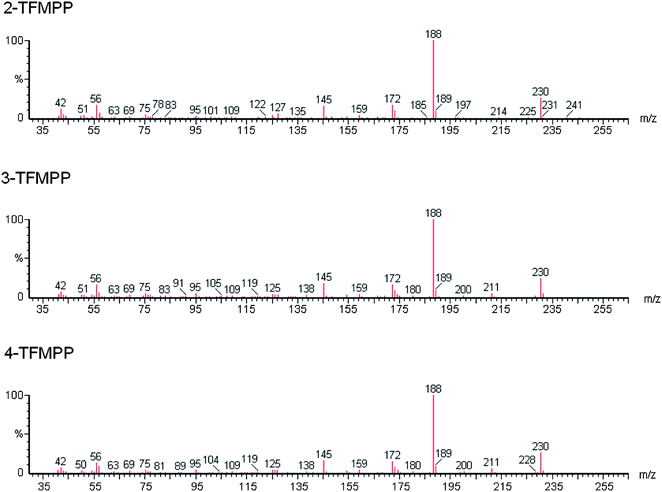
Studies pertaining to the analysis of 3-TFMPP and 4-FPP are currently limited. Most of the analytical methods published are on screening, detection and quantification of piperazines for metabolic and toxicological studies of urine and blood samples and not on the street form of the drug. In one study to determine BZP, (methoxyphenyl) piperazine (MeOPP), TFMPP, FPP and their isomers, a variety of techniques were applied; colour tests, thin layer chromatography (TLC), infrared spectroscopy (IR), gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS).18 However, a limitation to the methods was the inability to separate the three FPP isomers. These limitations were recognised by other authors.19 Isomers possess similar chemical properties resulting in similar physico–chemical profiles making it difficult to distinguish between them. In further studies 3-TFMPP and 4-TFMPP were found to have identical retention identical retention times and mass spectra.4,11 As such it was impossible to identify the specific isomer by GC-MS as required by some legislative systems.
In this study GC-MS was chosen as the method of analysis over LC-MS due to the wider availability of GC-MS on global basis. Further, a method was sought which allows complete chemical characterisation of the drugs in street samples of piperazines. In this paper we describe, for the first time, a GC-MS method for the separation and identification of the piperazines and congeners including the three isomers of each FPP and TFMPP. The method described has been successfully applied to street samples and provides operational laboratories a more effective way to identify, quantify and characterise piperazine based drugs.
Method
Chemicals and reagents
1-Benzylpiperazine, 1,4-dibenzylpiperazine, 1-(3-chlorophenyl)piperazine, 1-(2-fluorophenyl)piperazine,1-(4-fluorophenyl)piperazine, 1-(4-methylphenyl)piperazine, 1-(4-methylbenzyl)piperazine, methamphetamine hydrochloride, caffeine, cocaine hydrochloride, diazepam, 3,4-methylenedioxymethamphetamine hydrochloride, dapoxetine hydrochloride, nicotinamide, dextromethorphan hydrobromide, eicosane and were purchased from Sigma Chemical Company, U.K. 1-(3-trifluoromethylphenyl)piperazine was procured from Alfa Aesar, U.K. 1-(4-trifluoromethylphenyl)piperazine was from Fluka, U.K. 1-(3-fluorophenyl)piperazine and 1-(2-trifluoromethylphenyl)piperazine were from Chemos GMBH. 2-Methyl-propan-2-ol and pentane were from Fisher Chemicals.All chemicals were purchased and all work was carried out under Home Office Licence Ref no.: 00471 in compliance with the Misuse of Drugs Act, 1971.
Instrumentation and chromatographic conditions
GC-MS analysis was performed with a Perkin Elmer Clarus Turbomass Gold 500MS fitted with a Supelco Equity 5 GC capillary column (30 m × 0.25 mm i.d. × 0.25 μm layer thickness). For the optimised GC-MS system the injection temperature was 260 °C. The injection volume was 1.0 μL. The carrier gas was helium at a flow rate of 1 mL min−1 with a split ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The initial oven temperature was 60 °C (1 min), which was raised at 10 °C min−1 to 170 °C (2 min) then further increased at 15 °C min−1 to 280 °C (4 min). The transfer line and source temperature were 280 °C and 230 °C respectively. The ionisation energy was set at 70 eV with a m/z scan range of 40–620 AMU. The total run time was 25.3 min. Data was collected, analysed and processed using TurboMass™ 5.4 GC-MS software.
1. The initial oven temperature was 60 °C (1 min), which was raised at 10 °C min−1 to 170 °C (2 min) then further increased at 15 °C min−1 to 280 °C (4 min). The transfer line and source temperature were 280 °C and 230 °C respectively. The ionisation energy was set at 70 eV with a m/z scan range of 40–620 AMU. The total run time was 25.3 min. Data was collected, analysed and processed using TurboMass™ 5.4 GC-MS software.
Preparation of standards and samples
Stock solutions of each analyte were prepared at a concentration equivalent to 1 mg mL−1 free base in 2-methyl-propan-2-ol. The mass required of the salt was calculated from the molecular formula of each drug. For studies of retention time, precision and accuracy, standards at 0.1 mg mL−1 were used. For linearity studies the stock solutions were diluted to give 10 calibration standards for each analyte equivalent to the concentration range of 0.01–1 mg mL−1 free base containing 0.02 mg mL−1 of the internal standard, eicosane, to generate calibration, LOD and LOQ data. These values were calculated using standard IUPAC methods.20,21 Eicosane was chosen because it is chemically inert with respect to the analytes in this study.Preparation and analysis of street samples
A physical description and photograph of the street samples were recorded. Between 3 and 5 tablets from each sample were provided by Cambridge Constabulary. The average mass (mg) was recorded and photographs taken with a Nikon D90 digital camera. The sample was crushed to a fine powder and homogenised using the “cone and quarter” method. A sample of the powder (30 mg) was dissolved in 7 mL 2-methyl-propan-2-ol in a 10 mL volumetric flask, the sample placed in an ultrasonic bath for 30 minutes and then diluted to volume. After thorough mixing the solution was centrifuged at 3000 rpm for 3 minutes to remove the solid material. A 1 mL sample of the supernatant was diluted tenfold and analysed using the optimised GC-MS method.Results and discussion
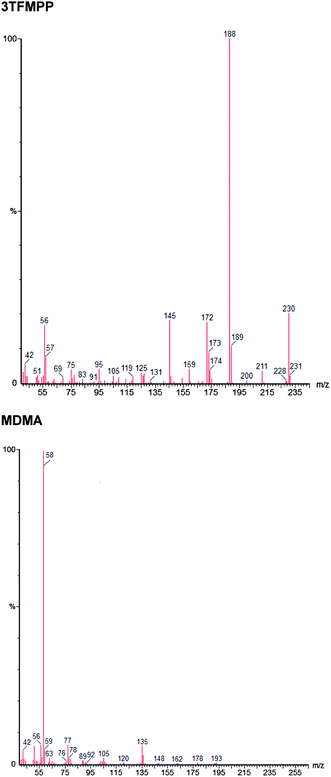
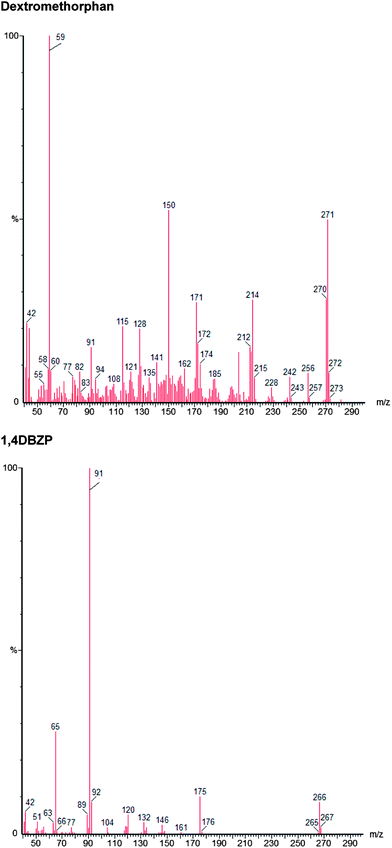
The method was optimised and validated for each of the drugs to be analysed in accordance with International Conference on Harmonisation (ICH) guidelines22 and Centre for Drug Evaluation and Research (CDER).23 The choice of solvent for the presentation of the drug samples to the instrument has been made to minimize analyte decomposition prior to and during analysis. The instrument operational parameters were optimised to give the best chromatographic separations and optimal peak shape. The raw data is available from the authors. 2-Methyl-propan-2-ol was chosen as the solvent for delivery of the drugs to the GC-MS because, as a tertiary alcohol, it was the only solvent in which all drugs under consideration were stable.24The chromatographic separation achieved is shown in Fig. 1 and relative retention index, calculated as retention time of the analyte/retention time eicosane and index data is given in Table 2. The isomers 2-FPP, 3-FPP and 4-FPP are separated with retention times of 12.88 min (2-FPP), 14.05 min (3-FPP) and 13.56 min (4-FPP) respectively. Additionally, the isomers of TFMPP are also separated with retention times of 12.12 min (2-TFMPP), 13.78 min (3-TFMPP) and 14.82 min (4-TFMPP) respectively. This separation is required because the isomers of FPP have near identical mass spectra (Fig. 2) as do the isomers of TFMPP (Fig. 3). There is some co-elution of 3-TFMPP (13.78 min) and MDMA (13.83 min) but they can both be identified through the use of mass spectrometry and selected ion monitoring (Fig. 4). The same applies to the co-elution of dextromethorphan (20.50 min) and DBZP (20.53 min) (Fig. 5). This chromatographic method allows identification and quantification of the native drugs and diluents commonly found in street samples of piperazine based amphetamine type stimulants (ATS) and satisfies the requirements for chemical characterisation of these drugs.
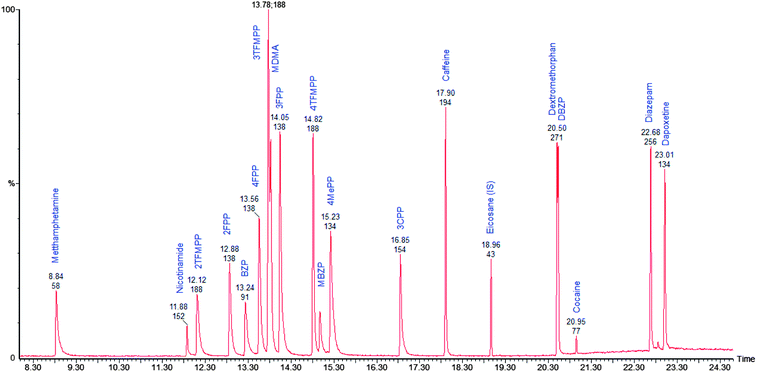 | ||
| Fig. 1 Total ion chromatogram of mixed drug standard. The separated compounds are labelled with their respective retention times. | ||
| Compound | Retention time (min) | Relative retention index (eicosane = 1.000) |
|---|---|---|
| Methamphetamine | 8.84 | 0.466 |
| Nicotinamide | 11.88 | 0.627 |
| 2-TFMPP | 12.12 | 0.639 |
| 2-FPP | 12.88 | 0.679 |
| BZP | 13.24 | 0.698 |
| 4-FPP | 13.56 | 0.715 |
| 3-TFMPP | 13.78 | 0.727 |
| MDMA | 13.83 | 0.729 |
| 3-FPP | 14.05 | 0.741 |
| 4-TFMPP | 14.82 | 0.782 |
| MBZP | 14.98 | 0.790 |
| 4-MePP | 15.23 | 0.803 |
| 3-CPP | 16.85 | 0.889 |
| Caffeine | 17.90 | 0.944 |
| Eicosane | 18.96 | 1.000 |
| Dextromethorphan | 20.50 | 1.081 |
| DBZP | 20.53 | 1.083 |
| Cocaine | 20.95 | 1.105 |
| Diazepam | 22.68 | 1.196 |
| Dapoxetine | 23.01 | 1.214 |
The intra-day and inter-day precision of the quantification for each of the analytes is given in Table 3. The relative standard error for the intra-day trial show that the error lies in the range 0.3–1.80%, for the inter-day 1.10–1.73%.
| Substance | Intra-day (n = 6) | Inter-day (n = 9) | ||
|---|---|---|---|---|
| Standard deviation | % relative standard error | Standard deviation | % relative standard error | |
| Methamphetamine | 0.009 | 1.86 | 0.063 | 1.44 |
| Nicotinamide | 0.014 | 1.13 | 0.060 | 1.18 |
| 2-TFMPP | 0.016 | 1.15 | 0.060 | 1.45 |
| 2-FPP | 0.023 | 1.40 | 0.100 | 1.72 |
| BZP | 0.090 | 1.10 | 0.090 | 1.10 |
| 4-FPP | 0.024 | 1.15 | 0.100 | 1.46 |
| 3-TFMPP | 0.090 | 1.38 | 0.340 | 1.50 |
| MDMA | 0.040 | 1.31 | 0.040 | 1.17 |
| 3-FPP | 0.017 | 1.23 | 0.310 | 1.41 |
| 4-TFMPP | 0.020 | 0.83 | 0.140 | 1.25 |
| MBZP | 0.010 | 1.43 | 0.070 | 1.24 |
| 4-MePP | 0.003 | 0.62 | 0.500 | 1.41 |
| 3-CPP | 0.037 | 1.90 | 0.230 | 1.73 |
| Caffeine | 0.160 | 1.70 | 0.230 | 1.69 |
| Dextromethorphan | 0.020 | 1.48 | 0.080 | 1.28 |
| DBZP | 0.012 | 1.47 | 0.140 | 1.25 |
| Cocaine | 0.010 | 1.31 | 0.660 | 1.40 |
| Diazepam | 0.130 | 1.32 | 0.320 | 1.53 |
| Dapoxetine | 0.018 | 1.88 | 0.220 | 1.73 |
Linearity, limit of detection and limit of quantification
The linearity and detection limit data are presented in Table 4. The method provides the required sensitivity (ng range) and linearity (generally ranging from 1.0–40.0 ng free base on column) that the drugs in street samples can be quantified. Comparison of the validation results to the work of other researchers can only be limited as research on these drugs is rather limited especially for 4-FPP. However, a study was undertaken for the determination of piperazines by GC-MS.25 The study investigated BZP, 3-CPP, MBZP, 4-MPP and 3-TFMPP. The LOD's obtained were in the range 2.5–5.0 μg mL−1 (also stated as mg ml−1). Both their method and the one under study can be applied for qualitative and quantitative investigation of street drugs.| Substance | Linearity (ng free base on column) | Method detection limits (ng mL−1 free base on column) | ||
|---|---|---|---|---|
| Linearity range | Working range | LOD | LOQ | |
| Methamphetamine | 1.0–33.3 | 4.2–33.3 | 0.69 ± 0.040 | 2.10 ± 0.122 |
| Nicotinamide | 2.1–33.4 | 5.0–33.4 | 0.32 ± 0.016 | 0.98 ± 0.048 |
| 2-TFMPP | 2.5–40.0 | 3.8–40.0 | 0.97 ± 0.044 | 2.93 ± 0.133 |
| 2-FPP | 2.6–42.1 | 3.9–31.6 | 0.82 ± 0.037 | 2.48 ± 0.113 |
| BZP | 2.6–30.7 | 3.8–30.7 | 0.28 ± 0.014 | 0.86 ± 0.043 |
| 4-FPP | 1.3–41.7 | 3.9–41.7 | 0.36 ± 0.022 | 1.09 ± 0.065 |
| 3-TFMPP | 3.3–52.1 | 4.9–39.1 | 0.85 ± 0.040 | 2.58 ± 0.120 |
| MDMA | 1.0–16.2 | 2.0–16.2 | 0.26 ± 0.015 | 0.77 ± 0.069 |
| 3-FPP | 4.6–49.5 | 6.2–37.1 | 0.93 ± 0.049 | 2.79 ± 0.150 |
| 4-TFMPP | 3.8–40.8 | 7.7–30.6 | 1.16 ± 0.10 | 3.50 ± 0.317 |
| MBZP | 3.1–33.4 | 4.2–33.4 | 0.83 ± 0.046 | 2.54 ± 0.140 |
| 4-MePP | 3.8–40.0 | 6.0–40.0 | 1.01 ± 0.008 | 3.06 ± 0.023 |
| 3-CPP | 2.9–47.1 | 5.9–47.1 | 1.18 ± 0.059 | 3.58 ± 0.179 |
| Caffeine | 3.8–30.0 | 5.0–30.0 | 1.10 ± 0.13 | 3.35 ± 0.40 |
| Dextromethorphan | 2.0–31.4 | 2.5–31.4 | 0.52 ± 0.003 | 1.5 ± 0.010 |
| DBZP | 1.0–32.0 | 4.0–32.0 | 0.45 ± 0.005 | 1.36 ± 0.015 |
| Cocaine | 3.7–39.3 | 6.0–29.5 | 1.74 ± 0.11 | 5.26 ± 0.340 |
| Diazepam | 5.0–30.0 | 7.5–30.0 | 1.95 ± 0.035 | 5.90 ± 0.105 |
| Dapoxetine | 2.3–37.5 | 7.0–28.1 | 1.62 ± 0.062 | 4.90 ± 0.189 |
Application to the analysis of street samples
The method described above has been applied to street samples made available from Cambridgeshire Constabulary. These samples were genuine street samples for which the trial proceedings had been concluded. For these examples the piperazines occurred as mixtures and 3-TFMPP was common to all samples. The drug content observed are in line with those reported in the literature for UK seizures.11 It is interesting to note that where the piperazine concentration is low (sample A10) the caffeine concentration is considerably elevated presumably in an attempt to mask the low concentration of the piperazine drug. In this sample the drug content as a percentage of the mass of the table was ephedrine (10%), 3-TFMPP (2%) and caffeine (28%). It is also interesting to observe that the presence of BZP in the sample is always accompanied by the presence of DBZP. This artefact arises as a product of the reaction of BZP with benzyl chloride. Benzyl chloride and piperazine are the starting reagents for the one pot synthesis of BZP and the DBZP occurs when benzyl chloride is in excess by reaction with BZP. The GC-MS method can also be used to confirm the absence of controlled substances as illustrated for sample A6 (Table 5).Conclusion
A method has been described which separates piperazines and other drugs commonly found in street samples of piperazines and amphetamine type stimulants. The validated method achieves the necessary sensitivity and linear dynamic range to carry out chemical characterisation of the drugs being examined. On application to recent street samples in the Cambridgeshire area the most common piperazines were found to be BZP and 3-TFMPP. This is the first time that the isomers of FPP and TFMPP have been separated along with other drug congeners.References
- United Nations Office on Drugs and Crime (UNODC) (2013) New Psychoactive Substances, http://www.unodc.org/documents/scientific/NPS_2013_SMART.PDF, accessed 5th September 2013.
- European Monitoring Centre for Drugs and Drug addiction (EMCDDA), BZP and other Piperazines, 2009, www.emcdda.europa.eu/publications/drug-profiles/bzp, accessed 5th September 2013.
- European Monitoring Centre for Drugs and Drug addiction (EMCDDA), The state of the drugs problem in Europe, EMCDDA Annual report 2011, http://www.emcdda.europa.eu/attachements.cfm/att_143743_EN_EMCDDA_AR2011_EN.pdf, accessed 5th September 2013.
- S. Elliott and C. Smith, J. Anal. Toxicol., 2008, 32, 172–177 CrossRef CAS PubMed.
- R. F. Staack and H. H. Maurer, Curr. Drug Metab., 2005, 6, 259–274 CrossRef CAS PubMed.
- C. W Yeap, C. K. Bianb and A. F. L. Abdullaha, J. Environ. Health, 2010, 1, 38–50 Search PubMed.
- M. D. Arbo and M. L. Bastos, Piperazine compounds as drugs of abuse, drug and alcohol dependence, 2011, http://www.sciencedirect.com/science/article/pii/S0376871611004431, accessed 5th September 2013 Search PubMed.
- A. R. Winstock and J. D. Ramsey, Addiction, 2010, 105, 1685–1687 CrossRef PubMed.
- R. F. Staack, Lancet, 2007, 369, 1411–1413 CrossRef.
- Advisory Council on the Misuse of Drugs (ACMD), Consideration of the Novel Psychoactive Substances (Legal Highs), 2011, http://www.homeoffice.gov.uk/publications/agencies-public-bodies/acmd1/acmdnps2011?view=Binary, accessed 5th September 2013.
- S. P. Vorce, J. M. Holler, B. Levine and M. R. Past, J. Anal. Toxicol., 2008, 32, 444–450 CrossRef CAS PubMed.
- S. Kenyon, J. Button, J. Ramsey and W. H. David, Legal highs – analysis of tablets and capsules containing piperazines, 2010, http://www.iatdmct.org/images/File/young%20scientists/PiperazineTabletsCapsules_LTG_2006_SKenyon_Final.pdf, accessed 5th September 2013 Search PubMed.
- U.S. Department of Justice Drug Enforcement Administration (DEA), 2009 Microgram Bulletin 42(12).
- S. Yuk, Combating illegal foods and drugs containing unauthorized substances, 4th seminar of European Customs Chemists, Finland, 2010, http://www.tulli.fi/en/secc/material/2ndJune2010/02.06.10_OpeningPlenary_04_Yuk.pdf, accessed 5th September 2013 Search PubMed.
- S. Davies, D. M. Wood, G. Smith, J. Button, J. Ramsey and R. Archer, J. Med., 2010, 103, 489–493 CAS.
- R. F. Staack, G. Fritschi and H. H. Maurer, J. Mass Spectrom., 2003, 38, 971–981 CrossRef CAS PubMed.
- Advisory Council on the Misuse of Drugs (ACMD), Consideration of the Novel Psychoactive Substances (Legal Highs), 2011, http://www.homeoffice.gov.uk/publications/agencies-public-bodies/acmd1/acmdnps2011?view=Binary, accessed 5th September 2013.
- T. Inoue, T. I. Yuko and K. Kuwayama, J. Health Sci., 2008, 54, 615 CrossRef.
- M. Takahashi, M. Nagashima, J. Suzuki, T. Seto, I. Yasuda and T. Yoshida, Talanta, 2009, 77, 1245–1272 CrossRef CAS PubMed.
- M. Thompson, Is my calibration linear? Technical Brief no. 3, Analytical Methods Committee (AMC), Royal Society of Chemistry, 2005, http://www.rsc.org/amc, accessed 5th Septemebr 2013 Search PubMed.
- M. Thompson, S. L. R. Ellison and R. Wood, Pure Appl. Chem., 2002, 74, 835–855 CrossRef CAS.
- International Conference on Harmonisation (ICH), ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text And Methodology, Q2(R1), 2005, http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html, accessed 5th September 2013.
- Center for Drug Evaluation and Research (CDER), Reviewer Guidance: Validation of Chromatographic Methods, 2004, http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM134409.pdf, accessed 5th September 2013.
- C. Kuleya, The forensic analysis of piperazines, Ph.D. thesis, Anglia Ruskin University, 2013 Search PubMed.
- B. Bryska, D. Zuba and R. Stanaszek, Problems in Forensic Science, 2010, 81, pp. 101–113 Search PubMed.
| This journal is © The Royal Society of Chemistry 2014 |