Resonant waveguide grating (RWG): overcoming the problem of angular sensitivity by conical, broad-band illumination for fluorescence measurements†
Tarmo
Nuutinen
*a,
Petri
Karvinen
b,
Jussi
Rahomäki
c and
Pasi
Vahimaa
c
aDepartment of Biology, University of Eastern Finland, PO Box 111, 80101, Joensuu, Finland. E-mail: tarmo.nuutinen@uef.fi
bPaul Scherrer Institute, Laboratory for Micro- and Nanotechnology, Villigen, Switzerland
cDepartment of Physics and Mathematics, University of Eastern Finland, Joensuu, Finland
First published on 12th October 2012
Abstract
Most biomedical applications are based on the light–matter interaction; the measurement of absorption, scattering or fluorescence at the visible and near visible region of the electromagnetic spectrum. This can be enhanced with nanophotonical devices. Most often, metallic nanoparticles are used for this purpose. However, metallic – as well as non-metallic – nanostructures have their limitations. In this report we introduce an all-dielectric structure, namely resonant waveguide grating (RWG), which can respond to the demands of optical enhancement of measurements. RWG's are, however, notorious for their angular sensitivity, which can be problematic, particularly in terms of enhancing fluorescence. We introduce a solution to this problem, which could enable RWG's to be harnessed for the benefit of cost-efficient and sensitive fluorescence measurements in the field of life sciences. This report represents a 30-fold fluorescence enhancement using RWG within a conventional fluorescence microscope and the theoretical calculations support our idea.
Introduction
Recent developments in nano-optical structures for life sciences most often make use of metallic nanostructures. The most well-known example of this is probably Surface Enhanced Raman Spectroscopy (SERS), in which the enhancement of Raman scattering is based on excited surface plasmons on metallic nanostructures.1 The enhancement of fluorescence with metal structures is known as well.1 Typically, both cases make use of nanoparticles which are subjected to a non-metallic surface. This can, however, be problematic in terms of application due to the chemical heterogeneity of the surface. Furthermore, the metals need to be stabilized if they are not used immediately, since oxidation can result in a change or even loss in the optical and chemical function. In our previous study, we have shown up to a 530-fold enhancement of fluorescence with RWG when using a laser beam illumination.2 To our knowledge, such an enhancement factor has not yet been achieved in fluorescence measurements utilizing metallic nanostructures. These points make all-dielectric structures interesting in terms of further development and applications.However, RWG structures have certain properties which limit their employment in the field of biosciences. Most importantly, the function of RWG is highly dependent on the angle of incidence, the wavelength and the polarization of the incident light.3 This can, in some cases, be harnessed for analytics; surface bound molecules (analyte) can change the refractive index of the surface layer of RWG, which in turn can alter the angle where maximum reflection occurs – alternatively, the changes in the reflection spectrum can be measured when using a tunable laser with a constant angle of incidence4,5 or broadband illumination.6 Unfortunately, reflection of light provides little or no qualitative information in the case of complex samples (changes in the refractive index can be due to numerous different reasons) and hence it is suitable for specific assays only. Furthermore, when ultrasensitive detection of fluorescence is desired, such angular sensitivity can be problematic; a small change in the angle can result in a high loss in detected fluorescence; only a 0.5° change in the angle of incoming light can cause more than a 10-fold loss in fluorescence gain.2,7 In addition to the higher costs of light sources and the necessity of specialized equipment, the problem of angular sensitivity is probably one of the major reasons RWG is yet to be exploited in fluorescence-based medical tests or in lab-on-chip applications.
In this report we show how the weaknesses of RWG-based measurements can be concurred by simple innovation. By changing monochromatic illumination to broadband and non-collimated (conical) illumination it is possible to overcome the problem of angular sensitivity. This enables the practical use of other interesting optical properties of RWG in fluorescence measurements of biological samples – including RWG's function as a “build-in optical filter” and its capability to beam the emission to a certain angular space (e.g. to a detector). To demonstrate the effectiveness of our invention, we show how the label free detection of a protein is possible even in a conventional fluorescence microscope.
Results and discussion
Our set-up is shown in Fig. 1 and the actual microscope images are shown in Fig. 2a–c. To test the optical function and enhancement of EGFP (Enhanced Green Fluorescent Protein) fluorescence in a microscope, RWG with a 250 nm grating period was fabricated, as was done in the previous study.7 Shortly, the surface patterns were generated with an electron beam (Ebeam EBG5000 + ES HR) on a poly(methyl methacrylate) (PMMA) layer as a positive resist on the SiO2 substrate followed by the Cr lift-off, reactive ion etching (RIE) and thermal evaporation of the TiO2 layer. As the control, gratings incapable to support waveguide-mode resonance but having the same surface topography and the same surface chemistry were fabricated. EGFP was allowed to adsorb passively and non-specifically to the surfaces and the excess protein was washed off. The surface-bound protein fraction was illuminated and imaged using an epifluorescence microscope (Zeiss Axioplan 2) (Fig. 2a) with a 40× objective with 0.75 numeric aperture (NA) and suitable filters for the HBO lamp excitation and emission path. We measured approximately a 30-fold higher fluorescence from the grating surface when compared to the flat surface (Fig. 2d). To test if this was due to higher adsorption, a control grating with the same topography but without the capability to support waveguide-mode resonance was measured. In this case, only a 1.2–1.5-fold increase in signal was recorded in comparison with the unstructured surface (Fig. 2c).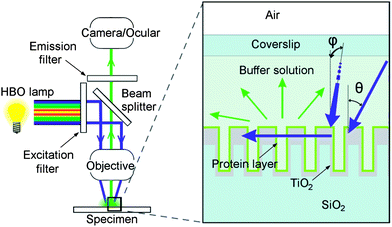 | ||
| Fig. 1 Schematic presentation of the present study. On the left, a fluorescence microscope is presented. With changeable filter sets, the light from the arc-discharge mercury lamp (HBO) can be filtered. Both excitation and emission bands travel through focusing optics. On the right, two blue arrows represent the incoming light with two variable conical angles θ and φ (the latter one incomes from behind the image plane and hence parallel to grating lines), while the horizontal blue arrow represents the light in-coupled to the grating structure (cross-section). While travelling inside the grating it excites fluorescent proteins (protein layer on the top of the structure) which then emit green light (green arrows). | ||
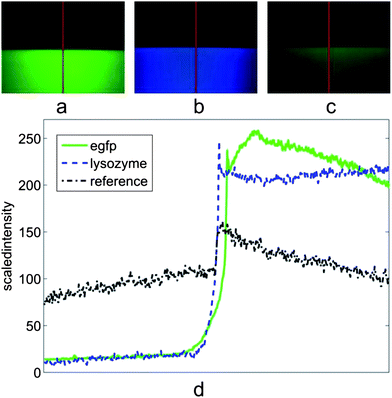 | ||
| Fig. 2 Actual fluorescence microscope images and local emission intensities. (a) Surface with bound EGFP protein layer, lower part with RWG structure and upper without RWG. (RWG with 250 nm period, filters: band-pass 450–490 nm for excitation, high-pass 515 nm for the emission and 510 nm beam splitter i.e. filter set 09 from Zeiss.) (b) Similar to (a) but the surface is covered with a lysozyme protein layer. (RWG with 200 nm period, filters: 365/12 nm excitation band, 397 nm long pass for the emission and 395 nm beam splitter, filter set 01, Zeiss.) (c) Control structure with the same topography as in (a) but with no optical functionality (lower part) and the surface without any structure (upper part). (d) The scaled intensity of sections (red lines) in (a)–(c). (a–c) 40× objective with 0.75 numeric aperture (NA). | ||
To test the detection of protein lacking highly fluorescent chromophores typical of the fluorescent proteins, a grating operating at the UV region was designed and manufactured. This grating was manufactured using the same processes but by having a grating period of only 200 nm, which was then covered similarly with the protein lysozyme instead of EGFP (by passive adsorption from 1% lysozyme at pH 8.00). The sample was subjected to the same set-up, but this time the filter set 01 (Zeiss) was employed (365/12 nm excitation band and 397 nm long pass for the emission with 395 nm beam splitter). Without the grating structure, we could not record a visible signal with “reasonable” exposure times (<10 s) from a flat surface. However, on the RWG we did found a clear blue colour originating from the lysozyme even with reasonable exposure times (∼1 s). This occurs in spite of the fact that amino acid residues mainly responsible for the lysozyme fluorescence (tryptophan, tyrosine and phenylalanine) barely adsorb at the wavelengths used in this experiment (see ESI† for details). After subtracting the average background noise from the picture (Fig. 2c) it can be estimated that again roughly a 27-fold increase in the signal gain was reached (Fig. 2d). This is considerably a high value, because in an analogous study, but with metallic structures, a 2–3-fold enhancement was reached.8
For the theoretical part, enhancement was studied by calculating the reflection efficiency in different angular, spectral and polarization states of incoming light in relation to the numeric aperture of the objective (Fig. 3 and 4). The reflection efficiency correlates with the amount of in-coupled light and hence with the intensity of the excitation field and also with the amount of emitted fluorescence. More precisely, the reflection efficiency correlates with the redistributed intensity of the excitation field inside and within near-proximity of the grating structure due to the nature of the leaky-mode phenomena where only the 0th orders propagate and higher orders excite purely evanescent modes. Further, these evanescent modes interfere constructively and destructively with the 0th reflected and transmitted orders, optimally leading to 100% reflection of incident light. Under this condition, maximum fluorescence excitation is reached as well. In these simulations, RWG's ability to enhance the excitation field was estimated by calculating the reflection efficiency with large angular space (±50° to ±50°) and using monochromatic light (in Fig. 3, RWG is “illuminated” with 470 nm wavelength). To estimate the reflection efficiency with broader band 3, 40 similar calculations were performed over the band, with 1 nm steps, and then averaged; this results in 0.05–0.2 reflection efficiency on average depending on the combination and on the direction of polarization in relation to the grating structure (Fig. 4). Interestingly, it seems that by using TE (Transverse Electric) polarization, slightly higher reflection efficiency could be reached on average than by using TM (Transverse Magnetic) or a combination of both (Fig. 4). However, for TE and TM the location of maximum field intensities inside the grating structure is different, and hence the actual enhancement factors for the two polarization states might be again different. At this point, it should also be reminded that in the case of set-up for lysozyme, there is a strong peak in the mercury spectrum at 365 nm from the lamp and the bandwidth (of the excitation filter) is now only 12 nm. The optical function can be something between those seen in Fig. 3 and 4. Nevertheless, a similar enhancement factor was again reached. For this reason, the most crucial factor for the efficiency of our invention may be the angular space of incoming light (and the collection of emission with appropriate angles). And indeed, the enhancement factor varies slightly depending on the objective employed, its magnification and NA, and on the polarization state as well (unpublished).
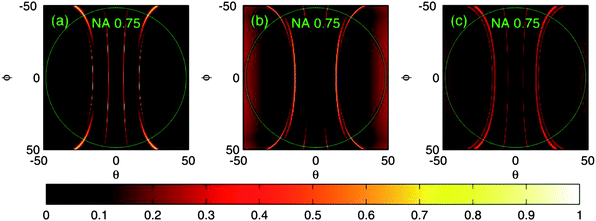 | ||
| Fig. 3 Reflection efficiencies as a function of conical incident angles θ and φ when using monochromatic light for illumination. Incident light has a wavelength of 470 nm. (a) TM-polarization, (b) TE-polarization and (c) both TM and TE together. The green circles correspond to the angular limits for the objective with a NA of 0.75. | ||
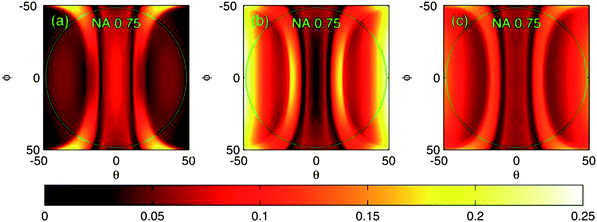 | ||
| Fig. 4 Reflection efficiencies as a function of conical incident angles θ and φ when using broadband (40 nm) illumination. The reflection efficiencies have been averaged over the wavelength band of 450–490 nm. (a) TM-polarization, (b) TE-polarization and (c) both TM and TE together. | ||
It should be borne in mind that fluorescence emission is partly in-coupled to the grating as well and depending on its wavelength and polarization it is out-coupled in a certain angle.7 This “beaming effect” might be notable when using imaging optics. This effect can also improve the signal to noise ratio, because all background noise does not couple to the grating to the same extent, for example the background noise with either a much longer or shorter wavelength. Such build-in filtering could be beneficial especially in lab-on-chip applications and in multi-colour measurements. In addition to new grating designs, possible future directions could include the integration of the light source (e.g. light-emitting diode, LED) into a chip/RWG. The possibility to mass-produce RWG structures is noteworthy and thus makes their exploitation in biomedical purposes attractive.
Conclusions
We have shown in practise how RWG could be used in enhancing biological measurements using a conventional light source even in the case of an unlabelled sample. This was demonstrated with lysozyme which lacks highly fluorescent chromophores and hence is impossible to detect in our set-up without the aid of our RWG-based optical enhancement. We have also shown that theoretical calculation supports this idea. Furthermore, this study implicates that any light sources (e.g. LED) with a bandwidth wider than that of lasers could be exploited as well. Our technical innovation implicates that using cost-effective and simple illumination and imaging or detecting and also integration of very low cost auxiliaries into a chip is possible for high throughput fluorescent measurements.Notes and references
- J. N. Anker, W. P. Hall, O. Lyandres, N. C. Shah, J. Zhao and R. P. Van Duyne, Nat. Mater., 2008, 7, 442 CrossRef CAS.
- P. Karvinen, T. Nuutinen, J. Rahomäki, O. Hyvärinen and P. Vahimaa, Opt. Lett., 2009, 34, 3208 CrossRef.
- S. S. Wang and R. Magnusson, Appl. Opt., 1993, 32, 2606 CrossRef CAS.
- J. J. Wang, L. Chen, S. Kwan, F. Liu and X. Deng, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.–Process., Meas., Phenom., 2006, 26, 3006 Search PubMed.
- A. M. Ferrie, Q. Wu and Y. Fang, Appl. Phys. Lett., 2010, 97, 223704 CrossRef.
- Y. Fang, Sensors, 2007, 7, 2316 CrossRef CAS.
- P. Karvinen, T. Nuutinen, O. Hyvärinen and P. Vahimaa, Opt. Express, 2008, 16, 16364 CrossRef.
- H. Szmacinski, K. Ray and J. R. Lakowicz, Anal. Biochem., 2009, 385, 358 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ay25470k |
| This journal is © The Royal Society of Chemistry 2013 |
