DOI:
10.1039/C2AN35226E
(Paper)
Analyst, 2013,
138, 111-117
Multiplex flow cytometric immunoassay for serum biomarker profiling of recombinant bovine somatotropin
Received
17th February 2012
, Accepted 27th June 2012
First published on 28th June 2012
Abstract
Recombinant bovine somatotropin (rbST) is licensed for enhancing milk production in dairy cows in some countries, for instance the United States, but is banned in Europe. Serum biomarker profiling can be an adequate approach to discriminate between treated and untreated groups. In this study a multiplex screening tool of a small set of biomarkers for pinpointing recombinant bovine somatotropin (rbST) (ab)use was developed and evaluated: insulin-like growth factor 1 (IGF-1), IGF binding protein 2 (IGFBP2) and rbST-induced antibodies were selected as rbST dependent markers and combined in one parallel assay format. For this, the color-encoded microspheres were used in a suspension array, with a dedicated flow cytometer. Serum samples obtained from an animal experiment with rbST-treated and untreated dairy cows were measured with the developed triplex immunoassay and biomarker responses on rbST treatment were evaluated. This resulted in characteristic treatment-dependent responses for all three individual biomarkers. Combining these results with the statistical prediction model k-nearest neighbours (kNN), resulted in good discrimination of treated and untreated animals: an overall sensitivity (true positive rate) of 89.1% and an overall specificity (true negative rate) of 97.7% were reached. Therefore, this is the first multiplex method which can be applied with high confidence for screening of unknown herds of cattle pinpointing at rbST (ab)use.
1 Introduction
Recombinant bovine somatotropin (rbST) can be used to enhance growth and lactating performance in cattle. Within the EU, rbST is banned since 2000,1 therefore, routine screening methods are urgently needed. A liquid chromatography-mass spectrometry (LC/MS) method for direct rbST detection in blood samples was developed,2 however it showed a small detection window, due to the short half-life of rbST in blood.3 Moreover, the similarity with the endogenous hormone (bST, also called growth hormone), the low concentrations of bST and rbST in serum, and strong fluctuations of bST hamper the direct detection. Therefore, detection of rbST-dependent biomarkers having a longer half-life offers a promising alternative, as already reported for steroid abuse and in sports doping.4–8 As rbST strongly influences the growth hormone/insulin like growth factor I (GH/IGF-I) axis, the following biomarkers are considered as indicative for administration of rbST: insulin-like growth factor-1 (IGF-1) and its binding proteins IGFBP2 and 3, rbST-induced antibodies, and several markers of bone and collagen turnover.9–12 So far, immunoassays detecting single rbST related biomarkers were developed on different platforms like radio immunoassays (RIA),9,13,14 enzyme linked immunosorbent assays (ELISA),15–19 western blot techniques (WB)20,21 and flow cytometric immunoassays (FCIA).22–24 Looking at single biomarkers, by using the above mentioned techniques, an indication of potential rbST abuse might be obtained. However, a much more powerful screening tool can be designed by combining multiple biomarkers into a multiplex assay format. The advantage of biomarker screening in the serum of dairy cows using flow cytometry in comparison with surface plasmon resonance (SPR) based techniques was demonstrated recently.22 Using color encoded microspheres in a suspension array format, in theory, 100 different analytes can be detected simultaneously with high throughput in minimal sample volume. In this study we evaluated the suitability of this technique for multiplex detection of rbST related biomarkers: IGF-1, its binding protein IGFBP2 and rbST-induced antibodies. This set was selected from the literature as it includes two biomarkers with a quick response upon rbST treatment (IGF-I and IGFBP2) and one with a long half-life (rbST-induced antibodies), i.e. together offering the possibility of a prolonged detection window. The development of a biomarker-based method for rbST (ab)use required the analysis of a large population of untreated cow samples to determine endogenous background levels, and the biological variation of each biomarker. Decision limits were then established. Next, the applicability of the developed triplex assay was demonstrated with serum samples from rbST-treated and untreated cows. Using the statistical prediction tool k-nearest neighbours (kNN), the origin of serum samples, treated or untreated, was predicted based on single biomarker analysis and combined biomarker analysis. Finally, both biomarker analysis approaches were compared on their capabilities for pinpointing rbST (ab)use in dairy cattle.
2 Experimental
2.1 Materials and instruments
Posilac® 500 mg single-dose syringes and syringes with only the slow-release formula were purchased from Monsanto Company (St Louis, MO). Hydrochloric acid, potassium phosphate, sodium azide, sodium chloride, sodium hydroxide, sodium phosphate, Tween-20 and the ultrasonic cleaner were purchased from VWR International (Amsterdam, The Netherlands) and glycine was from Duchefa (Haarlem, The Netherlands). The N-hydroxysulfosuccinimide sodium salt (sulfo-NHS) was supplied by Fluka (Steinheim, Switzerland) and sodium dodecyl sulfate (SDS) by Serva (Heidelberg, Germany). Bovine serum albumin (BSA), insulin-like growth factor-I (IGF-I; human recombinant), 2-(N-morpholino)ethanesulfonic acid (MES hydrate) and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) were purchased from Sigma-Aldrich Chemie (Zwijndrecht, The Netherlands). Monsanto rbST standard was obtained from the National Hormone & Peptide Program (NHPP) of Dr Parlow (Torrance, CA). Insulin like growth factor binding protein-2 (IGFBP2; bovine recombinant) was purchased from IBT (Reutlingen, Germany). Mouse anti-IGF-1 was supplied by Spring Bioscience (clone SPM406, Fremont, CA) and the rabbit anti-IGFBP2 was from USBiological (Swampscott, MA). R-Phycoerythrin (PE)-labeled goat anti-bovine immunoglobulins (GAB-PE) were from Santa Cruz Biotechnology (Santa Cruz, CA) and R-phycoerythrin (PE)-labeled goat anti-mouse immunoglobulins (GAM-PE) and goat anti-rabbit immunoglobulins (GAR-PE) were purchased from Prozyme (San Leandro, CA). MultiScreen HTS filter plates were purchased from Millipore (Amsterdam, The Netherlands). Protein LoBind Tubes (1.5 mL) and a table centrifuge model 5810R were supplied by Eppendorf (Hamburg, Germany). The Luminex 100 IS 2.2 system consisting of a Luminex 100 analyzer and a Luminex XY platform programmed to analyze a 96-well plate was purchased from Applied Cytometry Systems (ACS, Dinnington, Sheffield, South Yorkshire, UK). SeroMAP microspheres (sets 025, 050 and 078) and sheath fluid were purchased from Luminex (Austin, TX). The Snijder test tube rotator was purchased from Omnilabo International (Breda, The Netherlands). The microtiter vari-shaker was purchased from Dynatech (Guernsey, UK).
2.2 Sample materials
Eight 5 year old Holstein dairy cows were divided into two groups. After two weeks adaptation, the treatment given consisted of subcutaneous injections of 500 mg rbST in a slow-release formula for the first group (a–d, referred to as rbST-treated) and the slow-release formula only for the second group (e–h, referred to as untreated). The cows were injected four times at two week intervals and subsequently twice with a one week interval. During the two week adaptation period, blood samples were collected weekly. During the treatment period blood samples were collected a day before, a day after and a week after injection and after the last injection blood samples were collected weekly for four more weeks. Unfortunately, one untreated cow (denoted e) died in the beginning of the animal experiment, due to swollen hocks, which led to general inflammation and sepsis. Therefore, in this experiment results could be obtained only for 4 treated and 3 untreated cows. The experimental procedure was authorized by the ethical committee of the Faculty of Veterinary Medicine, Ghent University.
In addition, blood samples were taken from 20 healthy, lactating cows varying in the age range of two to five years, in different stages of their lactating cycle, to reflect a normal population of untreated dairy cows. Based on the origin of these cows, the assumption of being untreated with rbST was justified.
After blood collection, all blood samples were placed at room temperature for 4 h to coagulate. Then, samples were centrifuged for 10 min at 3000g, and serum samples were collected and stored at −80 °C until further use.
2.3 Pretreatment of serum samples
For the generic flow cytometric immunoassay (FCIA) sample preparation procedure,22,24 serum samples were pretreated by adding 25 μL glycine solution (27.5 mM glycine pH 0.5 (pH adjusted by addition of HCl)) to 25 μL of serum sample or standard solution in a polypropylene tube under constant vortexing. Samples were then incubated at room temperature for 60 min. After incubation, 50 μL glycine–SDS solution (400 mM glycine, 0.3% m/v SDS, pH 10 (pH adjusted by addition of NaOH)) was added under constant vortexing. Samples were further diluted with 0.1% BSA in PBST to a final dilution of 80 times. No further sample preparation was needed prior to the FCIA.
2.4 Microsphere preparation for the flow cytometric immunoassay (FCIA)
IGF-1, rbST standard and IGFBP2 were coupled to seroMAP microsphere sets 025, 050 and 078, respectively, according to Bremer et al.22 Briefly, for each microsphere set 2.5 × 106 microspheres were coupled with a two-step carbodiimide reaction using 500 μL of a 100 μg mL−1 protein solution in MES buffer for IGF-1 and rbST, and 500 μL of a 10 μg mL−1 protein solution in MES buffer for IGFBP2. After coupling, the microspheres were stored in a blocking buffer (PBS, 0.1% BSA, 0.02% Tween-20 and 0.05% NaN3) at 2–8 °C in the dark until use. Under these storing conditions, the microspheres were stable for more than one year.
2.5 FCIA procedure
Standards and sera were pretreated as described (Paragraph 2.3). One hundred μL of the pretreated and diluted serum samples or standard solutions were added to a filter bottom microtiter plate. Hereafter, a 10 μL antibody mixture containing 1500 times diluted anti-IGF-1 and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times diluted anti-IGFBP2 antibody was added and incubated for 15 min on a microtiter plate shaker. Then, microspheres (10 μL diluted suspension containing about 1250 microspheres per microsphere set) were added to each well and incubated for 1 h on a microtiter plate shaker. After incubation, the plate was centrifuged (1 min at 130g) and the microspheres were washed with 200 μL PBST. After washing, a 125 μL PE-labeled antibody mixture containing 625 times diluted GAM-PE, 1000 times diluted GAR-PE and 1000 times diluted GAB-PE was added and incubated for 30 min on a microtiter plate shaker. After this incubation step, the plate was centrifuged and 125 μL of PBST was added per well. Then, the microspheres were detected, according to bead assay type and PE-label in the flow cytometer (1 μL s−1 was measured until 100 events per microsphere set were reached with a maximum of 50 μL per well).
000 times diluted anti-IGFBP2 antibody was added and incubated for 15 min on a microtiter plate shaker. Then, microspheres (10 μL diluted suspension containing about 1250 microspheres per microsphere set) were added to each well and incubated for 1 h on a microtiter plate shaker. After incubation, the plate was centrifuged (1 min at 130g) and the microspheres were washed with 200 μL PBST. After washing, a 125 μL PE-labeled antibody mixture containing 625 times diluted GAM-PE, 1000 times diluted GAR-PE and 1000 times diluted GAB-PE was added and incubated for 30 min on a microtiter plate shaker. After this incubation step, the plate was centrifuged and 125 μL of PBST was added per well. Then, the microspheres were detected, according to bead assay type and PE-label in the flow cytometer (1 μL s−1 was measured until 100 events per microsphere set were reached with a maximum of 50 μL per well).
2.6 In-house validation study of the developed FCIA
As an in-house validation study the intra- and inter-assay precision levels of the individual biomarkers in the triplex FCIA were assessed. The intra-assay variation was calculated by averaging the percentaged standard deviation of each sample obtained by median fluorescence intensity (MFI) signals of a 10 times repeated triplex FCIA on 8 serum samples obtained from 7 cows. The inter-assay variation was calculated by averaging the percentage standard deviation of each sample obtained by duplicate measurements of the same 8 sera on 9 different days, using B/B0 results for the IGF-1 and IGFBP2 assay and normalized values as described in the next paragraph for rbST-induced antibodies.
2.7 Signal normalization for rbST-induced antibodies
Due to the lack of standard for rbST-induced antibodies, the daily variations in the assay performance and technical performance of the Luminex 100 IS 2.2 system, a normalization step to enable in-between-days comparison of signals from rbST-induced antibodies is needed. As the 8 sera used for the in-house validation study were measured in every experiment, MFI signals of these 8 sera were used for normalization: responses of the 8 sera were averaged and responses of the measured samples were divided by that average.
2.8 Assessment of decision limits
To be able to discriminate between treated and untreated dairy cows, a decision limit is needed for each biomarker. For assessing the decision limits with most accuracy, sera from 27 untreated dairy cows (20 untreated cows and 7 from the animal experiment during their adaption period) were measured with the triplex assay. For IGF-1 concentrations were used for the calculations, for IGFBP2, B/B0 values were used due to the absence of a pure standard for obtaining a calibration curve, and for rbST-induced antibodies, MFIs were used. Concentrations, B/B0 values and MFIs were averaged and the standard deviations determined respectively. Decision limits with 95% confidence were calculated. Theoretically, IGF-1 concentrations increase due to rbST treatment,10,12,25 therefore, 2 times the standard deviation was added to the average IGF-1 concentration. IGFBP2 concentrations theoretically decrease due to rbST treatment,12 which results in less inhibition of maximum MFI signals (B0) and consequently in higher B/B0 values. Therefore 2 times the standard deviation was added to the average B/B0 value. RbST-induced antibodies could be formed due to rbST treatment,23,24 therefore 2 times the standard deviation was added to the average MFI signal.
2.9 Statistics
To assess whether the combination of the three analysed biomarkers is already capable to predict rbST abuse, a k-nearest neighbours prediction model in the R environment26 was used. B/B0 values for IGF-1 and IGFBP2 as well as MFI signals for rbST-induced antibodies for every sample from the animal experiment were included in the data analysis. First of all, the whole dataset was divided into a training and test set by using a stratified repeated random sub-sampling approach, which means that 70% of the rbST-treated and 70% of the untreated samples were chosen for the training set and the remaining 30% of both groups for the test set. Subsequently, B/B0 values and MFI signals of the training set were auto-scaled and a kNN model was built using the training dataset. The optimal number of k (1 ≤ k ≤ 10) was chosen based on the bootstrapping approach leaving out 10% of the training data (randomly with replacement), which was repeated 10 times27 and the resulting model was tested by predicting the remaining auto-scaled test set data. Correctly and falsely predicted results were evaluated carefully. To obtain an average performance of the kNN model, this procedure was repeated 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times; each time different randomly chosen training and test sets were applied and an overall sensitivity (true-positive rate), specificity (true-negative rate) and misclassification rate could be calculated for every sample, for every time point and for the whole animal experiment.
000 times; each time different randomly chosen training and test sets were applied and an overall sensitivity (true-positive rate), specificity (true-negative rate) and misclassification rate could be calculated for every sample, for every time point and for the whole animal experiment.
3 Results and discussion
3.1 Development of the triplex FCIA
For the development of the triplex screening assay, three single immunoassays were combined: the previously developed IGF-1 assay,22 the assay for rbST-induced antibodies24 and a newly developed IGFBP2 assay. Despite the fact that IGF-1 and IGFBP2 are indirect competitive assays and the assay for rbST-induced antibodies is an indirect assay, one single straightforward approach in terms of pretreatment and incubation times was feasible. Therefore, the color-encoded microspheres, primary antibodies and secondary antibodies from the individual assays were simply mixed (Fig. 1). This approach, however, led to an increase in the MFI signal of approximately 150% for serum samples measured in the multiplex IGF-1 and IGFBP2 assays, compared to the same samples analysed in singleplex format, while no increased signal was observed for the standard solution (B0). This phenomenon might be caused by serum antibodies that directly bind to the microspheres unspecifically,28 whereas the standard solution only consisted of a 80 mg mL−1 BSA in PBS. No influence of multiplexing was found on the detection of rbST-induced antibodies. To further investigate the source of the increased signal, the influence of the individual primary antibodies and the individual secondary antibodies in combination with or without primary antibodies on all three microsphere sets, was tested. This pointed to unspecific binding of PE-conjugated secondary antibodies to all three microsphere sets, in particular GAR-PE and GAB-PE, as the cause of the increased MFI signals. Therefore, to decrease this background, the PE-coupled secondary antibodies were diluted more until MFI signals were just above 1000 MFI for the blank standard. Thus the main modification of the triplex conditions versus the singleplex assays were secondary antibody dilutions for GAM-PE, GAR-PE and GAB-PE of 625, 1000 and 1000 times, respectively, instead of the former 625, 375 and 100 times dilutions. By doing so, the increase in MFI signals upon multiplexing became less than 2%.
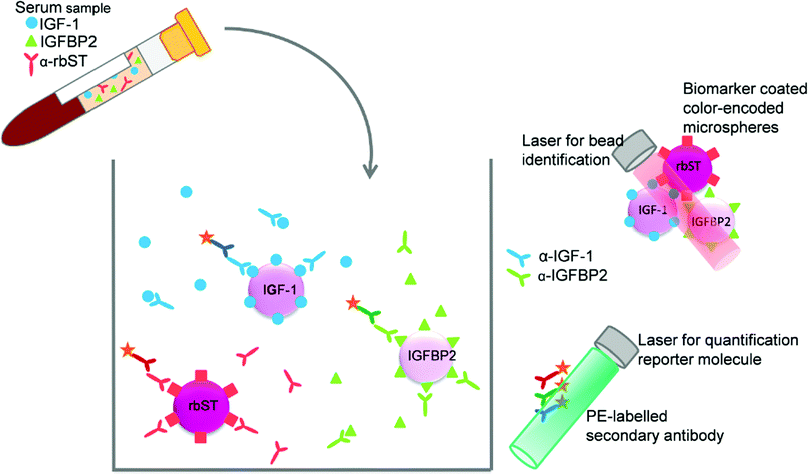 |
| | Fig. 1 Triplex assay format, the indirect competitive format for IGF-1 and IGFBP2 and indirect format for antibodies formed against rbST, all combined within one well. | |
3.2 In-house validation study of the developed triplex FCIA
For all three assays, high repeatability in both intra-assay and inter-assay variations were assessed. Average coefficients of variation of 5.4%, 5.4% and 6.5% in the intra-assay variation and 5.3%, 4.3% and 7.5% in the inter-assay variation for the detection of IGF-1, IGFBP2 and rbST-induced antibodies respectively were found. This is in very good agreement with the formerly found variations in the IGF-1 singleplex assay.22
3.3 Triplex FCIA applicability to real samples
As the ultimate goal is to detect rbST abuse with a biomarker-based method, the applicability of the triplex FCIA was tested.
3.3.1 Establishment of decision limits.
For determination of IGF-1 concentrations, a calibration curve was recorded and used to recalculate IGF-1 concentrations in sera of the 27 untreated cows (data not shown). Average IGF-1 concentrations of 94 ± 21 ng mL−1 were found, resulting in an IGF-1 decision limit of 136 ng mL−1. As no recombinant IGFBP2 suitable for obtaining a good calibration curve was available, a decision limit was determined on MFI signals normalized on the maximum MFI signal (B0). For IGFBP2, this resulted in a B/B0 of 0.43 ± 0.04 resulting in an IGFBP2 decision limit of 0.51. For the decision limit of rbST-induced antibodies, an average MFI signal of 193 ± 37 was found resulting in a decision limit of 266 MFI.
For evaluating the applicability of the assays, sera of the animal experiment (4 rbST-treated and 3 untreated dairy cows) were analysed and results were compared to the decision limits as shown in Fig. 2. As expected, for IGF-1 and IGFBP2, a rapid upcoming and decaying response, and for rbST-induced antibodies a response with a long half-life were observed. All three biomarkers showed specific characteristics in their response upon rbST treatment, together offering a wide detection window with great potential.
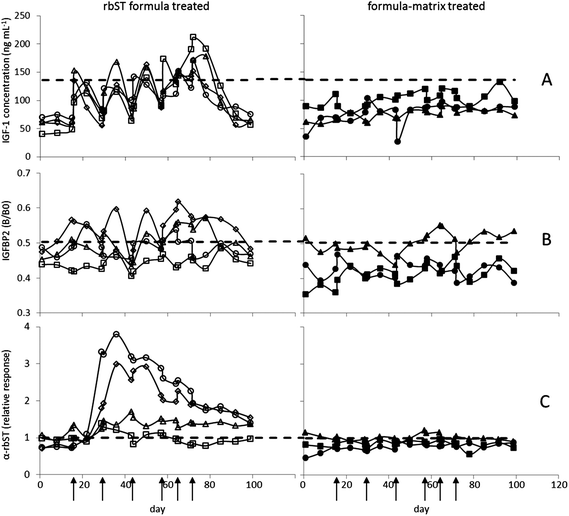 |
| | Fig. 2 Effect of rbST treatment on serum biomarker levels of dairy cows in time. Time points of treatment (rbST-formula or matrix-formula) are marked by arrows. IGF-1 concentrations (A), IGFBP2 B/B0 values (B) and normalized signals of rbST-induced antibodies (C) are shown for rbST treated animals a (◊), b (□), c (○) and d (Δ), and untreated animals f (■), g (▲) and h (●). | |
3.3.2 Biomarker IGF-1.
Following rbST treatment, IGF-1 concentrations were elevated in all four treated cows (Fig. 2A). This elevation was, however, only for a short period of time in all treated animals. Serum samples taken on the first day after the first treatment already showed an increase in IGF-1 concentration with the highest IGF-1 concentrations seen in sera taken one week after treatment. Then, concentrations declined towards the initial concentration as it can be seen in sera taken two weeks after treatment. In human serum this increase was also observed, however, only for two days after GH treatment,29 whereas similar responses were determined in lactating cows before.30 Although the increase in IGF-1 concentration is clearly seen in all 4 cows (a–d) after the last rbST treatment, only a part of the serum samples tested showed IGF-1 concentrations beyond the decision limit. Therefore, 37% of the serum samples were determined as true-positives and subsequently 63% were classified as false-negatives (Table 1). The untreated cows, as expected, showed no increase in IGF-1 concentration, leading to a true negative rate of 100%.
Table 1 Classification of serum samples from the animal experiment based on the single biomarker results in the triplex FCIA assay
| Biomarker |
Untreated |
rbST treated |
| True negative (%) |
False positive (%) |
True positive (%) |
False negative (%) |
| IGF-1 |
100 |
0 |
37 |
63 |
| IGFBP2 |
90 |
10 |
31 |
69 |
| α-bST |
86 |
14 |
80 |
20 |
3.3.3 Biomarker IGFBP2.
The rbST treatment was also reflected by the results from the IGFBP2 assay. As described in the literature, IGFBP2 concentrations decreased due to rbST treatment.9,31 Consequently, maximum MFI signals (B0) were inhibited less in sera of rbST-treated animals than in sera of the untreated animals yielding in general an increased B/B0 response due to the decreased IGFBP2 levels. The first and most pronounced IGFBP2 response upon treatment was found one week after the rbST treatment (Fig. 2B), i.e. later in time than for IGF-1. Two weeks after treatment IGFBP2 signals were back to original signals. In the literature, only responses to daily rbST injections were studied, resulting in a return to concentrations within 4 days after rbST treatment cessation.9 In this study however, responses to the slow release rbST formula were studied. The IGFBP2 assay showed responses upon rbST treatment in three out of the four rbST-treated cows (a, c and d), but only a part of the serum samples taken from treated animals showed a B/B0 higher than the decision limit. Therefore, 31% of the serum samples were classified true-positive, whereas subsequently 69% were tested as false-negative. The sera from untreated animals f and h, were all true negative; only some serum samples of the untreated animal g showed less inhibition, yielding a total of 90% true-negative samples and 10% false-positives (Table 1).
3.3.4 Biomarker rbST-induced antibodies.
Two weeks after the first rbST treatment, an increase in antibodies specific for rbST was clearly seen (Fig. 2C). This increase in rbST-induced antibodies was stable for a longer period of time, i.e. no MFI signal decline to start values in between rbST injections was seen. Even four weeks after the last rbST treatment, MFI signals for rbST-treated animals a, c and d were still beyond the decision limit, the maximum time point being investigated. This resulted in a true-positive rate of 80% and subsequently in a 20% false-negative rate. These results are in agreement with the literature17,32 where 70–80% of rbST treated cows responded with antibody production. The untreated cows, f and h tested negative and only for cow g some serum samples were found just above the decision limit. This resulted in a true-negative rate of 86% and a false-positive rate of 14% (Table 1).
3.3.5 Average classification of serum samples from (un)treated cows based on single and multiple biomarkers.
Summarizing, for IGF-1, IGFBP2 and rbST-induced antibodies respectively, on average 64%, 56% and 83% of the tested samples were classified correctly (Table 2). According to commission decision 2002/657/EC a 95% true positive rate is needed for screening assays. None of the single biomarkers on its own can pinpoint rbST abuse with that confidence. Combining results of the three biomarker assays, however, could increase the confidence rate for pinpointing rbST abuse. A statistical prediction model, the k-nearest neighbours (kNN) algorithm, was used to discriminate between rbST-treated and untreated animals. After building kNN models on all triplex serum sample data, an overall sensitivity (true-positive rate) of 89.1% and specificity (true-negative rate) of 97.7% were obtained (Table 3). Most of the false-negative results (10.9%) occurred two weeks after the beginning of the rbST treatment and three and four weeks after termination of the treatment. False-negative results at the beginning of the treatment period could be accounted to IGF-1 and IGFBP2 levels, which declined rapidly after injection, and the antibody titers, which did not increase that much after the first rbST treatment. After multiple treatments, as is to be expected in practice, the false-negative rate became lower. Three to four weeks after termination of the rbST treatment, the prediction power of the model diminished for the same reasons (Fig. 3). Overall a correct prediction of 93.6% was observed. Further improvements of this biomarker triplex screening method can be achieved by simply adding additional biomarkers.
Table 2 Average classification based on the single biomarker results
| Average prediction |
| Biomarker |
Classified correct (%) |
Classified incorrect (%) |
| IGF-1 |
64 |
36 |
| IGFBP2 |
56 |
44 |
| α-bST |
83 |
17 |
Table 3 Classification of serum samples based on all three biomarker results following kNN statistics
| |
kNN statistic prediction |
| Classified correct (%) |
Classified incorrect (%) |
| Untreated |
97.7 |
2.3 |
| rbST treated |
89.1 |
10.9 |
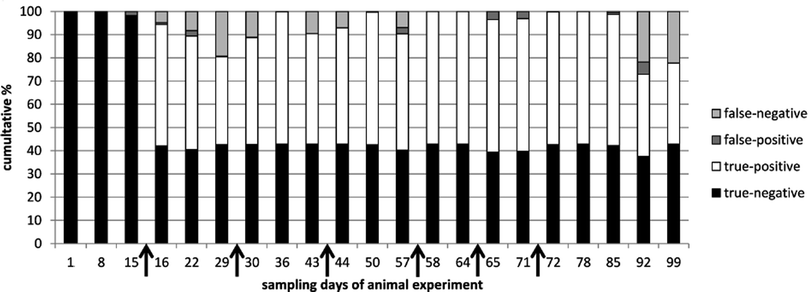 |
| | Fig. 3 Prediction of correct and incorrect classification, using kNN modelling. Results of all serum samples taken in the animal experiment were categorized by sampling day and used for building the model and predict classification. Time points of treatment (rbST-formula or matrix-formula) are marked by arrows. | |
4 Conclusion
A unique multiple biomarker FCIA assay has been developed to pinpoint rbST (ab)use in serum samples of dairy cows. Individual immunoassays could be combined into a robust triplex format with only minor modifications. Thus a reproducible and sensitive platform was obtained. The developed triplex FCIA enables pinpointing rbST abuse by combining results of three biomarkers, IGF-1, IGFBP2 and rbST-induced antibodies. The IGF-1 and IGFBP2 biomarkers responded rapidly after the first rbST injection, while responses for rbST-induced antibodies were characterized by a long half-life. For that reason, the combination of these three biomarkers resulted in a very long detection window. On average the individual biomarkers yielded correct classification of 64% for IGF-1, 56% for IGFBP2 and 83% for rbST-induced antibodies in serum samples. A kNN prediction model built on the combined triplex data enabled even a 93.6% correct prediction rate. Therefore, this triplex FCIA provides a detailed biomarker profile in serum, ultimately pinpointing rbST abuse in cattle with the highest possible confidence.
Acknowledgements
This project was financially supported by the Dutch Ministry of Agriculture, Nature and Food Quality (project 7202901). We kindly thank J. van Hende from the University of Ghent for performing the animal experiment and M. J. Groot and J. S. Ossenkoppele for their help with collection of sample material.
References
- D. Brinckman, AgBioForum, 2000, 3, 164–172 Search PubMed.
- M. H. Le Breton, S. Rochereau-Roulet, S. Chéreal, G. Pinel, T. Delatour and B. Le Bizec, J. Agric. Food Chem., 2010, 58, 729–733 CrossRef CAS.
- D. E. Bauman and R. G. Vernon, Annu. Rev. Nutr., 1993, 13, 437–461 CrossRef CAS.
-
G. Cacciatore, S. W. F. Eisenberg, C. Situ, M. H. Mooney, P. Delahaut, S. Klarenbeek, A. C. Huet, A. A. Bergwerff and C. T. Elliott, Anal. Chim. Acta, 2009, 637, 351–435 Search PubMed.
- J. Ding, E. O. List, S. Okada and J. J. Kopchick, Growth Horm. IGF Res., 2009, 19, 399–407 CrossRef CAS.
- M. H. Mooney, C. Situ, G. Cacciatore, T. Hutchinson, C. Elliott and A. A. Bergwerff, Biomarkers, 2008, 13, 246–256 CrossRef CAS.
- G. Pinel, S. Weigel, J. P. Antignac, M. H. Mooney, C. Elliott, M. W. F. Nielen and B. Le Bizec, TrAC, Trends Anal. Chem., 2010, 29, 1269–1280 CrossRef CAS.
- P. Teale, C. Barton, P. M. Driver and R. G. Kay, Bioanalysis, 2009, 1, 1103–1118 CrossRef CAS.
- W. S. Cohick, M. A. McGuire, D. R. Clemmons and D. E. Bauman, Endocrinology, 1992, 130, 1508–1514 CrossRef CAS.
- S. S. De Kock, J. P. Rodgers, B. C. Swanepoel and A. J. Guthrie, J. Endocrinol., 2001, 171, 163–171 CrossRef CAS.
- N. Fernandez, M. P. Molina, S. Balasch, A. Torres and F. Adriaens, J. Dairy Sci., 2001, 84, 2170–2176 CrossRef CAS.
- A. T. Kicman, J. P. Miell, J. D. Teale, J. Powrie, P. J. Wood, P. Laidler, P. J. Milligan and D. A. Cowan, Clin. Endocrinol., 1997, 47, 43–50 CrossRef CAS.
- W. S. Cohick, K. Plaut, S. J. Sechen and D. E. Bauman, Domest. Anim. Endocrinol., 1989, 6, 263–273 CrossRef CAS.
- A. Daxenberger, H. Sauerwein and B. H. Breier, Analyst, 1998, 123, 2429–2435 RSC.
- M. J. Khosravi, A. Diamandi, J. Mistry and P. D. Lee, Clin. Chem., 1996, 42, 1147–1154 CAS.
- S. Rochereau-Roulet, I. Gaudin, S. Chéreau, S. Prévost, G. André-Fontaine, G. Pinel and B. Le Bizec, Anal. Chim. Acta, 2011, 700, 189–193 CrossRef CAS.
- C. M. Zwickl, H. W. Smith, R. N. Tamura and P. H. Bick, J. Dairy Sci., 1990, 73, 2888–2895 CrossRef CAS.
- J. Jaouhari, F. Schiele, S. Dragacci, P. Tarallo, J. P. Siest, J. Henny and G. Siest, Clin. Chem., 1992, 38, 1968–1974 CAS.
- H. Tanaka, M. Kuwada, M. Shiraki and K. Katayama, J. Immunol. Methods, 1986, 94, 19–24 CrossRef CAS.
- S. Mohan and D. J. Baylink, J. Clin. Endocrinol. Metab., 1995, 80, 637–647 CrossRef CAS.
- G. Pinel, R. Buon, F. Aviat, C. Larré, G. André-Fontaine, F. André and B. Le Bizec, Anal. Chim. Acta, 2005, 529, 41–46 CrossRef CAS.
- M. G. E. G. Bremer, N. G. E. Smits, W. Haasnoot and M. W. F. Nielen, Analyst, 2010, 135, 1147–1152 RSC.
- S. K. J. Ludwig, N. G. E. Smits, M. G. E. G. Bremer and M. W. F. Nielen, Food Control, 2012, 26, 68–72 CrossRef CAS.
- N. G. E. Smits, M. G. E. G. Bremer, S. K. J. Ludwig and M. W. F. Nielen, Drug Test. Anal., 2012, 4, 362–367 CrossRef CAS.
- L. Castigliego, G. Iannone, G. Grifoni, R. Rosati, D. Gianfaldoni and A. Guidi, J. Dairy Res., 2007, 74, 79–85 CrossRef CAS.
-
R. Development Core Team, R. Foundation for Statistical Computing, Vienna, Austria, 2009 Search PubMed.
- B. Efron, Ann. Math. Stat., 1979, 7, 1–26 Search PubMed.
- T. Waterboer, P. Sehr and M. Pawlita, J. Immunol. Methods, 2006, 309, 200–204 CrossRef CAS.
- J. D. Wallace, R. C. Cuneo, P. A. Lundberg, T. Rosén, J. O. Jørgensen, S. Longobardi, N. Keay, L. Sacca, J. S. Christiansen, B. Å. Bengtsson and P. H. Sönksen, J. Clin. Endocrinol. Metab., 2000, 85, 124–133 CrossRef CAS.
-
C. Bertozzi, D. Portetelle, M. Pirard, I. Parmentier, V. Haezebroeck, R. Renaville and A. Burny, Focus on Biotechnology, ed. M. Hofman, J. Anné, M. Cuyper and J. W. M. Bulte, Springer, Netherlands, 2002, vol. 5, pp. 99–110 Search PubMed.
- L. Vleurick, R. Renaville, M. VandeHaar, J. L. Hornick, L. Istasse, I. Parmentier, C. Bertozzi, C. Van Eenaeme and D. Portetelle, J. Dairy Sci., 2000, 83, 452–458 CrossRef CAS.
- P. J. Eppard, G. J. Rogan, B. G. Boysen, M. A. Miller, R. L. Hintz, B. G. Hammond, A. R. Torkelson, R. J. Collier and G. M. Lanza, J. Dairy Sci., 1992, 75, 2959–2967 CrossRef CAS.
Footnote |
| † Contributed equally to the manuscript. |
|
| This journal is © The Royal Society of Chemistry 2013 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times diluted anti-IGFBP2 antibody was added and incubated for 15 min on a microtiter plate shaker. Then, microspheres (10 μL diluted suspension containing about 1250 microspheres per microsphere set) were added to each well and incubated for 1 h on a microtiter plate shaker. After incubation, the plate was centrifuged (1 min at 130g) and the microspheres were washed with 200 μL PBST. After washing, a 125 μL PE-labeled antibody mixture containing 625 times diluted GAM-PE, 1000 times diluted GAR-PE and 1000 times diluted GAB-PE was added and incubated for 30 min on a microtiter plate shaker. After this incubation step, the plate was centrifuged and 125 μL of PBST was added per well. Then, the microspheres were detected, according to bead assay type and PE-label in the flow cytometer (1 μL s−1 was measured until 100 events per microsphere set were reached with a maximum of 50 μL per well).
000 times diluted anti-IGFBP2 antibody was added and incubated for 15 min on a microtiter plate shaker. Then, microspheres (10 μL diluted suspension containing about 1250 microspheres per microsphere set) were added to each well and incubated for 1 h on a microtiter plate shaker. After incubation, the plate was centrifuged (1 min at 130g) and the microspheres were washed with 200 μL PBST. After washing, a 125 μL PE-labeled antibody mixture containing 625 times diluted GAM-PE, 1000 times diluted GAR-PE and 1000 times diluted GAB-PE was added and incubated for 30 min on a microtiter plate shaker. After this incubation step, the plate was centrifuged and 125 μL of PBST was added per well. Then, the microspheres were detected, according to bead assay type and PE-label in the flow cytometer (1 μL s−1 was measured until 100 events per microsphere set were reached with a maximum of 50 μL per well).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times; each time different randomly chosen training and test sets were applied and an overall sensitivity (true-positive rate), specificity (true-negative rate) and misclassification rate could be calculated for every sample, for every time point and for the whole animal experiment.
000 times; each time different randomly chosen training and test sets were applied and an overall sensitivity (true-positive rate), specificity (true-negative rate) and misclassification rate could be calculated for every sample, for every time point and for the whole animal experiment.



