A simple method for the determination of enantiomeric composition of propranolol enantiomers
Yanli
Wei
*a,
Hui
Kang
a,
Yanfang
Ren
a,
Guojie
Qin
b,
Shaomin
Shuang
a and
Chuan
Dong
*a
aResearch Center of Environmental Science and Engineering, School of Chemistry and Chemical Engineering, Shanxi University, Taiyuan 030006, P. R. China. E-mail: weiyanli@sxu.edu.cn; Fax: +86 3517011011; Tel: +86 3517011322
bInstitute of Horticulture, Shanxi Academy of Agriculture Science, Taiyuan 030031, P. R. China. Fax: +86 3517828066; Tel: +86 3517828066
First published on 31st October 2012
Abstract
A simple method of room temperature phosphorescence is proposed for the determination of propranolol enantiomeric composition based on β-cyclodextrin as the chiral host and bromocyclohexane as the heavy atom. Enantiomeric complexes are formed upon binding of the propranolol enantiomer to β-cyclodextrin, and the sign of the resultant phosphorescence signals allows for the identity of the propranolol enantiomers configuration to be determined successfully.
Most chiral pharmaceuticals have nearly identical physical and chemical properties, but physiological and pharmacological activities have some obvious differences owing to their differences of optical activity, as a result, the determination of the enantiomeric purity of chiral pharmaceuticals is vital to the personal safety and drug quality.1 More importantly, a single isomer can improve the performance of the treatment. Therefore, the determination of the enantiomeric composition of chiral pharmaceuticals plays an important role in the fields of pharmaceutical synthesis, clinical medicine and life science.
By now, the enantiomeric composition values of chiral pharmaceuticals are most commonly ascertained using either high performance liquid chromatography (HPLC) or gas chromatography (GC).2–4 Although these chromatography techniques have high accuracy, they are unable to meet the needs of rapid determination. Moreover, increasingly large numbers of samples also inherently increase the amount of solvent used, preparation time required, and cost incurred. In addition, several other methods including NMR,5 UV-vis6 and calorimetry7 have been employed to determine the enantiomeric composition. However, most of these techniques suffer from poor sensitivity, chemical derivatization of the analytes and complicated synthesis of the chiral host. It is still desirable to develop more feasible methods to detect the enantiomeric composition. Among various techniques available for enantiomeric composition detection, fluorescence based methods are of significant interest due to their simplicity, speediness, sensitivity, and inexpensiveness.8 As a sister technology to fluorescence spectroscopy, room temperature phosphorescence (RTP) is also a good choice for enantiomeric composition detection since it possesses some distinctive advantages such as fewer spectral interferences, longer emission wavelength, larger Stokes shift, and longer and easily measurable triplet state lifetimes. Based on RTP technique, our group focused on developing assays that chirally discriminate between enantiomers in various chiral media based on their difference in phosphorescence lifetime.9–11 The phosphorescence lifetime difference can be used to perform the chiral discrimination, however, it cannot provide quantitative information on the enantiomeric composition. Herein, we report rapid detection of propranolol enantiomeric composition based on its phosphorescence in the presence of β-cyclodextrin (β-CD) and bromocyclohexane, which can achieve the determination of enantiomeric components in the concentration range of 0–100%.
Propranolol, as one of the most commonly used β-adrenergic blocker drugs, the S-isomer exhibits the desired pharmaceutical activity, whereas R-isomer has a high contraception effect and can be used as an effective contraceptive. Hence, the treatment of heart disease has poor efficiency and side effects due to the racemate of propranolol. So it is vital to determine the propranolol enantiomeric composition for the heart system disease treatment. As the chiral host, cyclodextrin has been used for the asymmetric synthesis to achieve the perfect stereocontrol in photoreactions.12 In a previous study, we examined the chiral discrimination of propranolol in γ-CD system, in which the larger cavity of γ-CD may permit oxygen to easily enter and result in a weaker phosphorescence.13 More importantly, it is too expensive to apply in the field of pharmaceutical analysis. Herein, we identify that propranolol enantiomers can show stronger RTP in a oxygenated solution containing β-CD as the host molecule and bromocyclohexane presenting the heavy atom effect on propranolol. As far as we are aware, this is the first attempt to explore the room temperature phosphorescence of propranolol in β-CD system. In addition, the determination of propranolol enantiomeric composition can be achieved by recording their RTP intensity. Our proposed work provides a better convenient and simple method to analyse the enantiomeric composition for propranolol enantiomers.
No RTP signals of propranolol enantiomers are observed in individual β-CD or bromocyclohexane aqueous solution in the presence of oxygen. Fig. 1 depicts the phosphorescence spectra of R- and S-propranolol in the presence of CD and bromocyclohexane. Both R- and S-propranolol show an emission peak maximum at ca. 520 nm with the excitation peaks at 220 and 290 nm, inferring that a rigid tricomponent inclusion complex is formed from propranolol, β-CD and bromocyclohexane. Since the RTP spectra can avoid interference from the measurements when excited at 290 nm, this excitation wavelength is chosen for all the RTP intensity measurements.
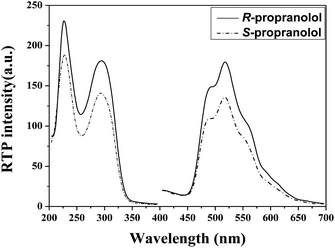 | ||
| Fig. 1 Phosphorescence spectra of 50 μM R- and S-propranolol in the presence of 3.2 mM β-CD and 14.7 mM bromocyclohexane. | ||
NMR is an important technique for exploring the interaction mechanism between chiral molecules and β-CD. To better understand the function model of the inclusion complex of propranolol/β-CD, 1H NMR spectra in the absence and presence of bromocyclohexane in D2O are performed and the changes of chemical shifts (Δδ) of various relevant protons in β-CD are summarized in Table 1. It can be seen that obvious upfield shifts for protons of β-CD occurred after forming inclusion complexes with propranolol, and the Δδ value of H-5 are much higher than that of H-3, suggesting that propranolol gets into the hydrophobic cavity from the narrower rim of the β-CD. In the tricomponent system of propranolol/β-CD/bromocyclohexane, Δδ of H-5 is also larger than that of H-3, indicating that bromocyclohexane also enters into the cavity from the primary side of the β-CD.
| Proton in β-CD | δ 0 | Δδ1 | Δδ2 | ||
|---|---|---|---|---|---|
| R-PPL | S-PPL | R-PPL | S-PPL | ||
| a δ 0 indicates the chemical shift of proton in β-CD. Δδi = δi − δ0 (i = 1, 2), where 1 and 2 correspond to the solution β-CD and propranolol, and β-CD, propranolol and bromocyclohexane, respectively. | |||||
| H-1 | 5.008 | −0.034 | −0.040 | −0.031 | −0.029 |
| H-2 | 3.580 | −0.023 | −0.062 | −0.074 | −0.061 |
| H-3 | 3.900 | −0.085 | −0.166 | −0.121 | −0.102 |
| H-4 | 3.504 | −0.051 | −0.145 | −0.154 | −0.147 |
| H-5 | 3.793 | −0.135 | −0.216 | −0.229 | −0.218 |
| H-6 | 3.837 | −0.094 | −0.121 | −0.134 | −0.127 |
Table 1 shows the chemical shift of protons (δ0) of β-CD and Δδi = δi − δ0 (i = 1, 2), where 1 and 2 correspond to the solutions of β-CD and propranolol, and β-CD, propranolol, and bromocyclohexane, respectively. It is interesting that the differences of H-4, H-5, H-6 between Δδ2 and Δδ1 for S-isomer system are slightly changeable, while those for R-isomer are significant, demonstrating that the addition of bromocyclohexane as a third component plays an important role in the detection of propranolol enantiomers. Herein, bromocyclohexane not only acts as a heavy atom perturber but also as a space regulator. As a heavy atom perturber, bromocyclohexane can enhance the S1 → T1 intersystem crossing and thus induce strong phosphorescence. On the other hand, as a space regulator, bromocyclohexane inserts into the cavity of β-CD and forms a tricomponent complex (propranolol/β-CD/bromocyclohexane). In addition, the changes of H-5 in both the binary and tricomponent systems are the largest as compared to other protons, i.e., a R- or S-propranolol molecule can interact differently with the H-5 of β-CD molecule, leading to the interaction difference between R-propranolol and S-propranolol with β-CD. The inclusion constant (K) and stoichiometric ratio of the inclusion complex of propranolol and β-CD can be determined by the non-linear least squares fitting of the experimental data obtained from the fluorescence measurement:14
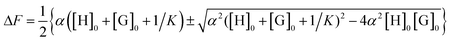 | (1) |
Experimental conditions are optimized to obtain the best detection efficiency for the enantiomeric composition of propranolol. Firstly, pH effects are taken into consideration. As shown in Fig. 2, the effects of pH on the RTP emission of propranolol enantiomers are very significant. Various pH phosphate buffer solutions, from 2.0 to 11.0, are examined in this system. RTP intensities of propranolol enantiomers increase warmly with the pH value of the system in the range of 2.0 and 3.0, but then their RTP signals gradually weaken with the increase in pH from 3.0 to 7.0 and decrease sharply when the pH is higher than 7.0. Finally, RTP signals almost disappear when the pH is higher than 11.0. Propranolol is a weak organic acid in aqueous solution and it has been reported that the pKa value of it is 5.0–6.5.15 As such, the existence of the protonated and deprotonated forms of propranolol depends on the pH of the solution. The RTP intensity of propranolol changes with pH implying that the RTP of propranolol mainly originates from its protonated species due to the coexistence of hydrogen bonding, hydrophobic interaction of the propranolol/β-CD/bromocyclohexane complex. Therefore, phosphate buffer solution at pH 3.0 is chosen for most RTP determinations.
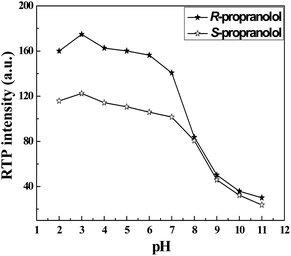 | ||
| Fig. 2 Effect of pH on RTP intensities of R- and S-propranolol (50 μM). | ||
The cavity of β-CD provides a hydrophobic microenvironment and avoids quenching of the excited triplet states of propranolol from collision with oxygen. Fig. 3 depicts the effect of β-CD concentration on the RTP intensities of R- and S-propranolol in the presence of bromocyclohexane. An increase in the RTP intensity of R- and S-propranolol with increasing concentration of β-CD is observed. RTP intensities of propranolol enantiomers increase gradually with the concentration of β-CD from 0.8 mM to 3.0 mM, and then, this change becomes slow and tends to reach a maximum at 3.4 mM β-CD, which demonstrates that the formation three-component inclusion compounds are practically finished. As a result of these studies, 3.2 mM β-CD concentration is selected for this work.
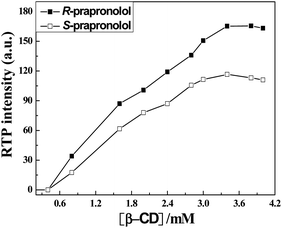 | ||
| Fig. 3 Effect of β-CD concentration on the RTP intensity of 50 μM R- and S-propranolol in the presence of 14.7 mM bromocyclohexane. | ||
Bromocyclohexane is a well known external heavy atom perturber for CD-induced RTP. It not only enhances the spin–orbit coupling interaction but also increases the probability of intersystem crossing from the excited singlet (S1) to the triplet (T1) states. The smaller molecular volume and lower polarity of bromocyclohexane makes it easier to enter into the cavity of β-CD.16 The fluorescence intensity of propranolol is quenched by bromocyclohexane but at the same time, the intersystem crossing efficiency is remarkably promoted, leading to the increase in triplet state emission. So the effect of bromocyclohexane on the RTP intensity of propranolol enantiomers is investigated in detail and the results are displayed in Fig. 4. It is obvious that RTP intensities of R- and S-propranolol sharply increase with addition of bromocyclohexane and reach a plateau in the concentration range of bromocyclohexane from 11.5 to 14.7 mM, and then a slight decrease of RTP intensity is observed with further increase of bromocyclohexane, which is possibly due to the fact that excess bromocyclohexane can quench the RTP of propranolol/β-CD/bromocyclohexane complex. As such, a suitable bromocyclohexane concentration of 14.7 mM is chosen for the present work.
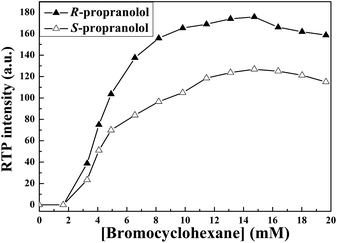 | ||
| Fig. 4 Effect of the concentration of bromocyclohexane on the RTP intensity of 50 mM R- and S-propranolol in the presence of 3.4 mM β-CD. | ||
Under the optimal experimental conditions, RTP spectra of mixtures of propranolol enantiomers are measured at different R-/S-propranolol ratios keeping the total concentration at 50 μM, and the results are shown in Fig. 5. It can be seen that RTP intensities of the mixture increase as the ratio of R-PPL increases. In addition, as can be seen in the inset of Fig. 5, the RTP intensities of propranolol enantiomer mixtures can be correlated linearly with the enantiomeric composition of R-PPL. The slope of the straight line, 0.6027, is 1.31 times larger than that in γ-CD. That is to say, the detection sensitivity for propranolol enantiomeric composition in β-CD system is higher than that in γ-CD system. On this basis, the enantiomeric composition of propranolol hydrochloride tablet has been measured using the proposed method. The contents of R- and S-propranolol are 5.03 mg and 4.97 mg, respectively, which agree with the labelled amount. In addition, the accuracy and the reliability of the method are further evaluated by recovery experiments with high, medium, and low concentration of R- and S-propranolol in the liner range and the results are shown in Table 2. The recoveries of R- and S-propranolol are in the range of 96.94–100.44%, which indicate that the proposed method is suitable for real samples. It can be concluded that the present method seems useful for the determination of enantiomeric composition in real samples. The proposed method is suitable for the determination of propranol enantiomers in propranol hydrochloride tablets with satisfactory results. It provides a rapid and simple method for the determination of propranolol enantiomers.
![RTP spectra of R- and S-propranolol with various ratios in the presence of 3.2 mM β-CD and 14.7 mM bromocyclohexane ([S-PPL]/[R-PPL]: (1) 1 : 0, (2) 4 : 1, (3) 3 : 2, (4) 2 : 3, (5) 2 : 3, (6) 0 : 1). Inset: phosphorescence intensity of mixed R-PPL and S-PPL versus the enantiomeric composition of R-PPL.](/image/article/2013/AN/c2an36003a/c2an36003a-f5.gif) | ||
Fig. 5 RTP spectra of R- and S-propranolol with various ratios in the presence of 3.2 mM β-CD and 14.7 mM bromocyclohexane ([S-PPL]/[R-PPL]: (1) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, (2) 4 0, (2) 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (3) 3 1, (3) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, (4) 2 2, (4) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, (5) 2 3, (5) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, (6) 0 3, (6) 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Inset: phosphorescence intensity of mixed R-PPL and S-PPL versus the enantiomeric composition of R-PPL. 1). Inset: phosphorescence intensity of mixed R-PPL and S-PPL versus the enantiomeric composition of R-PPL. | ||
| Samples | Added concentration (μmol L−1) | Found concentration (μmol L−1) | Recovery % | Average recovery % | RSD % |
|---|---|---|---|---|---|
| R-Propranolol | 1.02 | 1.00 | 98.04 | 99.23 | 1.19 |
| 5.06 | 5.03 | 99.21 | |||
| 9.02 | 9.06 | 100.44 | |||
| S-Propranolol | 0.98 | 0.95 | 96.94 | 97.99 | |
| 4.94 | 4.86 | 98.38 | |||
| 8.98 | 8.86 | 98.66 |
Conclusion
In this work, the use of β-CD/bromocyclohexane system to obtain strong and stable RTP of propranolol, to determine propranolol enantiomeric composition is reported for the first time. Propranolol can enter into the hydrophobic cavity of β-CD to form a host–guest complex, and a more stable inclusion complex, namely, β-CD/propranolol/bromocyclohexane can be formed in the presence of bromocyclohexane, which exhibits a strong RTP emission in the presence of oxygen. This enables easy determination of enantiomeric composition of propranolol enantiomers based on the RTP intensity only. It is anticipated that such new information would provide a better convenient and simple method to analyse the enantiomeric composition of propranolol enantiomers.Financial support from the National Natural Science Foundation of China (Grants 21175086), the Natural Science Foundation of Shanxi Province (no. 2010021010-2) and the China Postdoctoral Science Foundation (201104651, 20100471581) are gratefully acknowledged.
References
- D. W. Armstrong, T. J. Ward, R. D. Armstrong and T. E. Beesley, Science, 1986, 232, 1132–1135 CAS.
- C. J. Welch, M. H. Hyun, T. Kubota, W. Schafer, F. Bernardoni, H. J. Choi, N. J. Wu, X. Y. Gong and B. Lipshutz, Chirality, 2008, 20, 815–819 CrossRef CAS.
- R. J. Thompson, J. Liq. Chromatogr. Relat. Technol., 2005, 28, 1215–1231 CrossRef CAS.
- Y. Xiao, T. T. Y. Tan and S. C. Ng, Analyst, 2011, 136, 1433–1439 RSC.
- R. J. Ansell, D. Wang and J. K. L. Kuah, Analyst, 2008, 133, 1673–1683 RSC.
- Y. Wang, F. Zhang, J. Liang, H. Li and J. Kong, Spectrochim. Acta, Part A, 2007, 68, 279–283 CrossRef.
- F. P. Schmidtchen, Chem.–Eur. J., 2002, 8, 3522–3529 CrossRef CAS.
- S. Z. Zhang, J. W. Xie and C. S. Liu, Anal. Chem., 2003, 75, 91–97 CrossRef CAS.
- Y. L. Wei, S. F. Wang, J. B. Chao, S. B. Wang, C. Dong, S. M. Shuang, M. C. Paau and M. M. F. Choi, J. Phys. Chem. C, 2011, 115, 4033–4040 CAS.
- Y. L. Wei, C. Dong, D. S. Liu, S. M. Shuang and C. W. Huie, Biomacromolecules, 2007, 8, 761–764 CrossRef CAS.
- Y. L. Wei, W. H. Chan, A. W. M. Lee and C. W. Huie, Chem. Commun., 2004, 288–289 RSC.
- C. Yang, C. F. Ke, W. T. Liang, G. Fukuhara, T. Mori, Y. Liu and Y. Inoue, J. Am. Chem. Soc., 2011, 133, 13786–13789 CrossRef CAS.
- Y. L. Wei, Y. F. Ren, J. Li, S. M. Shuang and C. Dong, Analyst, 2011, 136, 299–303 RSC.
- Y. Inoue, K. Yamanoto, T. Wada, S. Everitt, X. M. Gao, Z. J. Hou, L. H. Tong, S. K. Jiang and H. M. Wu, J. Chem. Soc., Perkin Trans. 2, 1998, 1807–1816 RSC.
- J. Hermansson, A. Grahn and I. Hermansson, J. Chromatogr., A, 1998, 797, 251–263 CrossRef CAS.
- H. R. Zhang, S. Y. Guo, L. Li and M. Y. Cai, Anal. Chim. Acta, 2002, 463, 135–142 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
