A comparative study of cellular uptake and cytotoxicity of multi-walled carbon nanotubes, graphene oxide, and nanodiamond†
Xiaoyong
Zhang
*ab,
Wenbing
Hu
a,
Jing
Li
a,
Lei
Tao
b and
Yen
Wei
b
aLaboratory of Physical Biology, Shanghai Institute of Applied Physics, CAS, Shanghai 201800, China
bDepartment of Chemistry and Key Laboratory of Organic Optoelectronic & Molecular Engineering of Ministry of Education, Tsinghua University, Beijing, 100084, P.R. China. E-mail: Xiaoyongzhang2010@hotmail.com
First published on 16th April 2012
Abstract
Investigations of the interactions between carbon nanomaterials (CNMs) and living organisms and their subsequent biological responses are of fundamental significance for toxicity assessment and further biomedical applications. In this work, the cellular uptake and cytotoxicity of multi-walled carbon nanotubes (MWCNTs), graphene oxide (GO) and nanodiamond (ND) were examined and compared. We demonstrated that all of the CNMs were readily internalized by HeLa cells through nonspecific cellular uptake. Their cell uptake ratios showed significant differences in the following order: ND > MWCNTs > GO. A series of biological assays were used to evaluate the cytotoxicity of CNMs. It was found that CNMs showed dose- and time-dependent cytotoxicity to HeLa cells. However, cytotoxicity of CNMs was not associated with their cell uptake ratios. Among them, ND exhibited the highest cell uptake ratio and the least cytotoxicity. To the best of our knowledge, this is the first study which has quantitatively determined and compared the cell uptake ratios and cytotoxicities of MWCNTs, GO and ND. And we expect that these results described here could provide useful information for the development of new strategies to design efficient drug delivery nanocarriers and therapeutic systems as well as deep insights into the mechanism of CNMs’ cytotoxicity.
Introduction
With recent developments in nanoscience and nanotechnology, there has been a rapid expansion of nanomaterials with different sizes, compositions, and structural characteristics employed in various applications.1–8 The increasing interest in applications and the subsequent mass production of these engineered nanomaterials, however, has also brought much attention to their potential adverse effects on the environment and on human health.9–11 Among them, carbon nanomaterials (CNMs), including carbon nanotubes (CNTs), nanodiamond (ND), and graphene represent the most popular nanomaterials which have been extensively studied for their excellent physicochemical and biological properties12–14 and diverse applications.15–19 The evaluation of interactions between CNMs and living organisms and their consequently biological responses is therefore becoming one of the most dynamic research fields in nanotoxicology. In the past decade, considerable effort has been devoted to determining the toxicity of CNMs in vitro and in vivo.15–17 The preliminary results highlighted that numerous factors, such as metal contaminants, surface properties, aggregation state and cell types, will influence the toxicity of CNMs.18–27 However, the current results about the toxicity of CNMs remain fragmented and sometimes even contradictory, which should be addressed. For example, various materials and experimental conditions were adopted in different reports, leading to the toxicity results of CNMs being illusive and incomparable. Furthermore, previous studies were focused on the toxicity of C60 and CNTs, but limited information is known about the novel CNMs such as ND and graphene.18–27Compared with CNTs, ND and graphene are two relatively novel CNMs which have potential for use in a range of fields.28–43 It has been reported that these materials could serve as new alternatives and efficient tools for transporting and translocating therapeutic molecules such as bioactive peptides, proteins, nucleic acids and drugs to cells and organs. They are also regarded as promising candidates for other applications including biosensors, electronic devices, sustainable energy and conversion.44–52 To further extend their applications and regulate the potential future issues related to their widespread use, a fundamental and systematic understanding of the interactions of these novel materials with living organisms and their corresponding biological response is urgently needed.53,54 To the best of our knowledge, few reports have investigated and compared the cytotoxicity of these CNMs thus far.55 Moreover, no reports have previously quantitatively determined the cellular uptake ratios of MWCNTs, GO and ND, likely due to the lack of suitable detection techniques.
In this work, the cell uptake ratios of three types of CNMs (MWCNTs, GO, ND) were quantitatively determined using a radiolabeling technique.56–58 The key biological responses induced by these CNMs were evaluated by a series of biological assays, including the 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), malondialdehyde (MDA), superoxide dismutase (SOD), lactate dehydrogenase (LDH) and reactive oxygen species (ROS). We demonstrated that the cellular uptake ratios of CNMs were related to their structural characteristics. However, their cytotoxicity was not associated with their cell uptake ratios. We expect that these results will provide some useful information for better understanding of the interactions and cytotoxicity of CNMs and biological systems.
Results and discussion
The microstructural characteristics of CNMs are shown in Fig. 1A–C. The diameters of CNTs range from 20–40 nm, which is consistent with the data provided by the manufacturer. The outer wall and inner wall of the CNT can be clearly distinguished, suggesting that the CNT structure remained intact after chemical oxidation (Fig. 1A). The Trasmission Electron Microscopy (TEM) image of GO is displayed in Fig. 1B. Many flakes with single and few layers and a lateral range from several tens to hundreds of nanometers were observed. The individual ND is less than 10 nm, and they tend to aggregate into large clusters larger than 100 nm (Fig. 1C). | ||
| Fig. 1 Representative TEM images of CNTs (A), GO (B) and ND (C), the scale bar = 50 nm. | ||
Fourier Transform Infrared (FT-IR) Spectroscopy and TGA were also used to characterize the CNMs. In the FT-IR spectra, carboxyl groups were clearly identified in GO, both through the very broad 3400 cm−1 –OH stretching vibration and through the 1732 cm−1 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration. The existence of epoxy groups on the GO was demonstrated by the characteristic absorption bands at 1060 cm−1. Some bands at 1000–2000 cm−1 were also observed in ND and the CNTs, suggesting that oxygen-containing groups such as carboxyl groups, hydroxyl groups and epoxy groups were also induced on the surface of ND and CNTs after oxidation. However, the intensity of these bands was relatively weak, indicating that the intensity of functional groups existing on the surfaces of ND and CNTs is much lower than that of GO (Fig. 2A). The thermal stability of CNMs in air was characterized by TGA and different decomposition temperatures of CNMs were observed. The significant weight loss temperatures of CNTs, GO and ND were at 210, 421 and 540 °C, suggesting that these CNMs have different thermal stabilities. Furthermore, we could also calculate the density of functional groups on the CNMs. Based on the TGA results, the weight losses of CNTs, GO, and ND, which was ascribed to the functional groups, are about 11%, 9.2%, and 3%, respectively (Fig. 2B).
O stretching vibration. The existence of epoxy groups on the GO was demonstrated by the characteristic absorption bands at 1060 cm−1. Some bands at 1000–2000 cm−1 were also observed in ND and the CNTs, suggesting that oxygen-containing groups such as carboxyl groups, hydroxyl groups and epoxy groups were also induced on the surface of ND and CNTs after oxidation. However, the intensity of these bands was relatively weak, indicating that the intensity of functional groups existing on the surfaces of ND and CNTs is much lower than that of GO (Fig. 2A). The thermal stability of CNMs in air was characterized by TGA and different decomposition temperatures of CNMs were observed. The significant weight loss temperatures of CNTs, GO and ND were at 210, 421 and 540 °C, suggesting that these CNMs have different thermal stabilities. Furthermore, we could also calculate the density of functional groups on the CNMs. Based on the TGA results, the weight losses of CNTs, GO, and ND, which was ascribed to the functional groups, are about 11%, 9.2%, and 3%, respectively (Fig. 2B).
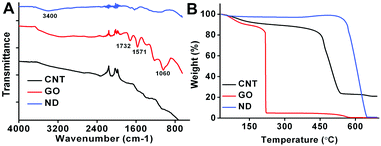 | ||
| Fig. 2 (A) FT-IR spectra of CNTs, GO, and ND; (B) TGA curves of CNTs, GO, and ND. | ||
The solution stabilities of CNTs, GO and ND in phosphate buffer solution (PBS) and fetal bovine serum (FBS) (RPMI-1640 cell culture medium with 10% FBS) are shown in Fig. 3. Different dispersibilities were evident in PBS and FBS for these CNMs. Among them, GO showed good stability in PBS. No significant precipitation was observed after standing for 6 h, however, most of the CNTs and ND were deposited at the bottom of the bottles. It can be also found that CNTs and ND showed enhanced dispersibility in the cell culture medium in the presence of 10% FBS. Compared with ND and CNTs, however, no enhancement phenomenon in FBS was found for GO (Fig. 3). Notably, all the CNMs are relatively stable in FBS over a period of 6 h, which is useful for evaluating their cytotoxicity.
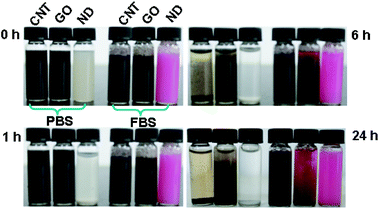 | ||
| Fig. 3 Optical photograph showing the solution stabilities of CNMs in PBS and FBS (RPMI-1640 cell culture medium containing 10% FBS), left standing for different amounts of time. | ||
The dispersibility of CNMs in PBS and FBS was further evidenced by zeta-potential and size distribution measurements. As shown in Table 1, the zeta-potentials of CNTs, GO and ND are −24.3 ± 1.1, −33.1 ± 1.2 and 12.3 ± 1.9 mV, respectively. These results further indicated the best stability of GO in PBS. Notably, all the zeta-potentials changed to positive in the presence of FBS, suggesting that the surface properties of CNMs were significantly changed after adsorption of serum proteins. Table 1 further shows that the sizes of the CNTs and ND are significantly reduced in the presence of FBS. However, GO shows the opposite trend, which is consistent with the optical photographs.
| Zeta-potential (mV) | Size distribution (nm) | |||
|---|---|---|---|---|
| PBS | FBS | PBS | FBS | |
| CNTs | −24.3 ± 1.1 | 49.7 ± 8.3 | 1391 ± 41.4 | 299 ± 9.9 |
| GO | −33.1 ± 1.2 | 36.9 ± 18.6 | 592 ± 10.9 | 1272 ± 56.2 |
| ND | 12.3 ± 1.9 | 52.1 ± 42.4 | 1728 ± 29.5 | 632 ± 21.5 |
Evaluation of CNMs’ cellular uptake by TEM observations
The cell uptake of CNMs by HeLa cells was first confirmed by TEM observations. As shown in Fig. 4, the CNMs were indeed internalized by HeLa cells and located in the cytoplasm, such as in the lysosomes, mitochondria and endoplasm. ND and CNTs were aggregated into large clusters in cells. Notably, some CNM aggregates were enwrapped by the elongated cell synapses, suggesting that the scale of CNM aggregates taken up by cells via that mechanism may be as large as several microns. Although a larger amount of ND and CNTs was taken up by cells, the cell membrane remained intact (Fig. 4B).59 In contrast, relatively little GO was taken up by cells under the same experimental conditions, and some vacuole formation and cell membrane rupture were observed after GO exposure (Fig. 4C). Furthermore, the cell uptake of CNMs was quantitatively determined by a radiolabeling technique.58 In this section, the cell uptake ratios of ND, CNTs, and GO in the serum-free cell culture medium and in the serum-containing cell culture medium are presented. The results indicated that these CNMs exhibit drastically different cell uptake ratios after 2 h incubation. The cell uptake ratios in the serum-containing cell culture medium are 7.8% ± 1.6%, 13.3% ± 1.3% and 25.6% ± 4.2% for GO, CNTs and ND, respectively, and a slight increase in cell uptake ratios was observed in the serum-free cell culture medium (Fig. 4D). The CNMs attached to the cell surface were differentiated from those being internalized into cells by treating the samples with PBS washing. Radioactive data on the washings from the cells showed no detectable radioactive material, suggesting that the cell uptake ratios of CNMs represent those CNMs that were either tightly bound to the cell membrane or actually internalized.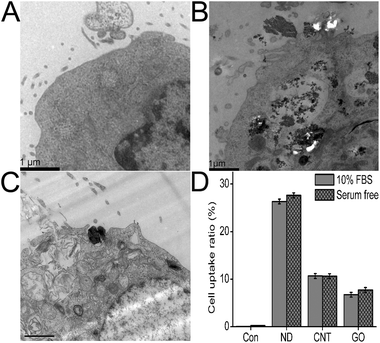 | ||
| Fig. 4 TEM images of HeLa cells after 2 h incubation with 40 μg mL−1 of CNM suspension. (A) Control, (B) ND, (C) GO; (D) cell uptake ratios of CNMs were quantitatively determined by a radiolabeling technique. | ||
Understanding the interaction of living organisms with nanoparticles is crucial for assessing their toxicity and further biomedical applications.60 Up to now, a large number of techniques, including radiolabeling techniques, fluorescence microscopy, TEM, and Raman spectroscopy were used to determine the cell uptake of nanomaterials.33,37,40,59,61,62 Although the cell uptake of these CNMs has been investigated,35,63–68 no reports have quantitatively determined and compared the cell uptake ratios of these CNMs due to the lack of suitable detection techniques. With the advantages of high sensitivity and low interference, radiolabeling techniques have been widely used for investigating the tracing of nanomaterials in vitro and in vivo.24,57,59,69 Herein, by utilizing an established radiolabeling technique, the cell uptake ratios of CNTs, GO, and ND were quantitatively determined and compared for the first time. Furthermore, according to the amounts of chemicals used in the labeling reaction, the atom ratio of 188Re to C was estimated roughly to be as low as 10−6, thus the radionuclide loaded on the CNMs will not significantly change their surface properties. Combined with the excellent in vitro stability of the labeling compounds (data not shown), we believe that the radiolabeling technique may be a general and powerful tool for evaluating the cell uptake of CNMs. And we expect that the results provided in this work may be useful for understanding the cytotoxicity of CNMs and their biomedical applications.
Cell viability and LDH release
The influence of CNMs on HeLa cells was directly observed by optical microscopy. As shown in Fig. S2,† cells showed significant different biological responses when exposed to 40 μg mL−1 of ND, CNTs, and GO for 24 h. We observed that ND showed relatively low cytotoxicity compared with that of CNTs and GO. It is worth noting that a lot of cell floating was observed after incubating with 40 μg mL−1 GO for 24 h (Fig. S2D†). The microscopy observations gave us the preliminary impression that GO was highly toxic to HeLa cells. MTT results showed the dose-dependent cytotoxicity of CNMs. As the dose increased, the survival rate of cells decreased correspondingly. MTT results also evidenced the differing cytotoxicity of CNMs. The cell viability was still more than 91%, even at a ND concentration of 80 μg mL−1. However, obvious cytotoxicity was found when cells were exposed to 20 μg mL−1 of CNTs and GO (Fig. 5A). Of note, ND showed the least cytotoxicity to HeLa cells although its cell uptake ratio is much higher than that of CNTs and GO, suggesting that the cytotoxicity of CNMs is not associated with their cell uptake efficiency.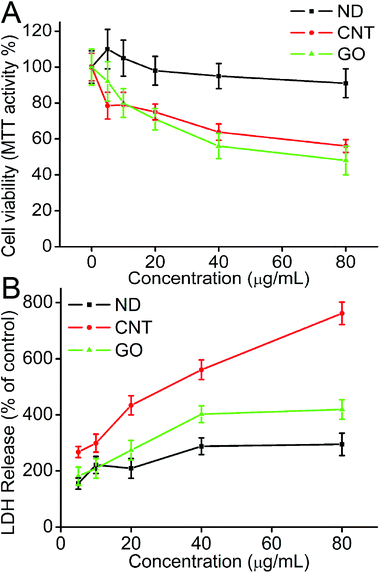 | ||
| Fig. 5 (A) Dose-dependent cytotoxicity of CNMs; the cell viability was determined by the MTT assay; (B) LDH release when cells were treated with different concentrations of CNMs. | ||
LDH is an important parameter which reflects the cell membrane injury induced by materials. The amount of LDH release induced by different CNMs is shown in Fig. 5B. A significant increase in LDH release was observed when cells were exposed to 80 μg mL−1 of CNTs. The value of LDH is about 8.5 fold when compared with the control cells. However, when cells are exposed to the same concentration of ND and GO, only 2.9 and 4.2 fold LDH increases were observed. The dramatic release of LDH by CNTs is speculated to be due to its needle-like structure, which may be more mobile and can more easily penetrate the cell membrane, resulting in greater cell membrane damage. The other possible reason is that the size of CNTs is the smallest in FBS among these CNMs (299 ± 9.9 nm, Table 1). Note that the LDH release is not associated with their cell uptake ratios, which is possibly due to the fact that CNMs are internalized into cells through different pathways.
MDA production and SOD depletion
The levels of MDA and SOD enzyme activity in the cell lysates were measured to evaluate the lipid peroxidation and oxidant stress induced by CNMs. Results showed that the level of MDA production in cell lysates was increased and the SOD enzyme activity was reduced after cells were exposed to 10 and 80 μg mL−1 of CNMs. The value of MDA remained at a very low level (0.85 nmol mg−1 protein) in the control group, and all the CNM samples showed a dose-dependent MDA production increase. Consistent with the MTT results, ND showed the minimum MDA increase. No significant MDA production increase was observed when cells were exposed to 80 μg mL−1 of ND. However, an obvious increase in MDA production was observed when cells were exposed to 80 μg mL−1 of CNTs and GO (Fig. 6A). On the contrary, the level of SOD enzyme activity showed the converse trend when compared with the results of MDA. The SOD activities of cells cultured in 80 μg mL−1 of ND, CNTs, GO were 37, 28, and 25 U mg−1 protein, respectively (Fig. 6B).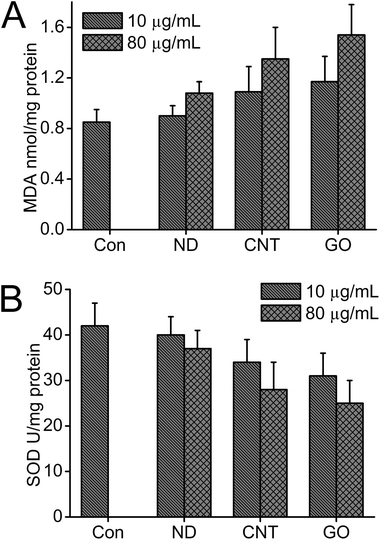 | ||
| Fig. 6 Effects of CNMs on MDA and SOD changes in cell lysates (A) MDA production and (B) SOD reactivity. | ||
ROS generation
Oxidative stress represents an imbalance between the production of ROS and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage.70 Previous results demonstrated that oxidation is a common mechanism to explain the damage of nanomaterials.70,71 In this study, we explored the generation of ROS to determine if it was involved in the toxic mechanism of different types of CNMs. Fig. 7 shows the level of the generated ROS to be time- and concentration-dependent when cells are exposed to CNMs. As shown in Fig. 7A, compared with the control, there was a 2.5 fold ROS increase induced by the CNTs when HeLa cells incubated with 80 μg mL−1 CNTs and a 3.6 fold increase for GO at the same concentration. Surprisingly, when cells were incubated with different concentrations of ND for 3 h, the fluorescence intensity which represents ROS generation decreased as the concentration of ND increased. The reasons may be ascribed to the ability of ND to scavenge ROS, or a large amount of ND taken up by cells could interfere with the detection of ROS. As the incubation time increased, the ROS levels were progressively increased. As shown in Fig. 7B, both CNTs and GO caused a significantly greater level (23 and 17 fold) of ROS generation when HeLa cells were incubated with 80 μg mL−1 of CNTs and GO for 24 h.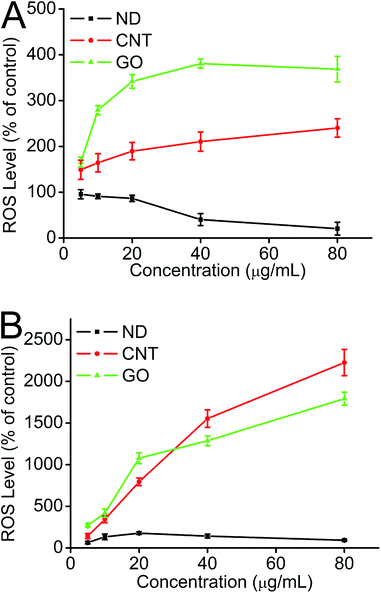 | ||
| Fig. 7 Generation of ROS from HeLa cells determined by the hydrolysis of DCFH-DA after incubation with CNMs for 3 h (A) and 24 h (B). | ||
CNMs have emerged as a promising class of nanomaterials, which have potential applications in a range of fields, but the practical applications of CNMs were severely hindered by their potential risks to human health and the environment.53,54 Previous studies have shown that many factors, including metal contaminants, aggregate states and surface functionalization, could influence the toxicity of CNTs.72 However, little information is known about the relatively novel CNMs, such as ND and GO.20,61 In the present study, the cytotoxicity of different CNMs was evaluated by cell morphology observation, MTT cell viability assay, biochemical index measurements and ROS generation. We demonstrated that the cytotoxicity of CNMs was structure-dependent. Among them, ND showed the best compatibility with HeLa cells, as evidenced by the cytotoxicity analysis. However, it is worth noting that the cytotoxicity of CNMs was not associated with their cell uptake ratios. All of these results suggested that ND may be the most promising candidate for biomedical applications, due to it having the highest cell uptake ratio and best biocompatibility.
Experimental
Preparation and characterization of CNMs
Preparation and characterization information about MWCNTs, GO and ND are described in the ESI.†Cell uptake of CNMs
The interaction between cells and CNM samples was investigated by TEM. HeLa cells were seeded at a density of 1 × 105 cells mL−1 in 6-well cell culture plates and incubated overnight to allow for cell adherence. Cells were washed with phosphate buffered saline (PBS) twice and exposed to 40 μg mL−1 CNM at 37 °C for 2 h. After incubation, the cells were washed with PBS three times, then collected with a cell scraper and centrifuged at 2000 rpm for 10 min. Cell aggregates were fixed in 2.5% glutaraldehyde for at least 2 h. After a further wash with PBS, they were then dehydrated in a graded gradient ethanol series and embedded in Epon618. Ultrathin sections of embedded cells were examined by TEM.The cell uptake ratios of CNMs were quantitatively determined by a radiolabeling technique. Here, 188Re labeled CNMs were prepared using the reduction method as described in our previous studies.58 After purification, the labeling compounds were dispersed by RPMI-1640 cell culture media with or without serum as stock solutions. HeLa cells were seeded in 1 × 105 cells mL−1 in 6-well cell culture plates and were cultured in RPMI-1640 containing 10% fetal bovine serum (FBS) and penicillin/streptomycin at 37 °C in 5% CO2 and 95% air. After 24 h of cell attachment, the cells were treated with 0.8 μCi 188Re–ND, 188Re–CNT, and 188Re–GO for 2 h at 37 °C in serum-free and serum-containing RPMI-1640 cell culture media. The cells were washed with PBS three times and then digested with 0.25% trypsin, and resuspended in 1 mL cell culture medium for further testing. Equal portions of stock solutions were taken as a reference for decay correction and counted simultaneously for evaluation of the cell uptake efficiency of these materials. Cell uptake assays were done in triplicate. Data were expressed as mean ± standard deviation (SD) of three independent experiments.
Cell viability of CNMs
The cytotoxicity of CNMs toward HeLa cells was evaluated by MTT assay as described by our previous report.59 Briefly, the MTT assay depends on the mitochondrial enzyme reduction of a tetrazolium dye to detect and determine cell viability. Cells were seeded in 24-well microplates at a density of 1 × 105 cells mL−1 in 1 mL of the respective media containing 10% FBS. After 24 h of cell attachment, the plates were washed with PBS and the cells were treated with 0, 5, 10, 20, 40, 80 μg mL−1 CNTs, GO, and ND prepared in 10% FBS containing media for 2 h. Then all samples were washed with PBS three times to remove the uninternalized CNMs, and cells were cultured with the complete cell culture medium for up to 24 h. MTT was added to evaluate the cytotoxicity induced by CNMs. The amount of dark blue formazan dye generated by the live cells was proportional to the number of live cells, and the absorbance at 570 nm was measured by using a microplate reader (Bio-Rad, model 680). Four replicate wells were used for each control and test concentration per microplate, and the experiment was repeated three times. Cell survival was expressed as absorbance relative to that of untreated controls. Results are presented as the mean ± SD.Determination of biochemical assays
HeLa cells were treated with different concentrations of CNMs as described above. Cell culture supernatants were collected, and LDH activity was measured using a colorimetric LDH assay kit (Nanjing Jiancheng Bioenginering Institute, China). To determine the activities of SOD and MDA, cells were exposed to 10 and 80 μg mL−1 CNTs, GO, and ND as described above, then cells were collected and lysed, the SOD activity was measured using a SOD assay kit (Nanjing Jiancheng Bioengineering Institute, China), and the MDA content was measured using a MDA assay kit (Nanjing Jiancheng Bioengineering Institute, China). Results are expressed as the mean ± SD. Biochemical assays for LDH, SOD, and MDA were performed in duplicate or triplicate for each specific sample. Therefore, all the data points are the mean of numbers that themselves are the mean of duplicate or triplicate measurements for these parameters. It is worth pointing out that all the biochemical index data were normalized by the cell viability of CNMs.Detection of ROS generation
The ability of CNMs to induce intracellular ROS formation was determined using DCFH-DA assay according to our previous work.59 In brief, HeLa cells were cultured in 24-well plates and incubated with different concentrations (5, 10, 20, 40, 80 μg mL−1) of ND, CNTs and GO for 3 h and 24 h, respectively. After being washed three times with PBS to remove the uninternalized CNMs, the cells were subsequently incubated in 500 μL of a working solution of 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma), a fluorogenic probe commonly used to detect intracellular generation of ROS at 37 °C for 30 min. Fluorescence data of oxidized DCFH-DA were recorded by using a filter based microplate reader (Tecan Infinite F200, Tecan AG, Switzerland) with the excitation and emission wavelengths set at 485 and 535 nm, respectively. The values were expressed as a percentage of fluorescence intensity relative to control wells and normalized to the absorbance in the control cells. All the procedures were performed without exposure to light, and the data were normalized to the same numbers of cells.Conclusions
The cell uptake and cytotoxicities of MWCNTs, GO, and ND were systematically determined and compared. Our results demonstrated that CNMs were readily taken up by HeLa cells with significantly different cell uptake efficiencies. Among them, ND exhibited the highest cell uptake ratio, MWCNTs showed a medium cell uptake ratio, and GO displayed the lowest cell uptake ratio. However, the cytotoxicity of CNMs was not associated with their cell uptake ratios. ND possessed the highest cell uptake ratio while exhibiting the best biocompatibility with HeLa cells. Although the cell uptake ratio of MWCNTs was apparently higher than that of GO, their cytotoxicity showed no significant difference. These results suggested that ND might be a promising candidate for further biomedical applications due to it having the highest cell uptake efficiency and best biocompatibility. On the other hand, although GO has been investigated for biomedical applications such as cell imaging and drug delivery, much effort is required to improve its cell uptake efficiency and decrease its cytotoxicity.Acknowledgements
This work was supported by the National Science Foundation of China (No. 10905086, 10975179), the Shanghai Municipal Natural Science Foundation (No. 08ZR1422700, 08JC1422600), the Ministry of Health (No. 2009ZX10004-301), the CAS Innovation Program, and the MOST973 Program (No. 2006CB705605).Notes and references
- L. Dong, R. R. S. Gari, Z. Li, M. M. Craig and S. Hou, Carbon, 2010, 48, 781–787 CrossRef CAS.
- P. Ayala, R. Arenal, M. Rümmeli, A. Rubio and T. Pichler, Carbon, 2010, 48, 575–586 CrossRef CAS.
- S. S. Yu and W. T. Zheng, Nanoscale, 2010, 2, 1069–1082 RSC.
- Y. Huang, X. Dong, Y. Shi, C. M. Li, L. J. Li and P. Chen, Nanoscale, 2010, 2, 1485–1488 RSC.
- C. Soldano, A. Mahmood and E. Dujardin, Carbon, 2010, 48, 2127–2150 CrossRef CAS.
- W. Hu, C. Peng, W. Luo, M. Lv, X. Li, D. Li, Q. Huang and C. Fan, ACS Nano, 2010, 4, 4317–4323 CrossRef CAS.
- J. Li, Y. Zhu, W. Li, X. Zhang, Y. Peng and Q. Huang, Biomaterials, 2010, 31, 8410–8418 CrossRef CAS.
- X. Cai, J. Hao, X. Zhang, B. Yu, J. Ren, C. Luo, Q. Li, Q. Huang, X. Shi and W. Li, Toxicol. Appl. Pharmacol., 2010, 243, 27–34 CrossRef CAS.
- R. H. Hurt, M. Monthioux and A. Kane, Carbon, 2006, 44, 1028–1033 CrossRef CAS.
- C. Lam, J. T. James, R. McCluskey, S. Arepalli and R. L. Hunter, Crit. Rev. Toxicol., 2006, 36, 189–217 CrossRef CAS.
- N. Lewinski, V. Colvin and R. Drezek, Small, 2008, 4, 26–49 CrossRef CAS.
- Y. P. Sun, K. Fu, Y. Lin and W. Huang, Acc. Chem. Res., 2002, 35, 1096–1104 CrossRef CAS.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS.
- A. Krueger, Chem.–Eur. J., 2008, 14, 1382–1390 CrossRef CAS.
- Z. Liu, S. Tabakman, K. Welsher and H. Dai, J. Nanopart. Res., 2009, 2, 85–120 CAS.
- Y. Xing and L. Dai, Nanomedicine, 2009, 4, 207–218 CrossRef CAS.
- V. Vaijayanthimala and H. C. Chang, Nanomedicine, 2009, 4, 47–55 CrossRef CAS.
- D. B. Warheit, B. R. Laurence, K. L. Reed, D. H. Roach, G. A. M. Reynolds and T. R. Webb, Toxicol. Sci., 2004, 77, 117–125 CrossRef CAS.
- N. Shinohara, K. Matsumoto, S. Endoh, J. Maru and J. Nakanishi, Toxicol. Lett., 2009, 191, 289–296 CrossRef CAS.
- A. M. Schrand, H. Huang, C. Carlson, J. J. Schlager, E. ōsawa, S. M. Hussain and L. Dai, J. Phys. Chem. B, 2007, 111, 2–7 CrossRef CAS.
- C. M. Sayes, A. M. Gobin, K. D. Ausman, J. Mendez, J. L. West and V. L. Colvin, Biomaterials, 2005, 26, 7587–7595 CrossRef CAS.
- A. Casey, E. Herzog, F. M. Lyng, H. J. Byrne, G. Chambers and M. Davoren, Toxicol. Lett., 2008, 179, 78–84 CrossRef CAS.
- C. W. Lam, J. T. James, R. McCluskey and R. L. Hunter, Toxicol. Sci., 2004, 77, 126–134 CrossRef CAS.
- X. Zhang, J. Yin, C. Kang, J. Li, Y. Zhu, W. Li, Q. Huang and Z. Zhu, Toxicol. Lett., 2010, 198, 237–243 CrossRef CAS.
- Y. Zhang, S. F. Ali, E. Dervishi, Y. Xu, Z. Li, D. Casciano and A. S. Biris, ACS Nano, 2010, 706–710 Search PubMed.
- Y. Chang, S. T. Yang, J. H. Liu, E. Dong, Y. Wang, A. Cao, Y. Liu and H. Wang, Toxicol. Lett., 2011, 200, 201–210 CrossRef CAS.
- W. Hu, C. Peng, M. Lv, X. Li, Y. Zhang, N. Chen, C. Fan and Q. Huang, ACS Nano, 2011, 5, 3693–3700 CrossRef CAS.
- H. Huang, E. Pierstorff, E. Osawa and D. Ho, ACS Nano, 2008, 2, 203–212 CrossRef CAS.
- R. A. Shimkunas, E. Robinson, R. Lam, S. Lu, X. Xu, X. Q. Zhang, H. Huang, E. Osawa and D. Ho, Biomaterials, 2009, 30, 5720–5728 CrossRef CAS.
- K. K. Liu, C. C. Wang, C. L. Cheng and J. I. Chao, Biomaterials, 2009, 30, 4249–4259 CrossRef CAS.
- X. Q. Zhang, M. Chen, R. Lam, X. Xu, E. Osawa and D. Ho, ACS Nano, 2009, 3, 2609–2616 CrossRef CAS.
- H. Huang, E. Pierstorff, E. Osawa and D. Ho, Nano Lett., 2007, 7, 3305–3314 CrossRef CAS.
- S. J. Yu, M. W. Kang, H. C. Chang, K. M. Chen and Y. C. Yu, J. Am. Chem. Soc., 2005, 127, 17604–17605 CrossRef CAS.
- Y. R. Chang, H. Y. Lee, K. Chen, C. C. Chang, D. S. Tsai, C. C. Fu, T. S. Lim, Y. K. Tzeng, C. Y. Fang and C. C. Han, Nat. Nanotechnol., 2008, 3, 284–288 CrossRef CAS.
- C. C. Fu, H. Y. Lee, K. Chen, T. S. Lim, H. Y. Wu, P. K. Lin, P. K. Wei, P. H. Tsao, H. C. Chang and W. Fann, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 727–732 CrossRef CAS.
- V. N. Mochalin and Y. Gogotsi, J. Am. Chem. Soc., 2009, 131, 4594–4595 CrossRef CAS.
- C. Shan, H. Yang, J. Song, D. Han, A. Ivaska and L. Niu, Anal. Chem., 2009, 81, 2378–2382 CrossRef CAS.
- S. He, B. Song, C. Z. Di Li, W. Qi, Y. Wen, L. Wang, S. Song, H. Fang and C. Fan, Adv. Funct. Mater., 2010, 20, 453–459 CrossRef CAS.
- C. H. Lu, H. H. Yang, C. L. Zhu, X. Chen and G. N. Chen, Angew. Chem., 2009, 121, 4879–4881 CrossRef.
- K. Yang, S. Zhang, G. Zhang, X. Sun, S. T. Lee and Z. Liu, Nano Lett., 2010, 10, 3318–3323 CrossRef CAS.
- Z. Liu, X. Sun, N. Nakayama-Ratchford and H. Dai, ACS Nano, 2007, 1, 50–56 CrossRef CAS.
- A. A. Bhirde, V. Patel, J. Gavard, G. Zhang, A. A. Sousa, A. Masedunskas, R. D. Leapman, R. Weigert, J. S. Gutkind and J. F. Rusling, ACS Nano, 2009, 3, 307–316 CrossRef CAS.
- Y. Wu, J. A. Phillips, H. Liu, R. Yang and W. Tan, ACS Nano, 2008, 2, 2023–2028 CrossRef CAS.
- S. Gilje, S. Han, M. Wang, K. L. Wang and R. B. Kaner, Nano Lett., 2007, 7, 3394–3398 CrossRef CAS.
- S. Alwarappan, A. Erdem, C. Liu and C. Z. Li, J. Phys. Chem. C, 2009, 113, 8853–8857 CAS.
- X. Wang, L. Zhi and K. Müllen, Nano Lett., 2008, 8, 323–327 CrossRef CAS.
- Q. He, H. G. Sudibya, Z. Yin, S. Wu, H. Li, F. Boey, W. Huang, P. Chen and H. Zhang, ACS Nano, 2010, 4, 3201–3208 CrossRef CAS.
- T. S. Huang, Y. Tzeng, Y. K. Liu, Y. C. Chen, K. R. Walker, R. Guntupalli and C. Liu, Diamond Relat. Mater., 2004, 13, 1098–1102 CrossRef CAS.
- J. Yan, Z. Fan, T. Wei, W. Qian, M. Zhang and F. Wei, Carbon, 2010, 48, 3825–3833 CrossRef CAS.
- Y. Li, W. Gao, L. Ci, C. Wang and P. M. Ajayan, Carbon, 2010, 48, 1124–1130 CrossRef CAS.
- J. Yan, T. Wei, B. Shao, Z. Fan, W. Qian, M. Zhang and F. Wei, Carbon, 2010, 48, 487–493 CrossRef CAS.
- B. Tian, C. Wang, S. Zhang, L. Feng and Z. Liu, ACS Nano, 2011, 5, 7000–7009 CrossRef CAS.
- L. Lacerda, A. Bianco, M. Prato and K. Kostarelos, Adv. Drug Delivery Rev., 2006, 58, 1460–1470 CrossRef CAS.
- C. Medina, M. J. Santos-Martinez, A. Radomski, O. I. Corrigan and M. W. Radomski, Br. J. Pharmacol., 2007, 150, 552–558 CrossRef CAS.
- A. M. Schrand, L. Dai, J. J. Schlager, S. M. Hussain and E. Osawa, Diamond Relat. Mater., 2007, 16, 2118–2123 CrossRef CAS.
- X. Zhang, Y. Zhu, J. Li, Z. Zhu, W. Li and Q. Huang, J. Nanopart. Res., 2011, 13, 6941–6952 CrossRef CAS.
- X. Zhang, J. Yin, C. Peng, W. Hu, Z. Zhu, W. Li, C. Fan and Q. Huang, Carbon, 2011, 49, 986–995 CrossRef CAS.
- X. Y. Zhang, J. Li, Y. Zhu, Y. J. Qi, Z. Y. Zhu, W. X. Li and Q. Huang, Nucl. Sci. Technol., 2011, 22, 99–104 CAS.
- Y. Zhu, W. Li, Q. Li, Y. Li, X. Zhang and Q. Huang, Carbon, 2009, 47, 1351–1358 CrossRef CAS.
- K. Kostarelos, L. Lacerda, G. Pastorin, W. Wu, S. Wieckowski, J. Luangsivilay, S. Godefroy, D. Pantarotto, J. P. Briand and S. Muller, Nat. Nanotechnol., 2007, 2, 108–113 CrossRef CAS.
- K. Wang, J. Ruan, H. Song, J. Zhang, Y. Wo, S. Guo and D. Cui, Nanoscale Res. Lett., 2010, 6, 1–8 Search PubMed.
- L. Zhan, G. Yanxia, Z. Xiaoyong, Q. Wei, F. Qiaohui, L. Yan, J. Zongxian, W. Jianjun, T. Yuqin, D. Xiaojiang and W. Wangsuo, J. Nanopart. Res., 2011, 13, 2939–2947 CrossRef.
- T. L. Wee, Y. W. Mau, C. Y. Fang, H. L. Hsu, C. C. Han and H. C. Chang, Diamond Relat. Mater., 2009, 18, 567–573 CrossRef CAS.
- O. Faklaris, V. Joshi, T. Irinopoulou, P. Tauc, M. Sennour, H. Girard, C. Gesset, J. C. Arnault, A. Thorel and J. P. Boudou, ACS Nano, 2009, 3, 3955–3962 CrossRef CAS.
- V. Vaijayanthimala, Y. K. Tzeng, H. C. Chang and C. L. Li, Nanotechnology, 2009, 20, 425103 CrossRef.
- C. Y. Fang, V. Vaijayanthimala, C. A. Cheng, S. H. Yeh, C. F. Chang, C. L. Li and H. C. Chang, Small, 2011, 7, 3363–3370 CrossRef CAS.
- A. M. Schrand, J. B. Lin, S. C. Hens and S. M. Hussain, Nanoscale, 2011, 3, 435–445 RSC.
- K. T. Al-Jamal, H. Nerl, K. H. Müller, H. Ali-Boucetta, S. Li, P. D. Haynes, J. R. Jinschek, M. Prato, A. Bianco and K. Kostarelos, Nanoscale, 2011, 3, 2627–2635 RSC.
- K. Yang, J. Wan, S. Zhang, Y. Zhang, S. T. Lee and Z. Liu, ACS Nano, 2011, 5, 516–522 CrossRef CAS.
- A. Nel, T. Xia, L. Madler and N. Li, Science, 2006, 311, 622–627 CrossRef CAS.
- K. Pulskamp, S. Diabaté and H. F. Krug, Toxicol. Lett., 2007, 168, 58–74 CrossRef CAS.
- G. Jia, H. Wang, L. Yan, X. Wang, R. Pei, T. Yan, Y. Zhao and X. Guo, Environ. Sci. Technol., 2005, 39, 1378–1383 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2tx20006f |
| This journal is © The Royal Society of Chemistry 2012 |
