Bioavailability and preliminary toxicity evaluations of alumina nanoparticles in vivo after oral exposure
Sheng-Tao
Yang
ab,
Tiancheng
Wang
c,
Erya
Dong
b,
Xin-Xin
Chen
b,
Kun
Xiang
b,
Jia-Hui
Liu
bd,
Yuanfang
Liu
bd and
Haifang
Wang
*b
aCollege of Chemistry and Environment Protection Engineering, Southwest University for Nationalities, Chengdu 610041, China
bInstitute of Nanochemistry and Nanobiology, Shanghai University, Shanghai 200444, China. E-mail: hwang@shu.edu.cn; Tel: +86-21-66138026
cDepartment of Clinical Laboratory, Third Hospital of Peking University, Beijing 100083, China
dBeijing National Laboratory for Molecular Sciences, Department of Chemical Biology, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China
First published on 1st May 2012
Abstract
Alumina nanoparticles (NPs) are among the most important nanomaterials and are widely used in diverse areas. In this study, we evaluated the bioavailability and toxicity of alumina NPs in mice after oral exposure, compared with traditional alumina powder. Our results indicated that negligible alumina NPs were absorbed post-exposure and alumina NPs did not influence the balance of essential trace elements, including Fe, Cu and Zn. Preliminary toxicological evaluations suggested that alumina NPs were of low toxicity. The body weights were similar among the mice exposed to alumina NPs, alumina powder and 0.9% NaCl aqueous solution. The low toxicity was also indicated by the unchanged serum biochemical parameters. The implications related to the ongoing safety evaluations and applications of alumina NPs are discussed.
1. Introduction
Alumina nanoparticles (NPs), which dominated approximately 20% of the nanomaterial world market in 2005, are among the most important nanomaterials. Alumina NPs have been widely used in a wide range of industries, such as ceramics, catalysis and optical polishing. In food, medicine, personal care and water treatment, alumina NPs are also indispensable.1–4 With the increase of annual production and applications, alumina NPs are becoming more prevalent in our daily life and the surrounding environment. Thus, the occupational and public exposures to alumina NPs have become inevitable. Therefore, a thorough investigation of their biological behaviors and potential toxicity should be carried out to ensure the safe applications of alumina NPs.5,6A detailed safety evaluation of alumina NPs is still lacking to date. A few pilot studies have investigated their biosafety using different models.7–13 Alumina NPs were of low toxicity to bacteria, cells, animals and plants.7–10 However, alumina NPs may induce toxicity upon entering the gastrointestinal tract, due to their increasing presence in food, medicine, water treatment and so on.1–4 Only a few studies have documented the oral toxicity of alumina NPs.11–13 Park et al. reported the absorption and moderate toxicity of alumina NPs to mouse brain after oral exposure.11 Balasubramanyam et al. found that alumina NPs induced genotoxicity to rats after oral exposure.12,13 However, the absorption, translocation and toxicity of alumina NPs after oral exposure are not yet well understood.
Herein, we studied the absorption and toxicity of alumina NPs in mice after oral exposure, compared with traditional alumina powder. The contents of aluminum and other trace elements in different tissues were analyzed by inductively coupled plasma-atomic emission spectrometry (ICP-AES). The toxicity of alumina NPs to mice was preliminarily evaluated by monitoring the body weight and measuring the serum biochemical parameters. Our results indicated that alumina NPs could not be absorbed after oral exposure and did not disturb the trace element balance. Very low toxicity of alumina NPs was presented in the preliminary toxicological evaluations. The implications regarding safety evaluations and applications of alumina NPs are discussed.
2. Materials and methods
2.1. Characterization of alumina NPs
Alumina NPs were purchased from Shanghui Co. (Shanghai, China). Traditional alumina powder was bought from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Other chemicals used were of analytical grade.The alumina NPs and alumina powder were carefully characterized before use. The shape and size were investigated by transmission electron microscopy (TEM, JEM-200CX, JEOL, Japan). The crystalline phase was characterized by X-ray diffraction (XRD, Rigaku, Tokyo, Japan). The specific surface area (SSA) was obtained by Brunauer–Emmett–Teller (BET) technique (ASAP2010, Micromeritics, USA). X-Ray fluorescence (XRF, S4-Explorer, Bruker, Germany) was adopted to analyze the purity. The suspensions of alumina NPs and alumina powder were prepared by dispersing alumina NPs and alumina powder in 0.9% NaCl solution under sonication (1 h, 40 kHz, 50 W). During the sonication, the water in the sonicator bath was replaced every 20 min to prevent the increase of temperature.
2.2. Animal administration
All animal experiments were performed in compliance with relevant national law, the institutional ethics committee regulations and guidelines on animal welfare (Animal Care and Use Program Guidelines of Peking University). Male ICR mice (∼25 g) were bought from Peking University Animal Centre, Beijing, China. The mice were housed in plastic cages (five mice per cage) and kept on a 12 h light–dark cycle. Food and water were provided ad libitum. Following acclimation, mice were randomly divided into groups of 3 for the bioavailability evaluations and groups of 5 for the toxicity assays.2.3. Bioavailability of alumina NPs
The mice were exposed to alumina NPs and alumina powder at a single dose of 500 mg kg−1 bodyweight (b.w.) by gavage (0.2 mL). Mice administered with 0.9% NaCl aqueous solution (0.2 mL) were taken as the control. At 1 d post-exposure, the mice were sacrificed and the tissues were collected for metal content determination.The tissues were washed with cold phosphate buffer (PBS) twice. After weighing, the tissue was cut into small pieces, soaked in 5 mL of HNO3 (14 mol L−1) and heated to boil. Thirty minutes later, there was 2 mL of solution left. Then, 1 mL of H2O2 was added dropwise under continuous heating. When no bubbles were generated, another 2 mL of HNO3 was added. The solution was kept boiling for 20 min. When there was 3 mL of solution left, another 1 mL of H2O2 was added dropwise. The solution became colorless. After it was cooled to room temperature, the solution was diluted to 10 mL with 2% HNO3 aqueous solution for determining the contents of Al, Fe, Cu and Zn by ICP-AES (Profile, Leeman, USA). The above process was performed without adding any tissue for preparing the blank samples.
2.4. Toxicity of alumina NPs
For the toxicity study, the mice were gavaged with alumina NPs and alumina powder at a single dose of 50 and 500 mg kg−1 b.w. (0.2 mL). Mice gavaged with 0.9% NaCl aqueous solution (0.2 mL) were taken as the control. The behaviors of the mice were recorded daily. At 1, 7 and 14 d post-exposure, mice were weighed and sacrificed. Blood samples were collected for serum biochemistry analyses.Serum samples were obtained from blood samples by centrifugation (3000 rpm for 10 min). The biochemical assays were performed on a Hitachi 7170A clinical automatic chemistry analyzer (Japan). Lactate dehydrogenase (LDH), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatinine (Cr), uric acid (UA), creatine kinase (CK) and total hemolytic complement levels (CH50) were measured using the commercial kits (Bühlmann Laboratories, Switzerland).
2.5. Statistical analysis
All data were presented as the mean of three or five individual observations with the standard deviation (mean ± SD). Significance was calculated using Student’s t-test. The difference was considered significant if p < 0.05.3. Results and discussion
3.1. Characterization of alumina NPs
Alumina NPs and alumina powder were carefully characterized. The alumina NPs and alumina powder were imaged by TEM (Fig. 1). Alumina NPs were rod-like, and alumina powder was irregular. Alumina NPs formed small aggregates, and nanosized rods were clearly recognized under higher magnification. In the TEM image of alumina powder there were some smaller particulates. Under higher magnification, some pores were observed in the alumina powder. The crystalline patterns of both samples were gamma-phase according to XRD analyses. BET analyses suggested that both samples had a large specific surface area (158 m2 g−1 for alumina NPs and 136 m2 g−1 for alumina powder). According to XRF measurements, the purity of alumina NPs was higher than 99.9%, and the purity of alumina powder was higher than 95%. The characterization data indicated that both samples were suitable for the bioavailability and toxicity evaluations.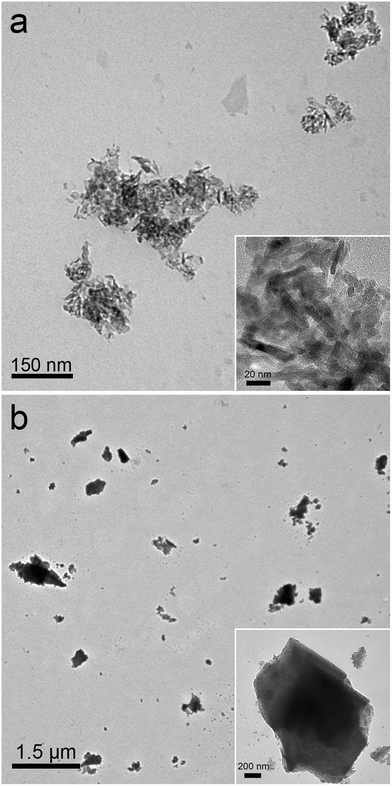 | ||
| Fig. 1 Representative TEM images of alumina NPs (a) and alumina powder (b). | ||
3.2. Bioavailability of alumina NPs
Alumina NPs and alumina powder were administrated to mice by gavage. As shown in Fig. 2, the Al content in different organs was similar to that in the corresponding organs of the control group. This indicated that there was no statistically significant uptake of alumina NPs after gavage exposure. Alumina powder was not absorbed by mice, either. We have highlighted the absorption at 1 d post-exposure here, because most alumina had left the gastrointestinal tract at that time.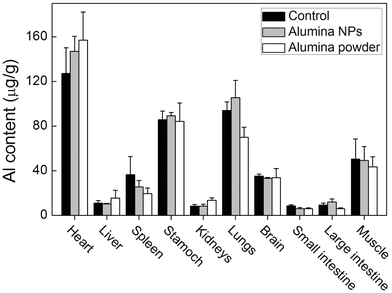 | ||
| Fig. 2 Bioavailability of alumina NPs and alumina powder in mice at 1 d after gavage exposure (n = 3). | ||
It seems that the absorption behaviors of different alumina NPs are different. Balasubramanyam et al. and Park et al. reported the absorption of alumina NPs after oral exposure separately. Balasubramanyam et al. only found alumina NPs in rat kidneys after gavage exposure (500 mg kg−1 b.w.).12,13 At higher doses (1000 and 2000 mg kg−1 b.w.), alumina NPs were found in many organs, including liver, spleen, heart, kidneys and brain. Park et al. studied the absorption of alumina NPs by mice following a 28 d oral administration with a dose of 60 mg kg−1 b.w. per day.11 At the end of this administration period, Al content had increased in brain, thymus, lungs and kidneys, but decreased in liver, spleen and testis. In our study, we did not find the absorption or down-regulation of Al in tissues after a single dose of 500 mg kg−1 b.w. Thus, the currently available data are inconsistent.
It is well-known that the physicochemical properties of NPs affect their absorption after oral exposure.14–17 For example, we found that hydroxylated carbon nanotubes (CNTs) were absorbed efficiently, but taurine functionalized CNTs could not be absorbed.14,15 Wang et al. reported that zinc oxide NPs (120 nm) accumulated more in bone than zinc oxide NPs (20 nm) after oral exposure.16 Therefore, we inferred that the inconsistent absorption data might be due to the different properties of alumina NPs. Because of the lack of systematic characterization in literature, we could not compare these alumina NP samples item by item. The shape and size of alumina NPs, the animal models (rats and mice), the administration methods (single dose and multiple doses) and the administration doses might contribute to the inconsistent absorption results.11–13 In future evaluations, careful characterization of NP samples should be performed to enable the comparison among different labs as the parameters regulating the absorption of alumina NPs need to be revealed.
3.3. Influence of alumina NPs on the element balance
The exposure to alumina NPs may influence the balance of trace elements of mice. To address this issue, we measured the trace element contents in organs, focusing on the three most important essential metals (Fe, Cu and Zn). Their imbalance will induce serious damage to the body. The competitive transport of Al3+ and the toxicity of alumina NPs may alter the trace element balance. As shown in Fig. 3, alumina NPs did not impact the Fe content in different organs. The exposure to alumina NPs led to a slight increase of Cu content in liver, but Cu content in other organs was not influenced. Alumina powder did not affect the Cu content in any organ. Zn content was not affected by alumina NPs either, but a slight decrease of Zn content in lungs was observed after the exposure to alumina powder. Overall, both alumina NPs and alumina powder had only a very tiny influence on the trace element balance in mice.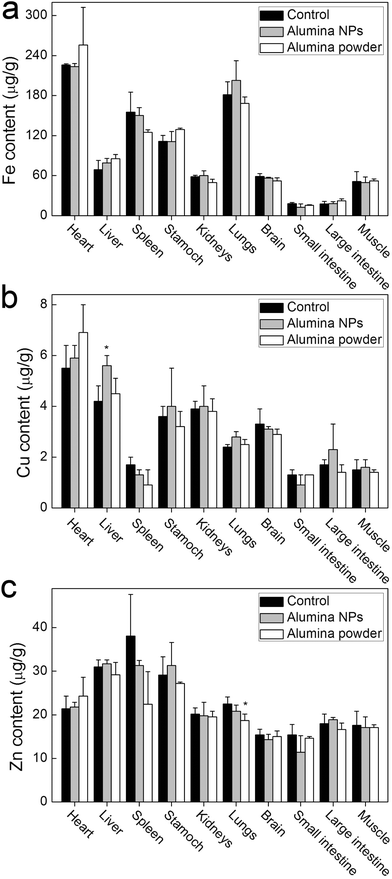 | ||
| Fig. 3 Influence of alumina NPs and alumina powder on the content of Fe, Cu and Zn in mice 1 d after gavage exposure (n = 3); *p < 0.05 compared with the control group. | ||
Element balance is a very important issue. The imbalance of essential elements leads to toxicity.18 The influence of NPs on element balance has not been well documented. Previously, Gao et al. reported that copper NPs increased the K level and changed the distribution pattern of Fe and Zn in Caenorhabditis elegans.19 Our results suggest that alumina NPs have a limited influence on the trace elements in mice after oral exposure. To this end, alumina NPs are expected to be less toxic than copper NPs.
3.4. Toxicity of alumina NPs
The acute toxicity of alumina NPs to mice was preliminarily evaluated. The mice behaved normally after the oral exposure to alumina NPs. Abnormalities, including lethargy, anorexia, vomiting or diarrhea, were not presented during the 14-d observation. The body weight increases were similar among the exposed groups and the control group (Table 1). Neither alumina NPs nor alumina powder affected the body weights of mice. The clinical behaviors indicated that both alumina NPs and alumina powder were of low toxicity.| Time (d) | Body weight (g) | ||||
|---|---|---|---|---|---|
| Control | NPs (L)a | NPs (H)a | Powder (L)a | Powder (H)a | |
| a L: low dose (50 mg kg−1 b.w.); H: high dose (500 mg kg−1 b.w.). | |||||
| 1 | 28.1 ± 1.9 | 27.4 ± 1.7 | 27.5 ± 2.2 | 28.9 ± 1.4 | 27.6 ± 1.2 |
| 7 | 33.0 ± 2.8 | 33.5 ± 2.7 | 31.1 ± 1.7 | 32.9 ± 2.7 | 32.7 ± 2.1 |
| 14 | 35.5 ± 1.7 | 34.3 ± 1.9 | 35.0 ± 3.0 | 35.6 ± 1.6 | 35.1 ± 3.5 |
The toxicity of alumina NPs was further evaluated by analyzing the serum biochemical parameters. LDH is a general indicator for functional damage. At 1 d post-exposure, LDH levels slightly increased after exposure to alumina NPs (500 mg kg−1 b.w.) and alumina powder (50 and 500 mg kg−1 b.w.). The increase in LDH levels indicated that alumina NPs and alumina powder induced some toxicity to mice. ALT and AST are two important and sensitive indicators for hepatic damage. The ALT level increased in the alumina NPs (50 mg kg−1 b.w.) group, suggesting that alumina NPs might induce hepatic toxicity at low dose. BUN, Cr and UA are indicators for renal toxicity. The BUN level increased slightly in the alumina NPs (50 mg kg−1 b.w.) group, which suggested possible renal toxicity of alumina NPs. CK, a general indicator for heart damage, was not affected at 1 d post-exposure of alumina NPs and alumina powder, indicating a non-toxic effect on the heart. Considering aluminum hydroxide is a very powerful immunologic adjuvant, thus, alumina might have an impact on the immunological activity of mice. The CH50 levels, an indicator of complement activation, were measured. The values of CH50 level suggested that alumina NPs and alumina powder did not influence the immunological activity after oral exposure (Fig. 4).
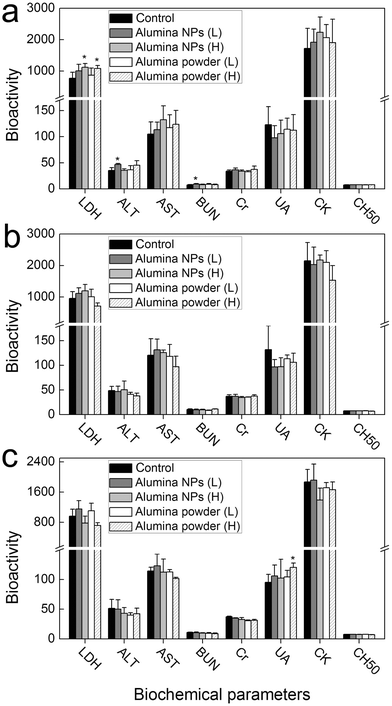 | ||
| Fig. 4 The influence of alumina NPs and alumina powder on the serum biochemical parameters of mice at 1 (a), 7 (b) and 14 (c) d post-exposure after oral exposure (n = 5). L: low dose (50 mg kg−1 b.w.); H: high dose (500 mg kg−1 b.w.); * p < 0.05 compared with the control group. | ||
With the time elapsed, the toxicity of alumina NPs faded. At 7 d post-exposure, no serum biological parameters were affected by alumina exposure. At 14 d post-exposure, only the UA level had increased in the alumina powder (500 mg kg−1 b.w.) group. Overall, the serum biochemical analyses indicated that alumina NPs induced low toxicity to mice at 1 d post oral exposure, which disappeared after that.
Toxicity evaluations of alumina NPs after oral exposure are currently scarce. Previously, Park et al. investigated the toxicity of alumina NPs following a 28 d oral administration.11 Alumina NPs enhanced the food and water consuming of the exposed groups, but slightly inhibited the body weight increase at 30 and 60 mg kg−1 b.w. per day. At 60 mg kg−1 b.w. per day, alumina NPs were toxic according to hematology analyses, serum biochemistry, transforming growth factor-β (TGF-β) and gene expression analyses. Another evaluation was performed by Balasubramanyam et al., where alumina NPs showed genotoxicity to blood cells and bone marrow after oral exposure.12,13 Our results suggest that alumina NPs induced low and reversible toxicity to mice. Nevertheless, the available data in our study and in the literature collectively suggest that the toxicity of alumina NPs is low. The very limited oral toxicity of alumina NPs is reasonable, since low toxicity of alumina NPs has already been evidenced using other models.7–13 We have reported that alumina NPs were of low toxicity and biocompatible with neural stem cells.8 The toxicity of alumina NPs to bacteria, fish and plants was also found to be low.7–10,20 The low toxicity of alumina NPs would enable the safe use of them in diverse areas.
Mechanistically, the toxicity of metal oxide NPs might come from the particles themselves or the dissolved metal ions.21–23 Aluminum is well known as a toxicant to the nervous system.24 However, alumina NPs hardly dissolved in a simulated biological environment in our previous cytotoxicity evaluation8 and only a very small portion of them dissolved in the stomach. Therefore, we infer that the toxicity of alumina NPs is mainly due to the particles, rather than dissolved aluminum ions. On the other hand, oxidative stress might be the toxicological mechanism of alumina NPs. We observed the generation of reactive oxygen species (ROS) in neural stem cells upon the exposure to high concentrations of alumina NPs.8 The oxidative damage was also observed in the studies of other metal oxide NPs.25 In future studies, the toxicological mechanism of alumina NPs in vivo should be systematically investigated.
4. Conclusion
In summary, we studied the absorption and toxicity of alumina NPs after oral exposure to mice, where no significant absorption and low toxicity of alumina NPs were observed. In addition, oral exposure to alumina NPs did not disturb the element balance. The oral toxicity of alumina NPs was very low and vanished with time in the course of the clinical observations and serum biochemistry. These insignificant biological behaviors may enable the safe applications of alumina NPs in diverse areas. Our results may stimulate more interest in the oral toxicity of NPs.Acknowledgements
We acknowledge financial support from the China Ministry of Science and Technology (973 Project No. 2011CB933402), the China Natural Science Foundation (No. 21071094), Key Project of Chinese Ministry of Education (211053) and the Fundamental Research Funds for the Central Universities, Southwest University for Nationalities (No. 11NZYBS06).References
- I. M. Sadiq, B. Chowdhury, N. Chandrasekaran and A. Mukherjee, Nanomed.: Nanotechnol., Biol. Med., 2009, 5, 282 CrossRef CAS.
- A. R. Studart, E. Amstad and L. J. Gauckler, Langmuir, 2007, 23, 1081 CrossRef CAS.
- A. Afkhami, M. Saber-Tehrani, H. Bagheri and T. Madrakian, Microchim. Acta, 2011, 173, 543 CrossRef CAS.
- B. Paul, W. N. Martens and R. L. Frost, J. Colloid Interface Sci., 2011, 360, 132 CrossRef CAS.
- G. Oberdorster, E. Oberdorster and J. Oberdorster, Environ. Health Perspect., 2005, 113, 823 CrossRef CAS.
- A. Nel, T. Xia, L. Madler and N. Li, Science, 2006, 311, 622 CrossRef CAS.
- W. Jiang, H. Mashayekhi and B. Xing, Environ. Pollut., 2009, 157, 1619 CrossRef CAS.
- E. Dong, Y. Wang, S.-T. Yang, Y. Yuan, H. Nie, Y. Chang, L. Wang, Y. Liu and H. Wang, J. Nanosci. Nanotechnol., 2010, 11, 7848 CrossRef.
- L. Chen, R. A. Yokel, B. Hennig and M. Toborek, J. Neuroimmune Pharmacol., 2008, 3, 286 CrossRef.
- L. Yang and D. J. Watts, Toxicol. Lett., 2005, 158, 122 CrossRef CAS.
- E.-J. Park, H. Kim, Y. Kim and K. Choi, Toxicol. Environ. Chem., 2011, 93, 120 CrossRef CAS.
- A. Balasubramanyam, N. Sailaja, M. Mahboob, M. F. Rahman, S. M. Hussain and P. Grover, Mutagenesis, 2009, 24, 245 CrossRef CAS.
- A. Balasubramanyam, N. Sailaja, M. Mahboob, M. F. Rahman, S. Misra, S. M. Hussain and P. Grover, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2009, 676, 41 CrossRef CAS.
- X. Deng, G. Jia, H. Wang, H. Sun, X. Wang, S. Yang, T. Wang and Y. Liu, Carbon, 2007, 45, 1419 CrossRef CAS.
- J. Wang, X. Deng, S.-T. Yang, H. Wang, Y. Zhao and Y. Liu, Nanotoxicology, 2008, 2, 28 CrossRef CAS.
- B. Wang, W. Feng, M. Wang, T. Wang, Y. Gu, M. Zhu, H. Ouyang, J. Shi, F. Zhang, Y. Zhao, Z. Chai, H. Wang and J. Wang, J. Nanopart. Res., 2008, 10, 263 CrossRef CAS.
- J. Wang, G. Zhou, C. Chen, H. Yu, T. Wang, Y. Ma, G. Jia, Y. Gao, B. Li, J. Sun, Y. Li, F. Jiao, Y. Zhao and Z. Chai, Toxicol. Lett., 2007, 168, 176 CrossRef CAS.
- C. Hotz, N. M. Lowe, M. Araya and K. H. Brown, J. Nutr., 2003, 133, 1563S CAS.
- Y. Gao, N. Liu, C. Chen, Y. Luo, Y. Li, Z. Zhang, Y. Zhao, B. Zhao, A. Iida and Z. Chai, J. Anal. At. Spectrom., 2008, 23, 1121 RSC.
- X. Zhu, L. Zhu, Z. Duan, R. Qi, Y. Li and Y. Lang, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2008, 43, 278 CrossRef CAS.
- S.-T. Yang, J.-H. Liu, J. Wang, Y. Yuan, A. Cao, H. Wang, Y. Liu and Y. Zhao, J. Nanosci. Nanotechnol., 2010, 10, 8638 CrossRef CAS.
- H. Wang, R. L. Wick and B. Xing, Environ. Pollut., 2009, 157, 1171 CrossRef CAS.
- Z. Chen, H. Meng, G. Xing, C. Chen, Y. Zhao, G. Jia, T. Wang, H. Yuan, C. Ye, F. Zhao, Z. Chai, C. Zhu, X. Fang, B. Ma and L. Wan, Toxicol. Lett., 2006, 163, 109 CrossRef CAS.
- R. A. Yokel, Neurotoxicology, 2000, 21, 813 CAS.
- N. Lewinski, V. Colvin and R. Drezek, Small, 2008, 4, 26 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
