Self-assembly of chiral block and gradient copolymers†
Meta M.
Bloksma
abcd,
Stephanie
Hoeppener
cd,
Cécile
D'Haese
e,
Kristian
Kempe
cd,
Ulrich
Mansfeld
cd,
Renzo M.
Paulus
bcd,
Jean-François
Gohy
e,
Ulrich S.
Schubert
*bcd and
Richard
Hoogenboom
*f
aLaboratory of Macromolecular Chemistry and Nanoscience, Eindhoven University of Technology, P. O. Box 513, 5600 MB, Eindhoven, The Netherlands
bDutch Polymer Institute (DPI), P. O. Box 902, 5600 AX, Eindhoven, The Netherlands. E-mail: ulrich.schubert@uni-jena.de
cLaboratory of Organic and Macromolecular Chemistry (IOMC), Friedrich Schiller University Jena, Humboldtstrasse 10, 07743, Jena, Germany
dJena Center for Soft Matter (JCSM), Friedrich Schiller University Jena, Humboldtstrasse 10, 07743, Jena, Germany
eBio and Soft Matter (BSMA), Institute of Condensed Matter and Nanoscience (IMCN), Université catholique de Louvain, Place L. Pasteur 1, Louvain-la-Neuve, Belgium
fSupramolecular Chemistry Group, Department of Organic Chemistry, Ghent University, Krijgslaan 281 S4, 9000, Ghent, Belgium. E-mail: richard.hoogenboom@ugent.be
First published on 10th November 2011
Abstract
Chiral micelles have a high potential for targeted drug delivery or chiral separation applications. In this contribution the self-assembly of chiral amphiphilic copolymers into chiral structures was investigated. Gradient copolymers could be obtained by statistically copolymerizing the hydrophilic 2-ethyl-2-oxazoline (EtOx) with the hydrophobic chiral R-2-butyl-4-ethyl-2-oxazoline (R-BuEtOx) or racemic RS-BuEtOx monomers. Self-assembly of the gradient enantiopure copolymers was studied by both cryogenic transmission electron spectroscopy (cryo-TEM) and dynamic light scattering (DLS) revealing the formation of spherical micelles in aqueous solution. Additionally, amphiphilic block copolymers were synthesized in a 1-pot-2-step manner. The type of self-assembled structure could be controlled by varying the hydrophobic to hydrophilic ratio within the block copolymer from spherical and cylindrical micelles to sheets and vesicles. When the enantiopure block was replaced by the corresponding racemic block, only spherical micelles could be observed, while the chiral block copolymers with similar hydrophobic content revealed cylindrical micelles.
Introduction
When a hydrophilic polymer segment is combined with a hydrophobic block, an amphiphilic block copolymer is created which can undergo self-assembly in aqueous solution. The type of nanostructure that is formed depends on the hydrophobic to hydrophilic ratio, the block lengths as well as the chemical composition. Spherical micelles, wormlike micelles and vesicles are widely reported self-assembled structures.1–5 Such architectures can be used as detergents and stabilizers as well as for the inclusion of organic molecules for drug delivery applications.6–82-Oxazolines can be polymerized in a living manner via the cationic ring-opening polymerization (CROP) under microwave-assisted conditions.9 Since both hydrophobic and hydrophilic poly(2-oxazoline)s can be synthesized, depending on the substituent on the second position, amphiphilic gradient or block copolymers can be prepared that self-assemble in water. The synthesis and self-assembly of both hydrophilic/lipophilic and hydrophilic/fluorophilic poly(2-oxazoline)s are reported in the literature.10–16
2-Oxazoline monomers that are substituted on the 4th or 5th position comprise a chiral center which is retained upon polymerization resulting in main-chain chiral polymers.17 In previous investigations it was already observed that the chiral main-chain poly-R-2-butyl-4-ethyl-2-oxazoline (p-R-BuEtOx) forms a chiral secondary structure in the solid phase and in solution.18,19 When this hydrophobic chiral polymer is combined with a hydrophilic polymer into an amphiphilic structure, micelles with a chiral hydrophobic core could be obtained in water. Such optically active micelles would be interesting given their potential application in chiral amplification, molecular recognition and catalysis.20
In this contribution we describe the copolymerization of 2-ethyl-2-oxazoline (EtOx) with the chiral R-2-butyl-4-ethyl-2-oxazoline. The kinetics of the copolymerization was determined and block copolymers with various block lengths were synthesized to investigate the influence of the hydrophilic to hydrophobic ratio as well as the monomer distribution on the self-assembled structures in water. The type of nanostructures formed was determined with cryogenic electron transmission microscopy (cryo-TEM) and dynamic light scattering (DLS). Moreover, also copolymers with the racemic RS-BuEtOx were synthesized to investigate the influence of chirality on the formation of the self-assembled supramolecular structures.
Experimental section
Materials and instrumentation
Acetonitrile (Aldrich) was dried in a solvent purification system (Pure Solv EN, Innovative Technology) before use as a polymerization solvent. Methyl tosylate (MeOTs, Aldrich) and 2-ethyl-2-oxazoline (EtOx, Aldrich) were distilled over barium oxide and stored under argon.Reactions were carried out in capped reaction vials designed for the Emrys Liberator microwave system (Biotage) equipped with an IR temperature sensor. The vials were dried in the oven at 105 °C and cooled under argon to room temperature before use.
GC measurements were performed on a Shimadzu GC-2010 equipped with a Restek Rtx-5 column, a FID detector and a PAL autosampler.
Size exclusion chromatography (SEC) was measured on a Shimadzu system equipped with a LC-10AD pump, a RID-10A refractive index detector, a SCL-10A VP system controller, and a PSS SDV pre/lin S column utilizing a chloroform–triethylamine–isopropanol (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture as eluent at a flow rate of 1 mL min−1 and a column temperature of 40 °C. A polystyrene (PS) calibration was used to calculate the molar mass values.
2) mixture as eluent at a flow rate of 1 mL min−1 and a column temperature of 40 °C. A polystyrene (PS) calibration was used to calculate the molar mass values.
Turbidity measurements were performed on a Crystal 16 from Avantium Technologies connected to a chiller (Julabo FP 40) at a wavelength of 500 nm. The sample concentration was 5 mg mL−1 in water and the transmittance was measured at a temperature range from 0 to 100 °C with a heating and cooling rate of 1 °C min−1. The cloud point temperature (CP) was defined as the temperature where the transmittance goes through 5%.
DLS experiments were performed on a Malvern CGS-3 apparatus equipped with a He–Ne laser with a wavelength of 633 nm. The micellar solutions were prepared by dissolving 5 mg of sample in 1 mL of ultrapure water and heating them up to 100 °C followed by cooling back to room temperature. Before the experiments the solutions were filtrated over a 0.2 μm syringe filter. The measurements were performed in water at 25 °C, at different angles and at different concentrations. The data were analyzed using the Cumulants method and the CONTIN method, which is based on an inverse-Laplace transformation of the data and provides access to a size distribution histogram for the analyzed micellar solutions. The polydispersity (PD) of the micelles was estimated from the Γ2/Γ12 ratio in which Γ1 and Γ2 represent the first and second cumulant, respectively. Hydrodynamic radii (Rh) were calculated from the diffusion coefficients by using the Stokes–Einstein approximation.
Cryogenic transmission electron microscopy (cryo-TEM) measurements were performed on a Philips CM120 operating at an acceleration voltage of 120 kV. Images were recorded with a bottom mounted 1k × 1k CCD camera. A drop of the polymer solution (5 mg mL−1) was placed on a perforated carbon grid in an in-house-built controlled environment vitrification system (CEVS), blotted and rapidly plunged into a cryogen reservoir containing liquid ethane. After preparation the samples were stored and measured at a temperature of approximately 180 °C to avoid the formation of crystalline ice layers.
Monomer synthesis
R-2-Butyl-4-ethyl-2-oxazoline (R-BuEtOx) and RS-BuEtOx were synthesized in the same way as described in a previously published paper.19Kinetic investigation of the microwave-assisted copolymerization
Two stock solutions with an initial total monomer concentration (M0) of 4 M of EtOx and R-BuEtOx or RS-BuEtOx together with the initiator MeOTs with a [EtOx]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BuEtOx]
[BuEtOx]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [MeOTs] ratio of 70
[MeOTs] ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were prepared in acetonitrile. The stock solution was divided into eight microwave vials (1 mL each), which were heated in the microwave to 140 °C for different times, cooled by compressed nitrogen and quenched by the addition of water after the desired polymerization time. The resulting mixtures were analyzed by GC and SEC to determine the monomer conversion and polymer molar mass (distribution), respectively.
1 were prepared in acetonitrile. The stock solution was divided into eight microwave vials (1 mL each), which were heated in the microwave to 140 °C for different times, cooled by compressed nitrogen and quenched by the addition of water after the desired polymerization time. The resulting mixtures were analyzed by GC and SEC to determine the monomer conversion and polymer molar mass (distribution), respectively.
Polymer synthesis
The block copolymers p-EtOx-b-R(S)-BuEtOx were synthesized by a two-step-one-pot protocol using a M0 of 4 M in acetonitrile and a total [M]/[I] (I = MeOTs) ratio of 100. The ratio EtOx![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R-BuEtOx was varied to investigate the structure dependence on the hydrophobic content. In the first step EtOx was polymerized at 140 °C under microwave irradiation for 6 to 9 min, depending on the [M]/[I] ratio. After the polymerization, a small sample of p-EtOx was taken with a syringe to determine the molar mass and polydispersity index (PDI) using SEC. Subsequently, the second monomer, R(S)-BuEtOx, was added with a syringe. After the second polymerization stage at 180 °C for 10 to 40 min, depending on the [M]/[I] ratio, the mixtures were quenched by the addition of water. A small sample was taken with a syringe to determine the molar mass and PDI values of the block copolymer using SEC. After drying, the polymers were redissolved in chloroform and precipitated in cold n-hexane or n-heptane before further characterization. Solubility tests were carried out to determine if the block copolymers revealed LCST behavior in water and the formation of micelles was determined by DLS and cryo-TEM.
R-BuEtOx was varied to investigate the structure dependence on the hydrophobic content. In the first step EtOx was polymerized at 140 °C under microwave irradiation for 6 to 9 min, depending on the [M]/[I] ratio. After the polymerization, a small sample of p-EtOx was taken with a syringe to determine the molar mass and polydispersity index (PDI) using SEC. Subsequently, the second monomer, R(S)-BuEtOx, was added with a syringe. After the second polymerization stage at 180 °C for 10 to 40 min, depending on the [M]/[I] ratio, the mixtures were quenched by the addition of water. A small sample was taken with a syringe to determine the molar mass and PDI values of the block copolymer using SEC. After drying, the polymers were redissolved in chloroform and precipitated in cold n-hexane or n-heptane before further characterization. Solubility tests were carried out to determine if the block copolymers revealed LCST behavior in water and the formation of micelles was determined by DLS and cryo-TEM.
Results and discussion
The polymerization kinetics of the statistical copolymerization of EtOx and R-BuEtOx or RS-BuEtOx in a one-step-one-pot system was determined at 140 °C, utilizing a monomodal microwave-synthesizer; an initial monomer concentration (M0) of 4 M and a total monomer to initiator ratio ([M]/[I]) of 100, i.e. [M]/[I] = 70 for EtOx and [M]/[I] = 30 for R-BuEtOx or RS-BuEtOx, with MeOTs as the initiator and acetonitrile as the solvent were utilized. Separate polymerizations were performed for different reaction times and the resulting mixtures were analyzed by gas chromatography (GC) and size exclusion chromatography (SEC) to determine the monomer conversion and molar mass distribution, respectively. A linear first order kinetic plot could be obtained for EtOx, R-BuEtOx and RS-BuEtOx up to at least 80% conversion, indicating a constant concentration of propagating species (Fig. 1a). In a previous investigation it was already observed that the polymerization of R-BuEtOx and RS-BuEtOx does not reach full conversion even after prolonged heating.19 Together with the linear increase of molar mass with conversion, the linear first order kinetics confirms a living copolymerization (Fig. 1b). The similar kinetic plots of R-BuEtOx and RS-BuEtOx demonstrate that the chirality of the monomers has no influence on the kinetics of the copolymerization. The SEC traces (see ESI†) reveal monomodal shapes and the PDI values stay below 1.4. The observed lower Mn compared to the theoretical Mn is most likely related to the compact structure of p-R(S)-BuEtOx leading to a smaller hydrodynamic volume compared to the utilized polystyrene SEC standards. From the first order kinetic plots the polymerization rate constants (kp) were calculated assuming complete initiation and the absence of termination. The kp of EtOx (52 ± 8 × 10 −3 L (mol × s)−1) is reduced by a factor 2 in the presence of the second monomer,21 while the kp of R-BuEtOx and RS-BuEtOx (11 ± 3 × 10 −3 L (mol × s)−1) increases with almost a factor 10.22 This behavior can be explained in the same way as reported for the copolymerization of MeOx with BuEtOx;22 during the non-ideal EtOx–BuEtOx copolymerization, the EtOx propagating species are better accessible than the BuEtOx propagating species, which is responsible for the faster incorporation of BuEtOx while the sterical hindrance of the BuEtOx propagating species slows down the polymerization of EtOx.![(a) Kinetic plots for the copolymerization of EtOx and R-BuEtOx or RS-BuEtOx initiated with methyl tosylate ([EtOx] : [BuEtOx] : [MeOTs] = 70 : 30 : 1) in acetonitrile ([M] = 4 M) at 140 °C. (b) Corresponding molar masses (Mn) against the conversion plot, including polydispersity indices. The black lines are linear fits of the data.](/image/article/2012/SM/c1sm06595e/c1sm06595e-f1.gif) | ||
Fig. 1 (a) Kinetic plots for the copolymerization of EtOx and R-BuEtOx or RS-BuEtOx initiated with methyl tosylate ([EtOx]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BuEtOx] [BuEtOx]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [MeOTs] = 70 [MeOTs] = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in acetonitrile ([M] = 4 M) at 140 °C. (b) Corresponding molar masses (Mn) against the conversion plot, including polydispersity indices. The black lines are linear fits of the data. 1) in acetonitrile ([M] = 4 M) at 140 °C. (b) Corresponding molar masses (Mn) against the conversion plot, including polydispersity indices. The black lines are linear fits of the data. | ||
After the full conversion of EtOx in ∼30 minutes, ∼62% R-BuEtOx or RS-BuEtOx is polymerized, indicating that after full conversion of EtOx still ∼20% of the chiral monomer polymerizes into a pure pBuEtOx hydrophobic block. When the monomer distribution along the chains of pEtOx-stat-R(S)-BuEtOx is calculated from the copolymerization kinetics by averaging the composition of each five consecutive monomers and fitting with a sigmoidal shape, it can be seen that at the beginning of the polymerization mainly EtOx is incorporated and the amount of R-BuEtOx or RS-BuEtOx in the polymer chain increases with increasing polymerization time until EtOx is fully converted and only R-BuEtOx or RS-BuEtOx is left to polymerize. As such, these polymers have an amphiphilic gradient monomer distribution going from rich in EtOx to pure BuEtOx (Fig. 2).
 | ||
| Fig. 2 Structural and schematic representations (top) as well as calculated monomer distributions (bottom) of the investigated (a) p-EtOx69-stat-RS-BuEtOx29 and (b) p-EtOx68-stat-R-BuEtOx31. | ||
Based on the kinetics, a gradient copolymer pEtOx70-stat-R-BuEtOx30 was synthesized in a larger amount. The SEC trace revealed a monomodal shape with a molar mass of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1 and a PDI value of 1.23. From turbidity measurements it can be observed that the statistical copolymer has a cloud point temperature (CP) of 66 °C when dissolved in water at a concentration of 5 mg mL−1, which is similar to block copolymers that contain EtOx.23 This CP is also similar to high molar mass pEtOx at high concentrated solutions indicating close proximity of the chains as is also the case in micellar structures. The polymer solution was further investigated with cryo-TEM and DLS to determine if supramolecular aggregates have formed. Cryo-TEM indeed revealed the presence of spherical micelles with a core diameter of ∼7 nm together with some larger aggregates (Fig. 3a) and DLS confirmed this result by demonstrating the presence of aggregates with a hydrodynamic radius (Rh) of 12 nm. Moreover, the DLS signal was not angular dependent, proving the spherical nature of the formed micelles (Fig. 3b). Also the low polydispersity (PD) of 0.03 measured with DLS is in agreement with the formation of well-defined spherical micelles for this statistical copolymer. The formation of spherical micelles is indeed expected from the hydrophilic/hydrophobic ratio of the pEtOx70-stat-R-BuEtOx30copolymer. These results support the assumption that an amphiphilic gradient copolymer is formed, which was able to form well-defined spherical micelles in water.
100 g mol−1 and a PDI value of 1.23. From turbidity measurements it can be observed that the statistical copolymer has a cloud point temperature (CP) of 66 °C when dissolved in water at a concentration of 5 mg mL−1, which is similar to block copolymers that contain EtOx.23 This CP is also similar to high molar mass pEtOx at high concentrated solutions indicating close proximity of the chains as is also the case in micellar structures. The polymer solution was further investigated with cryo-TEM and DLS to determine if supramolecular aggregates have formed. Cryo-TEM indeed revealed the presence of spherical micelles with a core diameter of ∼7 nm together with some larger aggregates (Fig. 3a) and DLS confirmed this result by demonstrating the presence of aggregates with a hydrodynamic radius (Rh) of 12 nm. Moreover, the DLS signal was not angular dependent, proving the spherical nature of the formed micelles (Fig. 3b). Also the low polydispersity (PD) of 0.03 measured with DLS is in agreement with the formation of well-defined spherical micelles for this statistical copolymer. The formation of spherical micelles is indeed expected from the hydrophilic/hydrophobic ratio of the pEtOx70-stat-R-BuEtOx30copolymer. These results support the assumption that an amphiphilic gradient copolymer is formed, which was able to form well-defined spherical micelles in water.
 | ||
| Fig. 3 Spherical micelle formation of pEtOx70-stat-R-BuEtOx30 indicated by (a) cryo-TEM (5 mg mL−1 in water) and (b) DLS (5 mg mL−1 in water at 25 °C at an angle of 90°) revealing a single distribution with a Rh of 12 nm (PD 0.03). | ||
Besides these gradient copolymers, pure diblock copolymers were prepared by the sequential monomer addition method. To investigate the influence of the ratio between the hydrophilic and hydrophobic blocks on the self-assembled structure in water, six block copolymers with varying ratios of EtOx![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R-BuEtOx were synthesized (Table 1, I–VI) under microwave-assisted conditions using MeOTs as initiator and acetonitrile as solvent. The hydrophilic monomer EtOx was first polymerized at 140 °C (SEC traces are presented in Fig. 4a) followed by the addition of the second hydrophobic monomer, R-BuEtOx, which was polymerized at 180 °C to shorten the required polymerization time (SEC traces are presented in Fig. 4b).
R-BuEtOx were synthesized (Table 1, I–VI) under microwave-assisted conditions using MeOTs as initiator and acetonitrile as solvent. The hydrophilic monomer EtOx was first polymerized at 140 °C (SEC traces are presented in Fig. 4a) followed by the addition of the second hydrophobic monomer, R-BuEtOx, which was polymerized at 180 °C to shorten the required polymerization time (SEC traces are presented in Fig. 4b).
| Polymer | M n a EtOx/g mol−1 | PDIaEtOx | M n a copolymer/g mol−1 | PDIacopolymer | DP a EtOx | DP a BuEtOx | DP a copolymer | BuEtOx a (wt%) |
|---|---|---|---|---|---|---|---|---|
| a Determined by SEC in CHCl3 using PS standards. The calculated DP and wt% represent a rough estimation. | ||||||||
| pEtOx67-R-BuEtOx12-I | 6600 | 1.06 | 8500 | 1.07 | 67 | 12 | 79 | 22 |
| pEtOx62-R-BuEtOx16-II | 6100 | 1.13 | 8600 | 1.16 | 62 | 16 | 80 | 29 |
| pEtOx64-R-BuEtOx30-III | 6400 | 1.06 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.12 | 64 | 30 | 94 | 42 |
| pEtOx59-R-BuEt Ox31-IV | 5900 | 1.06 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.11 | 59 | 31 | 90 | 45 |
| pEtOx56-R-BuEtOx38-V | 5900 | 1.05 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.16 | 56 | 38 | 94 | 49 |
| pEtOx54-R-BuEtOx34-VI | 5400 | 1.12 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.20 | 54 | 34 | 88 | 50 |
| pEtOx45-RS-BuEtOx16-VII | 4500 | 1.14 | 7000 | 1.12 | 45 | 16 | 61 | 36 |
| pEtOx41-RS-BuEtOx19-VIII | 4100 | 1.09 | 7000 | 1.18 | 41 | 19 | 60 | 41 |
 | ||
| Fig. 4 SEC traces (CHCl3) of (a) the first block, pEtOx, and (b) the block copolymers after purification. | ||
To investigate the influence of the chiral structure on the formation of the self-assembled structures, block copolymers based on EtOx and the racemic RS-BuEtOx have been synthesized as well (Table 1, VII–VIII); the self-assembled structures have also been investigated with cryo-TEM and DLS.
The resulting block copolymers still contain some pEtOx homopolymers, which could be removed to a large extent by precipitating the block copolymer in cold n-heptane or cold n-hexane. However, still some pEtOx homopolymers were left for most copolymers. Purifying the block copolymer for a second time did not significantly reduce the amount of unreacted pEtOx. Nonetheless, the presence of some pEtOx homopolymers will not interfere with the self-assembly studies since it will fully dissolve in water.
Turbidimetry revealed that the transmittance before the CP strongly varies with the composition of the copolymers. In general, the transmittance is lower for polymers with a higher hydrophobic content indicating the presence of larger self-assembled structures that scatter away part of the light, which is also evident from the translucent appearance of the solutions. The CP obtained from turbidity measurements of the block copolymers are similar to the CP of the statistical copolymer and varied between 60 and 70 °C (Fig. 5) indicative of a self-assembly behavior as explained for the gradient copolymer. The presence of self-assembled structures was investigated in further detail using cryo-TEM and DLS.
 | ||
| Fig. 5 Transmittance curves of the second heating run from turbidity measurements revealing CP's between 60 °C and 70 °C for the gradient and block copolymers. | ||
All the synthesized block copolymers contain a major fraction of hydrophilic pEtOx component and should be therefore prone to form spherical micelles. However, preliminary investigations revealed that the formed structures were clearly dependent on the micelle preparation method used. This observation indicated that non-equilibrium micelles are formed in the present study and that kinetically trapped transient morphologies could be therefore observed. In order to allow a comparison of the obtained results, a standard sample preparation protocol has been validated (see the Experimental section) and further used for all the investigated block copolymer solutions. Using this preparation protocol, some spherical micelles with a mean diameter of ∼25 nm together with large vesicular structures which can reach sizes of several hundreds of nanometres were observed for the pEtOx67-b-R-BuEtOx12-I copolymer (Fig. 6a). Moreover, the vesicles deviate significantly from the usually found perfectly round shape and exhibited characteristic protrusions, which could indicate the abstraction of smaller vesicles or micelles or may be related to fusion processes. Those vesicles could be considered as transient structures trapped during the sample preparation process, the ultimate structures being small spherical micelles in agreement with the composition of the copolymer.
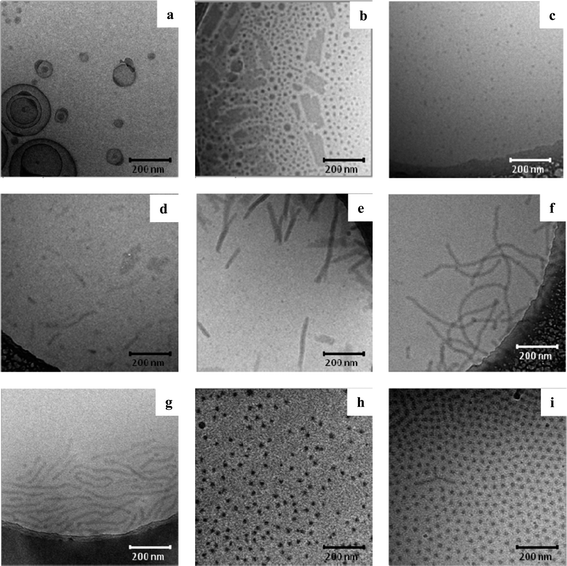 | ||
| Fig. 6 Variation of the hydrophobic content of the copolymer building blocks and influence on the resulting supramolecular aggregates. (a) pEtOx67-b-R-BuEtOx12-I forms micelles and vesicles. (b) pEtOx62-b-R-BuEtOx16-II results in the formation of micelles and 2-dimensional sheet structures or (c) spherical micelles depending on the sample preparation. (d) With further increasing the hydrophobic content (pEtOx64-b-R-BuEtOx30-III) the tendency to form 2-dimensional sheets is more pronounced and hardly spherical micelles are found. (e) pEtOx59-b-R-BuEtOx31-IV shows vertically aligned sheets as evidenced by the clearly resolved internal structures of the aggregates. Even higher hydrophobic contents of (f) pEtOx56-b-R-BuEtOx38-V and (g) pEtOx54-b-R-BuEtOx34-VI yield in the formation of worm-like micellar structures, where no internal structure can be resolved. (h) pEtOx45-b-RS-BuEtOx16-VII and (i) pEtOx41-b-RS-BuEtOx19-VIII are block copolymers containing the racemic block which both form spherical micelles. | ||
When the hydrophobic content of the chiral block copolymer is increased, large structures are always observed with cryo-TEM. PEtOx62-b-R-BuEtOx16-II mainly resulted in the formation of spherical micelles and thin sheet structures (Fig. 6b). Again, it might be that the sheet structures are transient morphologies caused by the sample preparation method. Indeed, a second sample was prepared revealing only the presence of spherical micelles with a core diameter of ∼7 nm (Fig. 6c). It might be speculated that sheets, fused micelles and vesicles are non-equilibrium morphologies due to the nonergodicity effect.24
By further increasing the hydrophobic content, the formation of sheet-like structures is clearly favored (pEtOx64-b-R-BuEtOx30-III in Fig. 6d). For the copolymers discussed in the following with higher hydrophobic content, it might be assumed that spherical micelles are no longer the final equilibrium morphology but that rod-like micelles and vesicles or other morphologies now represent the more stable state for the system. This is in particular evident if the ‘fiber-like’ aggregates in Fig. 6d are analyzed. They show an internal structure which can be attributed to the formation of a layered structure in a presumably bilayer-like fashion. The hydrophobic part of the molecules is thereby centered in the middle of the sheet's cross-section whereas the hydrophilic EtOx blocks are facing towards the liquid interface (Fig. 7). In this sample hardly any residual spherical micelles are observed. The tendency to form layered sheet structures is also revealed by analyzing pEtOx59-b-R-BuEtOx31-IV (Fig. 6e) where a comparable internal layered structure of vertically aligned sheets is observed. It has to be mentioned here that the orientation of the sheets significantly influences the resolution capabilities of the TEM investigations as only perfectly aligned sheets result in a good contrast, where the different layers can be clearly resolved. pEtOx56-b-R-BuEtOx38-V (Fig. 6f) and -VI (Fig. 6g) have the highest hydrophobic content and in these polymer solutions also extended structures were observed (Fig. 7c). However, it could be revealed that the formed supramolecular aggregates are fiber-like objects with a diameter of ∼12 nm, rather than 2-dimensional sheets as could be confirmed by tilting the series that were acquired to investigate the three dimensional structure of the objects (see ESI†). Since pEtOx56-b-R-BuEtOx38-V and pEtOx54-b-R-BuEtOx34-VI provide a comparable hydrophobic content, which resulted in similar structures, the reproducibility of the structure formation could be demonstrated. It is commonly observed that with the increase of the hydrophobic content spherical micelles, wormlike micelles and eventually sheets and/or vesicles are obtained. However, the formation of fiber-like structures is less common. It has been reported that when EtOx is copolymerized with a peptide, also fibrous structures can be observed when this block copolymer with a relatively high hydrophobic content is dissolved in a helicogenic solvent.25 As a consequence, the formation of a fiber-like structure might be ascribed to the formation of an ordered chiral structure of the core, resulting in a rod–coil amphiphilic block copolymer which can aggregate in diverse less common morphologies. Also other rod–coil block copolymers can form tubular-like structures in a selective solvent.26
 | ||
| Fig. 7 (a) Zoom-in of Fig. 6d, revealing the bilayer sheet structure of pEtOx64-b-R-BuEtOx30-III and (b) schematic representation of the bilayer sheets wherein the internal red helices represent the R-BuEtOx segment and the blue chains the EtOx segment sticking into the aqueous solution. (c) Zoom-in of the fiber-like structure of pEtOx56-b-R-BuEtOx38-III–V. | ||
To further evaluate the effect of the chiral block on the self-assembly process, pEtOx45-b-RS-BuEtOx16-VII and pEtOx41-b-RS-BuEtOx19-VIII were also investigated. The cryo-TEM images in Fig. 6h and i reveal that both block copolymers form spherical micelles with a core diameter of ∼13.5 nm. Since the micelles of pEtOx41-b-RS-BuEtOx19-VIII are hexagonally packed, also the length of the hydrophilic part can be estimated. The average distance between the micelles is ∼22 nm, indicating that the length of the pEtOx corona of one micelle is ∼11 nm, suggesting that the size of one micelle is ∼35.5 nm in diameter.
The obtained spherical micelles formed by the racemic block copolymers and the enantiopure block copolymers have a comparable size. However, in contrast to pEtOx-b-R-BuEtOx-III no sheets are obtained for pEtOx41-b-RS-BuEtOx19-VIII, while they have a similar hydrophobic content. Therefore it seems that the enantiopure block does have an influence on the supramolecular structure formed indicating the importance of the chiral block.
The polymer solutions have been characterized by DLS as well. Since only a poor influence of the concentration has been noted, all measurements have been collected at 5 mg mL−1 in water. To ascertain the diffusive behavior of the species in solution, DLS data have been collected at different angles (see ESI†). In contrast to the cryo-TEM results, DLS suggested the presence of spherical micelles for pEtOx67-b-R-BuEtOx12-I with a hydrodynamic radius (Rh) of 13 nm (Table 2). Also the narrow PD associated with this polymer solution is in agreement with the formation of well-defined spherical micelles. However, the presence of large vesicles was not detected by DLS for this sample. This might be due to the filtration step that has been used for sample preparation in DLS experiments or to aggregation due to the cryo-TEM sample preparation method. The large vesicles could have been retained on the filter or transformed into spherical micelles due to the high shear operating during filtration. This observation confirms the hypothesis that spherical micelles are indeed the final equilibrium structures expected for this sample. Also spherical micelles are suggested by DLS for pEtOx62-b-R-BuEtOx16-II with a Rh of 19 nm and a narrow PD of 0.06 which corresponds to the second cryo-TEM sample.
| Polymer | BuEtOx (wt%) | Angular dependence | PD | R h/nm, at 90° |
|---|---|---|---|---|
| a nm = not measured. | ||||
| pEtOx67-R-BuEtOx12-I | 22 | No | 0.11 | 13 |
| pEtOx62-R-BuEtOx16-II | 29 | No | 0.06 | 19 |
| pEtOx64-R-BuEtOx30-III | 42 | Yes | 0.25 | 86 |
| pEtOx59-R-BuEtOx31-IV | 45 | Yes | 0.30 | 116 |
| pEtOx56-R-BuEtOx38-V | 49 | Yes | 0.33 | 95 |
| pEtOx54-R-BuEtOx34-VI | 50 | Yes | 0.21 | 116 |
| pEtOx45-RS-BuEtOx16-VII | 36 | Uncertain | nma | 15 and 85 |
| pEtOx41-RS-BuEtOx19-VIII | 41 | No | 0.08 | 17 |
For the next samples with a higher hydrophobic content, DLS results confirm in all cases the formation of non-spherical large structures as ascertained by the angular dependence of the DLS measurements. Moreover, the average sizes associated with these samples are much larger than for the previously discussed polymer solutions (Table 2) and fall in the 100 nm range. PDs associated with the aggregates formed by these polymer solutions are much broader and CONTIN histograms show large, sometimes bimodal, distributions (see ESI†). All these observations point toward the formation of non-spherical micellar aggregates for pEtOx-b-R-BuEtOx with a hydrophobic content of 42 wt% or more, as also observed by cryo-TEM.
DLS confirmed the formation of spherical micelles found in pEtOx45-b-RS-BuEtOx16-VII and pEtOx41-b-RS-BuEtOx19-VIII and that micelles formed by both polymers have approximately the same size. Also the Rh of 17.5 nm measured for pEtOx41-b-RS-BuEtOx19-VIII corresponds to the diameter of ∼35.5 nm measured with cryo-TEM. However, DLS revealed two distributions for pEtOx45-b-RS-BuEtOx16-VII, which indicates that, besides the formation of spherical micelles, also larger, non-spherical aggregates are present in solution, although such aggregates have not been observed with cryo-TEM. These larger aggregates are probably dynamic clusters of micelles which have been observed frequently for poly(2-oxazoline) micelles.23
Conclusions
The statistical copolymerization of EtOx with R-BuEtOx in a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ratio resulted in the formation of an amphiphilic gradient copolymer that self-assembles into spherical micelles based on cryo-TEM and DLS investigations. During the non-ideal copolymerization the EtOx propagating species are better accessible, which increased the polymerization rate of BuEtOx while the sterical hindrance of the BuEtOx propagating species slowed down the polymerization of EtOx. The polymerization kinetics revealed similar copolymerization behavior of EtOx with R-BuEtOx or RS-BuEtOx and the monomer composition gradient goes from an EtOx rich part to a pure BuEtOx segment.
30 ratio resulted in the formation of an amphiphilic gradient copolymer that self-assembles into spherical micelles based on cryo-TEM and DLS investigations. During the non-ideal copolymerization the EtOx propagating species are better accessible, which increased the polymerization rate of BuEtOx while the sterical hindrance of the BuEtOx propagating species slowed down the polymerization of EtOx. The polymerization kinetics revealed similar copolymerization behavior of EtOx with R-BuEtOx or RS-BuEtOx and the monomer composition gradient goes from an EtOx rich part to a pure BuEtOx segment.
Block copolymers with varying compositions of EtOx and R-BuEtOx were prepared in a two-step-one-pot reaction under microwave-assisted conditions. These thermo-responsive block copolymers form different types of self-assembled structures, depending on the hydrophobic to hydrophilic ratio. Block copolymers with a higher hydrophobic content resulted in more stable non-spherical structures, while for low hydrophobic contents spherical micelles coexist with other morphologies. The multiple self-assembled structures found in some cryo-TEM samples might represent nonequilibrium morphologies due to the nonergodicity effect, possibly due to the formation of an ordered chiral hydrophobic core, which results in an amphiphilic rod-coil block copolymer. DLS investigations confirmed the formation of spherical micelles at relatively low hydrophobic content and non-spherical aggregates at higher hydrophobic/hydrophilic ratio. Block copolymers based on EtOx and the racemic RS-BuEtOx only formed spherical micelles in aqueous solution with a similar size compared to the block copolymers containing the enantiopure chiral block, indicating the influence of the chiral block on the obtained morphology.
The type of self-assembled structures that are formed can be controlled by the hydrophobic to hydrophilic ratio and such amphiphilic chiral block copolymers might be useful for separating enantiomeric mixtures in aqueous solution or for drug delivery applications.
Acknowledgements
The authors thank the Dutch Polymer Institute (DPI) and the Netherlands Scientific Organisation (NWO; Veni-grant for RH and Vici-award for USS) for financial support. K. Kempe is grateful to the Landesgraduiertenfoerderung Thueringen for financial support. J.-F. Gohy thanks BELSPO for financial support in the frame of IAP 6/27: Functional Supramolecular Systems and to the P2M Programme from the ESF.References
- A. Blanazs, S. P. Armes and A. J. Ryan, Macromol. Rapid Commun., 2009, 30, 267–277 CrossRef CAS.
- N. Dan and S. A. Safran, Adv. Colloid Interface Sci., 2006, 123–126, 323–331 CrossRef CAS.
- I. W. Hamley, Soft Matter, 2011, 7, 4122–4138 RSC.
- D. A. Christian, S. Cai, D. M. Boven, Y. Kim, J. D. Pajerovski and D. E. Discher, Eur. J. Pharm. Biopharm., 2009, 71, 463–474 CrossRef CAS.
- J. F. Gohy, Adv. Polym. Sci., 2005, 190, 65–136 CrossRef CAS.
- H. Wei, S.-X. Cheng, X.-Z. Zhang and R.-X. Zhuo, Prog. Polym. Sci., 2009, 34, 893–910 CrossRef CAS.
- G.-H. Hsiue, C.-H. Wang, C.-L.-H. Wang, J.-P. Li and J.-L. Yang, Int. J. Pharm., 2006, 317, 69–75 CrossRef CAS.
- H. Cabral and K. Kataoka, Sci. Technol. Adv. Mater., 2010, 11, 014109, DOI:10.1088/1468-6996/11/1/014109.
- F. Wiesbrock, R. Hoogenboom, C. H. Ablen and U. S. Schubert, Macromol. Rapid Commun., 2004, 25, 1895–1899 CrossRef CAS.
- H. Schlaad, C. Diehl, A. Gress, M. Meyer, A. L. Demirel, Y. Nur and A. Bertin, Macromol. Rapid Commun., 2010, 31, 511–525 CrossRef CAS.
- R. Hoogenboom, Angew. Chem., Int. Ed., 2009, 48, 7978–7994 CrossRef CAS.
- R. Ivanova, T. Komenda, T. B. Bonne, K. Ludtke, K. Mortensen, P. K. Pranzas, R. Jordan and C. M. Papadakis, Macromol. Chem. Phys., 2008, 209, 2248–2258 CrossRef CAS.
- M. Lobert, R. Hoogenboom, C.-A. Fustin, J.-F. Gohy and U. S. Schubert, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5859–5868 CrossRef CAS.
- N. ten Brummelhuis and H. Schlaad, Polym. Chem., 2011, 2, 1180–1184 RSC.
- M. Hruby, S. K. Filippov, J. Panek, M. Novakova, H. Mackova, J. Kucka, D. Vetvicka and K. Ulbrich, Macromol. Biosci., 2010, 10, 916–924 CrossRef CAS.
- N. Adams and U. S. Schubert, Adv. Drug Delivery Rev., 2007, 59, 1504–1520 CrossRef CAS.
- M. M. Bloksma, U. S. Schubert and R. Hoogenboom, Macromol. Rapid Commun., 2011, 32, 1419–1441 CrossRef CAS.
- M. M. Bloksma, M. M. R. M. Hendriks, U. S. Schubert and R. Hoogenboom, Macromolecules, 2010, 43, 4654–4659 CrossRef CAS.
- M. M. Bloksma, S. Rogers, U. S. Schubert and R. Hoogenboom, Soft Matter, 2010, 6, 994–1003 RSC.
- J. Skey and R. K. O'Reilly, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3690–3702 CrossRef CAS.
- R. Hoogenboom, M. W. M. Fijten, H. M. L. Thijs, B. M. van Lankvelt and U. S. Schubert, Des. Monomers Polym., 2005, 8, 659–671 CrossRef CAS.
- R. Hoogenboom, A. J. Van der Linden, B. M. Van Lankvelt, M. M. Bloksma, M. W. M. Fijten, H. M. L. Lambermont-Thijs and U. S. Schubert, Macromolecules, 2011, 44, 4320–4325 CrossRef.
- H. M. L. Lambermont-Thijs, R. Hoogenboom, C.-A. Fustin, C. Bomal-D'Haese, J.-F. Gohy and U. S. Schubert, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 515–522 CrossRef CAS.
- Y. He, Z. Li, P. Simone and T. P. Lodge, J. Am. Chem. Soc., 2006, 128, 2745–2750 CrossRef CAS.
- S.-W. Kuo, H.-F. Lee, C.-F. Huang, C.-J. Huang and F.-C. Chang, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3108–3119 CrossRef CAS.
- J. Raez, I. Manners and M. A. Winnik, Langmuir, 2002, 18, 7229–7239 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEC traces, tilted cryo-TEM images, angular dependent DLS data and CONTIN size distribution histograms. See DOI: 10.1039/c1sm06595e |
| This journal is © The Royal Society of Chemistry 2012 |
