Reversible long range network formation in gold nanoparticle - nematic liquid crystal composites†
Jonathan
Milette
a,
Stephen J.
Cowling
b,
Violeta
Toader
a,
Cyrille
Lavigne
a,
Isabel M.
Saez
b,
R.
Bruce Lennox
a,
John W.
Goodby
*b and
Linda
Reven
*a
aCenter for Self-Assembled Chemical Structures (CSACS-CRMAA), Chemistry Department, McGill University, 801 Sherbrooke St. West, Montreal, Canada H3A 2K6. E-mail: linda.reven@mcgill.ca; Fax: +1-514-398-3797
bDepartment of Chemistry, University of York, York, YO10 5DD, UK. E-mail: jwg500@york.ac.uk; Fax: +1-44-(0)1904-432516
First published on 17th November 2011
Abstract
Nanoparticles (NPs) are dispersed into liquid crystals (LCs) to create ordered NP assemblies and thereby modify the LC and NP properties. Although low NP concentrations are normally used to avoid aggregation, high concentrations can lead to new organization through coupling of the interparticle attractive forces with the LC elastic properties. Gold nanoparticles (AuNPs) with mesogenic coatings, tailored to be highly miscible in the liquid phase of n-alkyl-cyanobiphenyl LCs, form reversible micron-scale networks on cooling at the clearing point by enrichment of the NPs at the nematic-isotropic liquid interfaces. The network topology and LC director field orientation are controlled by the cooling rate, surface alignment, film thickness, AuNP concentration and ligand shell composition. Thin film networks consisted of branches and circular areas of LC enriched in AuNPs. Nucleating nematic droplets evolve into homeotropic alignment of the host nematic matrix, accompanied by birefringent disclination lines and loops. Thick film AuNP networks in LCs form complex structures with stable radial director configurations in small domains and Schlieren domains elsewhere. Controlled formation of networks via the use of LC phase transitions offers an additional approach to produce quasi-periodic NP assemblies that are both long range and reversible in nature.
Introduction
The use of liquid crystals (LCs) as a dispersing medium for the assembly of nanoparticles (NPs) is emerging as a flexible method for the fabrication of nanostructures.1–3 NPs can induce local distortions in the LC director field, resulting in the directed rearrangement of the NPs and LC matrix to minimize the elastic perturbations. Combined with long-range van der Waals interactions, these forces may give rise to a wide variety of ordered structures that depend on the LC anchoring strength at the NP surface, the alignment of the LC matrix, the boundary conditions and other parameters. The elasticity and topological defects of the host LC have long been used to assemble microparticles in different geometries.4,5 However the LC-mediated colloidal interactions of micron-size particles, on the order of 103 kBT and several orders of magnitude larger than in aqueous colloidal suspensions, are normally too strong to permit the reversible association required to form well-defined structures. Reversible ordering of microparticles has been realized at LC-aqueous interfaces through the adsorption and desorption of surfactants.3 The weak and long range interactions of nanometre-sized particles offer better conditions for reversible self-assembly than using the LC forces alone.6 The LC mediated interactions between NPs can be further tuned by altering the covalently bound NP ligand shell to change the surface anchoring.7 Unlike microparticles, little is known about the local LC order and defect structures around NPs as they are too small for characterization by optical microscopy. Uncontrollable aggregation due to the high surface energies of NPs is another challenge. One approach has been to functionalize NPs with mesogenic ligands to promote miscibility.8 NPs decorated with bent core,9 discotic10,11 and nematic12,13 mesogens have been used to disperse NPs in LCs. Most of these studies have used relatively low concentrations of NPs to avoid phase separation. Concentrated dispersions, where colloidal crystallization can couple with the LC forces, offer the potential to produce interesting structures such as the gel-like materials formed by micron size polymer colloids dispersed in nematic matrices.14 We recently reported unprecedented miscibility of 4–5 nm gold NPs in the liquid phase of 5CB and 8CB (Fig. 1, a–b) through fine tuning a ligand shell composed of hexanethiol and 4′-(12-mercaptododecyloxy)biphenyl-4-carbonitrile [CBO(CH2)12SH]15 (Fig. 1, c–d). In this article we focus on the structures formed by high concentrations of these AuNPs in the nematic phases of 5CB and 8CB using polarized optical microscopy (POM). Upon cooling from the isotropic liquid to the nematic phase, the NPs reversibly self-assemble into highly regular networks whose morphologies and associated LC patterns are controlled by the cooling rate, concentration, and film thickness and elastic properties of the LC. | ||
Fig. 1 Liquid crystals (a) 4-n-pentyl-4′-cyanobiphenyl (5CB; K 22.5 N 35.5 Iso) and (b) 4-n-octyl-4′-cyanobiphenyl (8CB; K 21.4 SmA 33.4 N 40.4 Iso) are used to disperse mono and mix monolayer capped AuNPs. Ligands (c) alkanethiols (CH3(CH2)mSH, m = 5, 11 and (d) 4′-(n-mercaptoalkoxy)biphenyl-4-carbonitrile (CBO(CH2)nSH; n = 8, 12, 16) are used to functionalize the AuNPs. (e) Sample Au1 with a ligand shell made of CBO(CH2)12SH:CH3(CH2)5SH at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 surface ratio is the main AuNP sample used for this study. 1 surface ratio is the main AuNP sample used for this study. | ||
Materials and methods
AuNP-LC dispersions
The synthesis of the monolayer and mixed monolayer 4.7 nm dia. gold nanoparticles (AuNPs) via exchange reactions with 4-(N,N-dimethylamino)pyridine (DMAP) stabilized AuNPs is reported in an earlier publication.15 The procedure to prepare the AuNP - LC dispersions was adapted from Hegmann et al.13 Briefly, the LC was mixed with the AuNPs using dichloromethane, the solution was sonicated for 1 min and the solvent evaporated under a stream of Ar(g) (overnight) and vacuum (1 h). Some samples were subjected to overnight vacuum drying with heating to the isotropic phase to eliminate the possibility of residual solvent acting as a third component. These samples however showed the same behavior as those not subjected to prolonged vacuum drying.Polarizing optical microscopy
Prior to the preparation of the optical microscopy slides, the dispersion was sonicated above the nematic-isotropic phase transition and manually stirred at room temperature with the pipette used to transfer the material, which is in the nematic phase, to the glass slide. The dispersions were heated to the isotropic phase and, for thin cells, mixed by moving and pressing the two slides together. Polarized optical microscopes (Zeiss or Canon) with parallel and crossed polars were used and mounted with a Mettler FP900 heating stage or Instec processor/Mettler FP82 hot stage.All glass vials and microscope glass substrates were rinsed with aqua regia and generous portions of water, acetone and hexane. The untreated glass slides and cover slips were rinsed with water, acetone and hexane. This type of sample holder has little effect on the LC orientation and results in LCs with randomly oriented nematic and smectic domains (ESI, Fig. S1–S2†). Slides were also coated with a homeotropic (HT) or homogeneous (HG) LC aligning organic layer. HT slides were prepared by soaking the glass slides in a 10 mM toluene solution of octadecyltrichlorosilane at 60 °C for 30 min and rinsing them with generous portions of toluene, acetone and hexane. HG slides were prepared by spin coating (1000 rpm, 10min) a 0.5 wt%/vol formic acid solution of Nylon 6/6 on glass slides. Brushing the glass surface with a lint-free cloth was performed to align the deposited polyamine. Pure 5CB and 8CB aligned perpendicularly in HT slides or parallel in HG slides to the glass surface result in a bright and dark contrast under the parallel and cross polarizers respectively of the optical microscope (ESI, Fig. S1c–f†).
Results and discussion
Network formation
Initial studies examine the behaviour of the lower concentration (1 wt% Au) of the gold nanoparticles (AuNPs) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CBO(CH2)12SH:CH3(CH2)5SH mixed ligands) in 5CB. The POM images of this dispersion deposited between untreated glass slides are presented in Fig. 2. The appearance and evolution of the NP network as well as the accompanying LC patterns of the host nematic phase can be followed in a video (see ESI†). The features described below are individually labeled in an expansion of an image taken at TN-I (Fig. 3). After depositing the composite in the nematic phase between glass slides and prior to any heat treatment, the sample is inhomogeneous with large black gold aggregates in a background of small, randomly oriented nematic domains (Fig. 2a). After heating, the AuNPs completely disperse in the isotropic liquid phase (Fig. 2b–c) demonstrating the homogeneity of the mixture. Upon cooling to TN-I, spherical droplets of nematic LC are uniformly formed throughout the isotropic liquid (Fig. 2d). The nucleating nematic droplets initially exhibit a four-armed star of alternating bright and dark regions typical of a radial director configuration, presumably about a central AuNP aggregate. At the same time, a large quantity of AuNPs enrich the nematic-isotropic (NI) boundaries as seen by the dark contours of the droplets in a similar way to zone refining. As the size of the droplets increases (Fig. 2e–g), the walls carrying the AuNP aggregates gradually merge, creating areas rich in AuNPs. Under slightly uncrossed polarizers, these correspond to dark lines or branches of a network that are in contrast with the light bulk LC. At the end of the NI transition, circular areas enriched in AuNPs form. These features, that we describe as “nodes” since they seem to be located at the intersections of the branches, appear as dark circles under the polarized light (Fig. 2h). The birefringence patterns evolve as the LC situated in the middle of the droplets becomes homeotropically aligned. This alignment persists below the clearing point, as confirmed when the dispersion is viewed under parallel and crossed polars (Fig. 4 a–b). As the nematic droplets expand and coalesce, an additional LC pattern emerges. Disclination lines run between some of the nodes. Some of these lines disappear in a progressive fashion from one node to the other, shortly after the droplets have merged to form the branches.
1 CBO(CH2)12SH:CH3(CH2)5SH mixed ligands) in 5CB. The POM images of this dispersion deposited between untreated glass slides are presented in Fig. 2. The appearance and evolution of the NP network as well as the accompanying LC patterns of the host nematic phase can be followed in a video (see ESI†). The features described below are individually labeled in an expansion of an image taken at TN-I (Fig. 3). After depositing the composite in the nematic phase between glass slides and prior to any heat treatment, the sample is inhomogeneous with large black gold aggregates in a background of small, randomly oriented nematic domains (Fig. 2a). After heating, the AuNPs completely disperse in the isotropic liquid phase (Fig. 2b–c) demonstrating the homogeneity of the mixture. Upon cooling to TN-I, spherical droplets of nematic LC are uniformly formed throughout the isotropic liquid (Fig. 2d). The nucleating nematic droplets initially exhibit a four-armed star of alternating bright and dark regions typical of a radial director configuration, presumably about a central AuNP aggregate. At the same time, a large quantity of AuNPs enrich the nematic-isotropic (NI) boundaries as seen by the dark contours of the droplets in a similar way to zone refining. As the size of the droplets increases (Fig. 2e–g), the walls carrying the AuNP aggregates gradually merge, creating areas rich in AuNPs. Under slightly uncrossed polarizers, these correspond to dark lines or branches of a network that are in contrast with the light bulk LC. At the end of the NI transition, circular areas enriched in AuNPs form. These features, that we describe as “nodes” since they seem to be located at the intersections of the branches, appear as dark circles under the polarized light (Fig. 2h). The birefringence patterns evolve as the LC situated in the middle of the droplets becomes homeotropically aligned. This alignment persists below the clearing point, as confirmed when the dispersion is viewed under parallel and crossed polars (Fig. 4 a–b). As the nematic droplets expand and coalesce, an additional LC pattern emerges. Disclination lines run between some of the nodes. Some of these lines disappear in a progressive fashion from one node to the other, shortly after the droplets have merged to form the branches.
 | ||
| Fig. 2 POM images of 1 wt% Au of AuNPs dispersed in 5CB during temperature cycling. When first prepared between untreated glass slides, the dispersion shows (a) gold aggregates in the nematic phase that (b) diffuse at the clearing point resulting in (c) an homogeneous isotropic phase. Upon cooling at 1°/min (d–h; see Fig. 3 for expansion of (f) red dash square), nematic droplets are formed at the TN-I transition that expand and coalesce as the temperature is decreased. The resulting AuNP network and LC patterns dissipate as the dispersion is heated back to the isotropic phase (i). (parallel polars; scale bars = 100μm). | ||
 | ||
| Fig. 3 POM image of 1 wt% Au of AuNPs dispersed in 5CB at TN-I (expansion of Fig. 1f red dash square). When cooling from the isotropic phase (a), nucleating nematic droplets appear at TN-I with a radial director field configuration (b) evolving into homeotropic alignment of the host nematic matrix while keeping radial configuration at the walls (c). There is an enrichment of the AuNPs at the nematic walls (d) that creates branches (e) and nodes (f) along with twist disclination lines (g) as the droplets coalesce. | ||
 | ||
| Fig. 4 POM images of network made at different cooling rates with 1 wt% Au of AuNPs dispersed in 5CB. The dispersions between untreated slides are in the nematic phase after cooling at (a–b) 1.0°/min and (c–f) 0.02°/min. The temperature is kept below the phase transition at time 0 (c–e) and 24 h after (f) the network formation. (c–d) and (e–f) are different regions of the same slide. (single arrow = parallel polars, crossed arrows = crossed polars; scale bars = 100μm). | ||
The AuNP network is stable for at least several weeks if the dispersion is kept in the nematic phase and not mechanically disturbed. If the dispersion is cooled to the crystalline state, then heated back to the nematic phase, the topology of the network remains intact but the host LC no longer displays the different patterns described above and is randomly oriented, i.e., loses homeotropic orientation and becomes homogeneous to reveal a birefringent texture (ESI, Fig. S5†). When heated back to the isotropic phase, the AuNPs completely redisperse (Fig. 2i). The composite can be repeatedly thermally cycled to reform the network, demonstrating that this self-assembly process is completely reversible.
The formation of a network rather than a simple phase separation involves a complex interplay between the interparticle attractive forces and the nematic visco-elastic forces. If the quasi-periodicity of the structure arises from the phase separation of similar sized clusters of AuNPs, the size, along with the density and the rate of growth of the clusters, the nucleating nematic droplets, and therefore the network dimensions, should depend on the cooling rate, particle concentration and LC elastic properties. These parameters are investigated below. As the nematic fills the spaces between the clustering NPs, the distances between the forming clusters cannot be too small since the system would become isotropic and yet it still tries to maintain nematic order. Thus the clusters are linked together via the director field, sitting at similar distances from each other due to the elastic continuum of the host LC and pinning points at the glass surfaces. Overall the nematic brings the NPs together locally and then organizes them over longer distances to form the branches and nodes that consist of AuNP rich LC.
LC director field orientation
As for the LC textures that accompany the network, homeotropic alignment of the bulk LC has also been observed for AuNP dispersions that do not form a network and has been studied by Hegmann and co-workers for a variety of nanoparticles and LC hosts13,16–19 The perpendicular alignment has been attributed to a partial phase separation of AuNPs to the LC-glass interface and is thus inversely related to the miscibility of the NPs in the LC phase and the film thickness. The temperature dependence of the AuNP miscibility has been used to thermally switch the LC alignment modes in alkanethiol AuNP nematic dispersions.18Highly miscible 1–2 nm LC functionalized AuNPs reported by Goodby12 display only partial homeotropic alignment at high concentrations, whereas Hegmann observed complete alignment for both alkanethiol and mixed CBO(CH2)10SH/alkanethiol functionalized AuNPs in 5CB and 8CB at concentrations of ≥5 wt%.17,18 Our 4.7 nm AuNPs which are less miscible than the 2 nm AuNPs in the nematic phase, produce homeotropic alignment in a lower concentration range of ≥1 wt%.
The birefringent stripes were initially proposed to arise from linear particle aggregates formed by the interaction of defect charge distribution.16 A more recent study combining polarizing optical and fluorescence confocal polarizing microscopies concluded that the birefringent stripes, induced by the nanoparticles, are due to twist disclination lines. These defect lines, located at the LC-substrate interface, are pinned at their ends and stabilized by NP agglomerates. No evidence for a concentration of the NPs in the vicinity of the birefringent stripes was found.20 Our data agrees with this scenario as all the birefringent stripes clearly run between two defect sites (circular nodes) but may or may not coincide with the branches.
Experimental control of the network
 | ||
| Fig. 5 POM images of 5 wt% Au of AuNPs dispersed in 5CB using thick cells. Untreated 20 μm thick glass cell results in a network of branches and no nodes with Schlieren texture mixed with homeotropically aligned LC (a–b). A cellular network is formed with stable radial director configuration (d, inset) when using untreated 70 μm thick cell (single arrow = parallel polars; crossed arrows = crossed polars; scale bars = 100μm; cooling rate = 1°/min). | ||
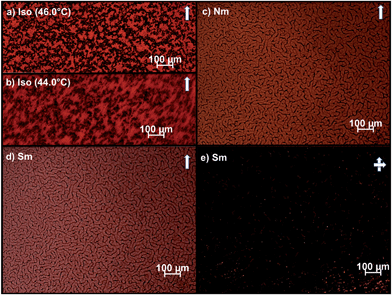 | ||
| Fig. 6 POM images of 15 wt% Au of AuNPs dispersed in 8CB. Insoluble aggregates present in the isotropic phase (a) disperse when approaching the clearing point (b) resulting in the formation of a tight network in the nematic (c) and smectic (d–e) phases. (single arrow = parallel polars; crossed arrows = crossed polars; scale bars = 100μm). | ||
Finally in the list of factors that influence the network, the composition of the AuNP ligand shell is the most sensitive parameter since well-defined networks only form if the AuNPs are completely dispersed in the LC isotropic phase. Unlike the smaller 1–2 dia. AuNPs, complete miscibility of the 4–5 nm AuNPs in isotropic n-alkylcyanobiphenol LCs was only realized through fine tuning of the ligand shell.15 AuNPs with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CBO(CH2)12SH:CH3(CH2)5SH surface ligands are fully miscible in isotropic 5CB and 8CB for concentrations up to at least 25 wt% Au. The AuNPs with 28% and 70% LC ligand shells produce poorly defined networks due to their lower miscibility in 5CB as discussed in our previous work15 (ESI, Fig. S3, S8†). Likewise, one component ligand shells of CH3(CH2)5SH or CBO(CH2)nSH, n = 8, 12, 16, formed no network or poorly defined networks due to low miscibility in the isotropic liquid (ESI, Fig. S4, S9†). Homeotropic alignment of the host LC was observed at fast cooling rates for all the AuNPs with ligands shells that partially or fully consist of CBO(CH2)12SH (ESI, Fig. S8–S9†).
1 CBO(CH2)12SH:CH3(CH2)5SH surface ligands are fully miscible in isotropic 5CB and 8CB for concentrations up to at least 25 wt% Au. The AuNPs with 28% and 70% LC ligand shells produce poorly defined networks due to their lower miscibility in 5CB as discussed in our previous work15 (ESI, Fig. S3, S8†). Likewise, one component ligand shells of CH3(CH2)5SH or CBO(CH2)nSH, n = 8, 12, 16, formed no network or poorly defined networks due to low miscibility in the isotropic liquid (ESI, Fig. S4, S9†). Homeotropic alignment of the host LC was observed at fast cooling rates for all the AuNPs with ligands shells that partially or fully consist of CBO(CH2)12SH (ESI, Fig. S8–S9†).
Related particle-LC systems
In another study of highly concentrated AuNP-LC dispersions, Goodby and co-workers found that 1–2 nm dia. AuNPs functionalized with CBO(CH2)nSH (n = 8–12) disperse up to at least 20 wt% in both the isotropic and LC phases of 5CB and 8CB.12 The high miscibility of these very small NPs is due to a dense coating of mesogens combined with retention of the void volume through splaying of the ligands. There is no broadening of the DSC peaks, only a slight lowering of the phase transition temperatures. No phase separation or LC defects other than the usual textures for the nematic and smectic phases are observed although it was noted that the samples become homeotropically aligned at high concentrations.Concerning the phase behavior of other types of NP-nematic dispersions, Da Cruz et al. observed the reversible clustering of 5–12 nm magnetic iron oxide NPs in 5CB at the nematic to isotropic liquid phase transition.21 However this system behaves very differently from the AuNPs in that the magnetic NPs accumulate inside non-isotropic droplets that co-exist with an unperturbed, empty nematic phase. In common with the AuNP dispersions, the size of the ferronematic droplets depends on the cooling rate with smaller droplets formed at higher rates. The DSC thermograms show broadening and shifting of the nematic to isotropic phase transition peak with increasing NP concentration that was attributed to unbound surfactant rather than interactions with the magnetic NPs. In contrast, the differential scanning calorimetry (DSC) thermograms of the AuNP-LC dispersions exhibit only small temperature shifts and broadening (ESI, Fig. S10†).
Cellular networks of micron size polymer colloids are also proposed to form at the isotropic liquid to nematic phase transition through expulsion of the colloids from the nucleating nematic droplets.14 Just as the AuNPs here must be completely dispersed in the isotropic liquid phase to produce a well-formed network, the polymer colloid dispersions are homogenized by stirring for several hours or longer in the isotropic liquid phase before cooling. The AuNP networks form larger nodes with fewer and thicker branches with slow cooling, and likewise, larger cells are produced in the polymer colloid networks when the nucleating nematic droplets have more time to coalesce before the network freezes into its final structure.22 Higher cooling rates result in smaller cells and hierarchical cellular structures.23 The polymer colloid networks are also reversible with slow dissolution of the cellular network with heating and re-forming of the structure upon cooling.24 Differences between the polymer colloid and AuNP networks include the topologies, mechanical properties, LC textures and the role of impurities. The topologies are strikingly different in that the polymer colloid networks are cellular structures with irregular walls whereas the AuNP networks are regular. The cellular networks are more mechanically stable, forming a soft solid, whereas the AuNP nematic dispersions remain a viscous liquid. LC textures other than the regular nematic Schlieren texture are not observed for the polymer colloid cellular networks. Finally, the formation of the polymer colloid cellular network was proposed to require a third component suggested to be residual hexane.25 The alkane impurity reduces the onset temperature of the phase separation and opens up a coexistence region of nematic (alkane poor) and isotropic (alkane rich) 5CB of several degrees, allowing for phase separation into a metastable network structure. No such third component appears to play a role in the AuNP networks as any residual solvent was removed by prolonged vacuum drying and unbound ligands were eliminated by thorough purification of the nanoparticles. Furthermore, Abbott and co-workers have prepared cellular polymer colloid networks using materials that rule out the possibility of a third component.26
Conclusions
In summary, gold nanoparticles with mesogenic ligands that disperse in the isotropic phase of nCB, reversibly phase separate at the clearing point into long range, quasi periodic structures. A more complete characterization of the network, as well as the formation mechanism, will be addressed by additional experiments and theoretical studies. The thick film POM data indicates that the formation of the network is a bulk rather than surface phenomena but this can be verified by fluorescence confocal microscopy. Whether there is any coexistence region of nematic (AuNP poor) and isotropic (AuNP rich) is an intriguing question given that this has been proposed, but remains unresolved, for both the polymer colloid cellular networks12 as well as for nanosized lyotropic inverse micelle dispersions in 5CB.27Deuteron NMR studies of isotopically labeled AuNPs and host 5CB are currently underway to explore this question along with the NP-LC interactions. Small angle X-ray data will fill in the picture of the local structure by providing the interparticle distances.A theoretical understanding of the short and long range forces operating at the phase transitions of NP-LC dispersions is key to developing this process as a rational method to form other types of nanoparticle assemblies. As a first step, the application of a mean-field thermodynamic model to the nematic dispersions indicates that the structure is determined essentially by the phase equilibrium behaviour and the nucleation rate of the nematic phase.28 Preliminary experimental studies with different LC phases show that completely different structures are formed. In the case of a smectic matrix, the AuNPs reversibly phase separate at the N-Sm phase transition into linear arrays on a microscopic scale. These more complex LC systems will undoubtedly yield even richer phase diagrams and a greater variety of nanoparticle structures than the nematic dispersions.
Acknowledgements
Funding for this research was provided by Fonds quebecois de la recherche sur la nature et les technologies (FQRNT) and the Natural Sciences and Engineering Research Council of Canada (NSERC). The authors thank Prof. Derek Gray for use of his optical microscope.Notes and references
- T. Hegmann, H. Qi and V. M. Marx, J. Inorg. Organomet. Polym. Mater., 2007, 17, 483 CrossRef CAS.
- H. K. Bisoyi and S. Kumar, Chem. Soc. Rev., 2011, 40, 306 RSC.
- Y. Bai and N. L. Abbott, Langmuir, 2011, 27, 5719 CrossRef CAS.
- H. Stark and D. Ventzki, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2001, 64, 031711 CrossRef CAS.
- I. Musevic, M. Skarabot, U. Tkalec, M. Ravnik and S. T. Zumer, Science, 2006, 313, 954 CrossRef CAS.
- G. M. Koenig, J. J. Pablo and N. L. Abbott, Langmuir, 2009, 25, 13318 CrossRef CAS.
- M. Skarabot and I. Musevic, Soft Matter, 2010, 6, 5476 RSC.
- J. W. Goodby, I. M. Saez, S. J. Cowling, V. Gortz, M. Draper, A. W. Hall, S. Sia, G. Cosquer, S.-E. Lee and E. P. Raynes, Angew. Chem., Int. Ed., 2008, 47, 2754 CrossRef CAS.
- V. M. Marx, H. Girgis, P. A. Heiney and T. Hegmann, J. Mater. Chem., 2008, 18, 2983 RSC.
- S. Kumar, S. K. Pal, P. S. Kumar and V. Lakshminarayanan, Soft Matter, 2007, 3, 896 RSC.
- X. Zeng, F. Liu, A. G. Fowler, G. Ungar, L. Cseh, G. H. Mehl and J. E. Macdonald, Adv. Mater., 2009, 21, 1746 CrossRef CAS.
- M. Draper, I. M. Saez, S. J. Cowling, P. Gai, B. Heinrich, B. Donnio, D. Guillon and J. W. Goodby, Adv. Funct. Mater., 2011, 21, 1260 CrossRef CAS.
- H. Qi, B. Kinkead, V. M. Marx, H. R. Zhang and T. Hegmann, ChemPhysChem, 2009, 10, 1211 CrossRef CAS.
- S. P. Meeker, W. C. K. Poon, J. Crain and E. M. Terentjev, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, 61, R6083 CrossRef CAS.
- J. Milette, V. Toader, L. Reven and R. B. Lennox, J. Mater. Chem., 2011, 21, 9043 RSC.
- H. Qi and T. Hegmann, J. Mater. Chem., 2006, 16, 4197 RSC.
- H. Qi, B. Kinkead and T. Hegmann, Adv. Funct. Mater., 2008, 18, 212 CrossRef CAS.
- H. Qi and T. Hegmann, ACS Appl. Mater. Interfaces, 2009, 1, 1731 CAS.
- B. Kinkead and T. Hegmann, J. Mater. Chem., 2010, 20, 448 RSC.
- M. Urbansk, B. Kinkead, T. Hegmann and H.-S. Kitzerow, Liq. Cryst., 2010, 37, 1151 CrossRef.
- C. Da Cruz, O. Sandre and V. Cabuil, J. Phys. Chem. B, 2005, 109, 14292 CrossRef.
- M. Roth, M. D'Acunzi, D. Vollmer and G. K. Auernhammer, J. Chem. Phys., 2010, 132, 124702 CrossRef CAS.
- J. Cleaver and W. K. Poon, J. Phys.: Condens. Matter, 2004, 16, S1901 CrossRef CAS.
- D. Vollmer, G. Hinze, B. Ullrich, W. C. K. Poon, M. E. Cates and A. B. Schofield, Langmuir, 2005, 21, 4921 CrossRef CAS.
- D. Vollmer, G. Hinz, W. C. K. Poon, J. Cleaver and M. E. Cates, J. Phys.: Condens. Matter, 2004, 16, L227 CrossRef CAS.
- A. Agarwal, E. Huang, S. Palecek and N. L. Abbott, Adv. Mater., 2008, 20, 4804 CrossRef CAS.
- Z. Chen and R. Nozaki, J. Chem. Phys., 2011, 134, 034505 CrossRef.
- E. R. Soulé, L. Reven, A. D. Rey, accepted for publication Mol. Cryst. Liq. Cryst. Special Issue: Proceedings of the 11th European Conference on Liquid Crystals, ECLC 2011.
Footnote |
| † Electronic supplementary information (ESI) available: POM images of AuNP LC dispersions, DSC thermograms. See DOI: 10.1039/c1sm06604h |
| This journal is © The Royal Society of Chemistry 2012 |
