Surface tension gradient control of bacterial swarming in colonies of Pseudomonas aeruginosa†
M.
Fauvart
a,
P.
Phillips
b,
D.
Bachaspatimayum
a,
N.
Verstraeten
a,
J.
Fransaer
c,
J.
Michiels
a and
J.
Vermant
*b
aCentre of Microbial and Plant Genetics, Katholieke Universiteit Leuven, B-3001, Leuven, Belgium
bDepartment of Chemical Engineering, Katholieke Universiteit Leuven, B-3001, Leuven, Belgium. E-mail: jan.vermant@cit.kuleuven.be
cDepartment of Metallurgy and Materials Engineering, Katholieke Universiteit Leuven, B-3001, Leuven, Belgium
First published on 20th October 2011
Abstract
Bacterial swarming is one of the most efficient methods by which bacteria colonize nutrient-rich environments and host tissues. Several mechanisms have been proposed to explain the phenomenon and the associated intricate macroscopic pattern formation, but so far no conclusive evidence has been presented that identifies the factors that control swarming. Vice versa, little is known about how swarming can be controlled. Here, by using a series of complementary genetic and physicochemical experiments and a simple mathematical analysis, we show how the bacterial swarming can be caused by a surface tension driven flow. The opportunistic pathogen, Pseudomonas aeruginosa, is studied, as it is relevant for such bacteria to control and arrest swarming. Moreover, P. aeruginosa bacteria secrete strong surface active components as part of their quorum sensing system. Our results demonstrate that surface tension gradient control can even be the dominant mechanism that drives swarming. It can be quantitatively predicted and can be expected to play a role in a wide variety of bacterial systems. The modeling reveals subtle dependencies on both the wetting conditions and the physical properties of the slime. Based on these dependencies, strategies can be devised to arrest swarming under certain conditions by simple physicochemical means.
Introduction
Most bacteria (90%) in nature occur under the form of dense colonies of bacteria surrounded by a bacterial slime, which is mainly composed of exopolysaccharides (EPS). The dynamics by which bacteria move around has been the topic of intensive research, given the key role in determining phenomena such as virulence and biofilm formation.1 More specifically, most attention has been given to the role of individual cell motility and the mechanism by which these microswimmers move in the EPS.2–4 It should be noted, however, that the most efficient mechanisms for bacteria to colonize surfaces are collective rather then individual in nature.5,6 As such, bacterial swarming is one of the fastest known modes of translocation on a surface and enables the rapid colonization of a nutrient-rich environment, thus playing an important role in survival strategies of bacterial colonies. Swarming is widespread in many Gram-negative and Gram-positive flagellated bacteria.6Three main mechanisms have been put forward to explain swarming. Some authors point to the role of the quorum sensing system, which is suggested to trigger a change of the cells from an undifferentiated vegetative state to multi-nucleated and hyper-flagellated swarmer cells, which form rafts of bacteria as their flagellar filaments become entangled.7,8 Swarming is seen as an example of dynamic self-assembly in microbiology.9 A repeated switching is suggested to give rise to the appearance of typical bulls eye patterns, for example in Proteus mirabilis.10 However, the types of patterns formed by colonies living on nutrient rich agar plates differ strongly, ranging from fractal-like in Serratia marcescens11 and Rhizobium etli12 to striking dendritic patterns in e.g. P. aeruginosa.13 To rationalize the rich variety of patterns formed by swarming colonies and the sensitivity of the pattern formation to the conditions in the substrate, the second explanation has been that swarming can be described by models which couple nutrient diffusion to bacterial density. Reaction-diffusion models, and extensions taking into account phenomena such as chemotaxis and lubrication fluid layers,14 are highly non-linear and can produce a wide range of morphologies. However, the modeling provides limited insight into the biological and physical mechanisms underlying swarming dynamics. So far, there have been no attempts to rationalize, predict or control the spreading velocities.
Conceptually adhering to a continuum modeling approach, the role of flows driven by surface tension, which are known as Marangoni flows, has been proposed as a third explanation. For the bacterium R. etli both the pattern formation and the spreading speeds were observed to be consistent with those expected for a Marangoni flow for surface tension gradients, film thicknesses and viscosities observed experimentally.12N-acylhomoserine lactone (AHL) molecules, the signaling molecules of quorum sensing in most Gram negative bacteria,15 were identified mainly by genetic knock out experiments as playing a dominant role in the swarming. This class of AHL molecules was proven to be surface active for biologically relevant concentrations and surface tension gradients can develop and cause the resulting Marangoni flows.12 More recently, experiments on Bacillus subtilis confirmed the generic character of the role of Marangoni flows in this specific case for biofilm dynamics. B. subtilis produces the molecule surfactin, a strong biosurfactant. Model experiments were used to show how the differential accumulation rates induced by the geometry of the bacterial film give rise to surfactant waves causing bacterial biofilms to climb up a wall.16 Within the continuum description, the bacteria act as little factories of surfactant production, and their spatial distribution or differentiation can lead to gradients of concentration. Although the role of these surface tension gradients has not yet received much attention, it is widely recognized that surface active components play an important role in motility (see e.g. Wilking et al. for a recent review17).
In the present work, we demonstrate that surfactant concentration gradient driven flows are a factor to be reckoned with and can even be the dominant mechanism controlling swarming of P. aeruginosa colonies. The quorum sensing system controls the production of rhamnolipids and swarming.13,18 Swarming is also accompanied by striking dendritic patterns. In this respect it is important that rhamnolipids are known to be strong biosurfactants and the present bacterial model system was selected and placed in conditions where the effects of surfactant production are maximized.19 Using a combination of genetic knock out experiments and green fluorescent protein (GFP) reporters for both the cell density and rhamnolipid production, the effect of surfactant production and quorum sensing on the swarming patterns and spreading velocities are investigated. The presence of surfactant waves under swarming conditions is shown using white light interferometry, in situ and for different instants during the swarming process. A simple mathematical model is used to explain the height increase and predict the spreading velocity. The physicochemical parameters that influence the phenomena are determined experimentally and can be used to predict or control the spreading velocities, even in such a way that swarming can be brought to a stop using simple counter-gradients of surfactant concentrations.
Materials and methods
Swarming assays were carried out by spot-inoculating 2.5 μl of a Pseudomonas aeruginosa culture grown overnight in trypticase soy broth at 37 °C onto plates containing swarming medium (0.5% agar, 0.2% glucose, 1 mM MgSO4, 0.05 g casamino acids, 3.4 g Na2HPO4·2H2O, 1.5 g KH2PO4, 0.25 g NaCl, pH 7). Inoculated plates were incubated at 37 °C. P. aeruginosa strain PA14 was kindly provided by Pierre Cornelis (Vrije Universiteit Brussel, Belgium). Rhamnolipid biosynthesis activity was monitored by measuring rhlA transcription using a GFP reporter fusion. A PA14-derivative carrying a chromosomal gfp fusion under transcriptional control of the rhlA promoter was obtained by subcloning the HindIII-SspI PrhlA-gfp fragment from pYL122f into mini-CTX220 and transferring the resulting construct to PA14 by electroporation. Cell density in swarming colonies was assessed by measuring fluorescence of an arabinose-inducible GFP reporter. A PA14-derivative carrying a chromosomal PBAD-gfp fusion was constructed by transferring pYL18321 into mini-CTX220 and transferring the resulting construct to PA14 by electroporation.Timelapse movies were recorded using a Nikon D80 camera (Nikon, NY, USA). Fluorescent images were obtained using a LT-9500 imaging system (Lightools Research, CA, USA) or an Olympus BX51W equipped with a Hamamatsu C8800 CCD. Image analysis was carried out using ImageJ (National Institutes of Health, MD, USA). White light interferometry was performed using a Wyko NT3300 interferometer (Veeco, AZ, USA). Samples were prepared by gel trapping, as described previously,22 to lock in the height profile at a give moment in time such that high resolution height profiles could be measured.
Rheological experiments were carried out on a rotational rheometer (MCR501 Paar Physica), equipped with cone and plate geometries of varying cone angles. Colonies were harvested from the agar plates, the bacteria were killed using UV and the samples were measured immediately to yield an effective viscosity of the colony and its medium. A double solvent lock was used and a Peltier plate and hood were used to ensure constant temperature. At low stresses significant wall slip was present, whereas at stresses above 0.2 Pa the results became independent of measurement geometry. Only data in this regime will be reported. The surface pressure measurements were carried out using a Wilhelmy plate and an electromagnetic balance and a Langmuir trough (from KSV instruments, Finland).
Swarming dynamics of P. aeroginosa
Model experiments of swarming behaviour are typically carried out on planar model and nutrient-rich subphases (see S.I. for details on the materials and methods†). When conducting swarming experiments, a rigorously defined protocol must be adhered to.23 The water content is critical—too little results in poor swarming and too much results in swimming. Hence swarm plates are typically poured to a standard thickness at relatively low temperature and allowed to dry in a laminar flow hood to remove water at the surface. The resulting spot inoculated colony on the agar plate is a circular ring, which is slightly thicker near the edges due to the well known coffee ring effect that occurs as a consequence of capillary flows during drying24 (see Fig. 1A). In Fig. 1 we further compare a sequence of visible light images of a swarming colony with signal intensity plots below, and this for the wild-type strain P. aeruginosaPA14 (left) compared to a rhlA mutant deficient in rhamnolipid production (right). As the colony starts to grow, the ring pattern first fills in, then grows out radially as the overall height of the colony starts to increase (sequences 1B–1D). For the wild-type the spreading of the colony is faster and the growth of the ring shaped pattern outwards is accompanied by increase of the reflected light intensity near the edges of the colony (see supporting information S1: suppmovie1a.mov†for a time-lapse series). A spectacular branching of the colonies into striking dendritic structures is subsequently observed (Fig. 1E–1G), the final stage of which is shown in Fig. 1H. This branching is absent in the rhamnolipid-deficient rhlA mutant.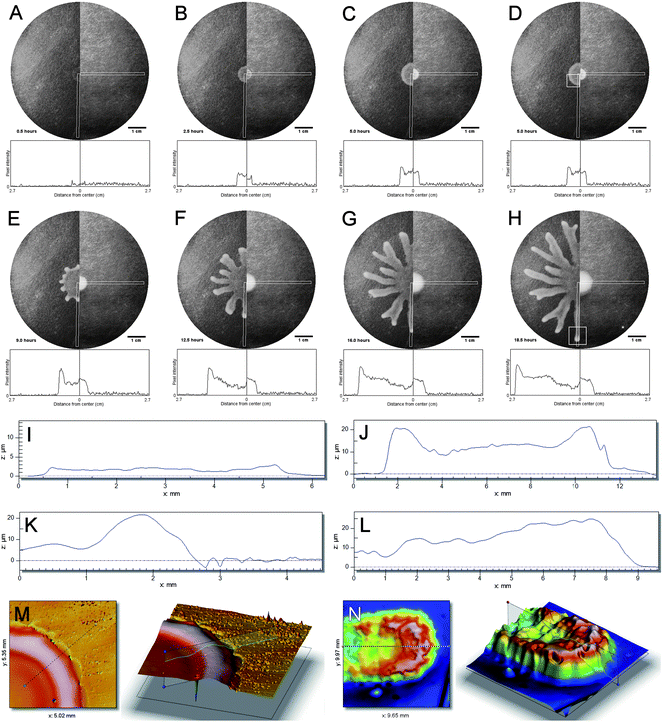 | ||
| Fig. 1 Swarming colonys edges are characterized by a height increase. A–H: Visible light images of a swarming colony with signal intensity plots below; images are in chronological order (0.5 h, 2.5 h, 5 h, 9 h, 12.5 h, 16 h, 18.5 h). Left half: wild-type strain P. aeruginosa PA14, right half: rhlA mutant deficient in rhamnolipid production. Gray rectangles indicate regions for which signal intensity was calculated after background subtraction. White square boxes in panels D and H indicate regions for which white light interferometry data is shown in panels K–N. See also supplementary movie 1.† I–L: height profiles of swarming colony recorded with white light interferometry; sampling time points correspond to panels A, B, D and H, respectively. M–N: 3D images generated from white light interferometry data shown in panels K and L, respectively. | ||
To prove more clearly that the higher intensity of the microscopy images near the edges of the colony is caused by an increase in height, the thickness of the bacterial films has been measured in situ using white light interferometry. Profiles I–L give the measured height profiles for the wild-type strain P. aeruginosaPA14 for the panels A, B, D and H, respectively. Whereas the initial spot inoculation and drying leads to a thin film of a few micrometres thick (panel I), the height of the colony gradually increases and a pronounced rim of roughly twice the height of the colony develops as is shown in Fig. 1J for a full cross section. A more detailed graph for half the cross section is given in panel K (corresponding to a situation as in panel D). Panel K was taken just before the branching instability takes place. A 3D height profile generated from white light interferometry data shown in panels K and L corresponds to the situation right at the onset of branching and within a tendril, respectively. The wave front near the edges of the colony is also present within a branch as is shown in the profile in Fig. 1 K and even more clearly in the 3D reconstructions. It should be pointed out that the mutant which does not produce the biosurfactant shows no increase of the height. Image analysis of the spreading colonies of the wild-type strain P. aeruginosa PA14 was used to measure the spreading velocities. The initial spreading velocities of the circular shaped colonies were on the order of 5–10 μm min−1. In the branches the spreading speeds up to values of 40 μm min−1. In the valleys between the branches, the spreading velocities of the colonies slow down. Similar to the case of R. etli,12 we could not detect a correlation between the velocities of the individual bacteria moving in the slime and the overall spreading velocity. More intricate local scale mechanisms such as coordinated flagellar motion25 were also not observed.
To identify the role of the bacteria in driving these collective mechanisms, we performed experiments with modified P. aeruginosa strains, allowing to detect the cell density or to provide information on the rhamnolipid production. Fig. 2A shows bright field images of a swarming colony. The bacteria in this experiment were genetically modified to produce a green fluorescent protein (GFP) and the corresponding fluorescence image is shown in Fig. 2B. The fluorescence is present throughout the colony and is most intense in the dendritic arms. It can be concluded that the fluorescence intensity mimics the intensity observed in the bright field images, and hence the difference in light (2A) and fluorescence (2B) intensity can be traced back to the differences in film thickness shown in Fig. 1. Hence it can be concluded that the bacteria are distributed uniformly throughout the colony. For a colony of bacteria expressing rhlA-gfp fusion to monitor rhamnolipid production, the fluorescence microscopy image in Fig. 2C also shows a fluorescence throughout the colony with higher intensity in the arms, clarifying that rhamnolipids are being produced everywhere in the colony. Fig. 2D shows a detailed image of the edge of the colony just before the onset of swarming. Although no direct quantitative comparison can be made it can be concluded that there are only limited or localized spatio-temporal variations in production of rhamnolipids. A recent microarray study has shown that rhlA production is down regulated in the dendrite tips, compared to the swarm center.26 This down-regulation, or the mere fact that there is a region of lower bacterial density near the very edges of the colony (where the bacteria cannot penetrate the film) can lead to local gradients in rhamnolipid concentration.
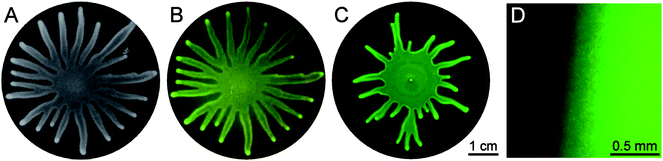 | ||
| Fig. 2 Swarming by Pseudomonas aeruginosa. A: swarmed state, visible light, B: swarmed state, GFP filter shows autofluorescence = cell density, C: swarmed state, GFP filter shows PrhlA activity = rhamnolipid production, D: zoom of swarmed state, GFP filter shows PrhlA activity = rhamnolipid production. | ||
In order to identify the possible role of nutrient diffusion in the subphase to bacterial density, which is a key element in some of the reaction diffusion approaches, we investigated if the colonies could swarm over a barrier of about 1 mm thickness. The setup is shown in Fig. 3A. A petri dish with an insert was filled with agar in such a manner that the insert was just covered with a very thin layer of agar. A planar interface was then obtained. Spot inoculation was carried out only on one side of the barrier. If nutrient diffusion would be an important issue, the barriers would have been ‘felt’ by the swarming colony and the swarming should have stopped. However, both 3B and C show that in the final swarmed state, the colony has swarmed over a barrier. In the experiments reported in Fig. 3C a dye molecule was added to the right hand side to demonstrate that there is no significant diffusion over the barrier. These experiments suggest that nutrient diffusion does not play a critical role in the swarming of P. aeruginosa.
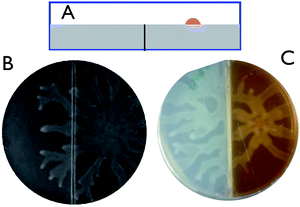 | ||
| Fig. 3 Swarming over a barrier. A: schematic cartoon of the dishes with an insert in the agar (side view). The colony was spot inoculated on the right. B: swarmed state, bright field image showing dendrils going over the barrier. C: swarmed state, the right hand element was colored with a food dye. | ||
Swarming as a Maragoni flow
In order to further investigate the role of the surface tension driven flows, we use a simplified view of the bacterial colony. We treat the bacterial film as a continuum liquid with surface active molecules present at the surface. We do not consider growth of the colony nor additional surfactant production, but explore if and how the measured spreading velocity and the shape of the bacterial colony can be compared to what is expected initially for Marangoni driven flows. The spreading velocity can then be predicted from a dimensional analysis, with the driving force being the surface tension gradients, which are proportional to the range of surface tension differences that can occur over a characteristic length scale L. The latter is being taken to be equal to the initial radius of the colony (5 mm). To assess the possible differences in surface tension, the surface pressure was measured as a function of the rhamnolipid concentration. Fig. 4 (inset) shows that surface pressures on the order of 30 mN m−1 are achieved at biologically relevant concentrations. The shear stress (τ) driving the flow is balanced by the viscous dissipation in the film which is controlled by the viscosity (η) and the relevant deformation gradient, given by the spreading velocity (U) divided by the average height of the colony H (10 μm). The balance can be written as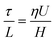 | (1) |
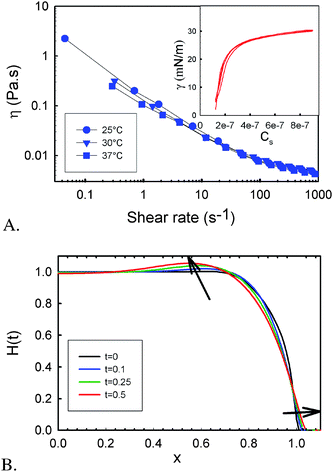 | ||
| Fig. 4 Physical characteristics of the biofilm and calculated height profiles. A: viscosity versus shear rate of the biofilm and (insert) surface pressure of the rhamnolipids versus the surface concentration (Cs in mol cm−2) for 2 consecutive compression expansion cycles. B: renormalized height profile of a spreading film. | ||
The viscosity of the bacterial colony has been measured and reveals a non-Newtonian behavior with a strong shear thinning, i.e. the viscosity decreases as the deformation gradient or stress is increased, as is shown in Fig. 4A. In the range of deformation gradients (0.1–1 s−1 given by the range of U/H) that are relevant for the spreading, the viscosity is of the order of 0.1 to 1 Pa s. As the orders of magnitude of all parameters of eqn (1) are known, the spreading velocity expected for a Marangoni driven flow is expected to be 0.5 to 5 μm s−1. The experimentally observed velocities before the patterns become unstable agree very well with the order of magnitude predicted from this simple analysis.
A slightly more elaborate analysis, still using a simplified continuum view of the bacterial colony, can be used to evaluate not only the velocities, but also the characteristic height increase near the edges. As the liquid film is thin (ε = H0/L0 ≪ 1), the lubrication approximation can be used, and only a 1D Stokes flow equation needs to be solved.27 The concentration gradients that drive the flow appear in the boundary conditions of the flow equations. The model is based on the Marangoni driven spreading of a monolayer of surfactant on the surface of a Newtonian liquid layer.28 Writing down the momentum and mass balances, a set of coupled PDE is obtained, to predict the spatial temporal variables, the height of liquid layer, H(x,t), and the surfactant concentration, Γ(x,t). The resulting equations are
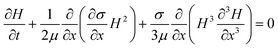 | (2) |
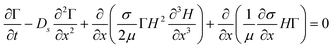 | (3) |
These equations reflect the balance between the Marangoni stresses that drive the motion and the viscous terms and the capillary stresses that oppose the creation of curvature, as well as a surface diffusive term, with the surface diffusivity Ds. To complete the formulation of the problem suitable initial conditions for both H(x,t) and Γ(x,t) have been selected. For H(x,0) a pancake like profile was assumed, which mimics the height profile that was measured experimentally using white light interferometry at the moment the colony starts to swarm and moves radially outwards. A similar profile was chosen for the concentration profile at the start of the calculations, based on the observations in the GFP experiments that the surfactant concentration follows the bacterial density which is assumed to be more or less uniform in the bulk but lower near the edges17 and the observations in literature that the expression of biosurfactant may be down regulated near the edges of the colony.26 The set of PDEs is rendered non-dimensional and solved numerically using a finite element method for the spatial discretisation. The resulting non-dimensional groups are the Péclet number (Pe = UL0/Ds), characterizing the relative importance of convection over diffusion. For the sizes and molecular weight of the rhamnolipids, the surface diffusion was estimated and Pe was estimated to be of the order of 5000. The capillary number (Ca = ηU/σ) can be estimated to be of order one, but a higher value (Ca = 500) was used to speed up the computations (hence the time scales are faster compared to the experimental time frame). The solution of the problem was found to be very sensitive to the boundary condition used near the edge of the colony. To mimic the experimental conditions as much as possible, we allow slip at certain length below the boundary, denoted as l, the slip length which was taken to be of order 0.05. We assume this to be a realistic boundary condition as there is water everywhere on the agar film. The boundary condition becomes:
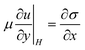 | (4) |
and
| u(x,−l,t) = 0 | (5) |
Fig. 4B shows the characteristic features for the initial Marangoni driven spreading for conditions which are assumed to be relevant for the present case. The height and length were normalized relative to the initial stage, the time scale will be faster than in the experiments due to choice of the dimensionless parameters. The calculations serve to demonstrate how some of the experimentally observed features during the initial stages of spreading are effectively reproduced. Specifically, a characteristic ‘bulge’ develops near the edge of the droplet, which is reminiscent of the features observed during the white light interferometry experiments in Fig. 2, especially in Fig. 2M. Such height increases have also been observed in experiments and calculations of the spreading of surfactant droplets atop of viscous liquids,28,29 and indirectly through bright field microscopy images for a bacterial biofilm of B. subtilis climbing up an inclined slope.16 The occurrence of the bulge near the edge of the colonies, both during the initial stages as within the fingers (see Fig. 1M and 1N and Fig. 4b), can be viewed as a signature of the start of Marangoni driven flow. Very similar to the formation of ‘tears of wine’,30 the process of Marangoni driven flows will speed up as a fingering instability develops along the front of the film. The new area which is being created can be expected to have a lower surface concentration of the surfactant, hence a higher surface tension. As a consequence, the liquid is pulled out into the branches and this leads a self-sustained propagation.
Although the pattern formation is not discussed in detail here, it can be pointed out that like the reaction-diffusion models, the set of equations describing the Marangoni flows is highly nonlinear and can be expected to give rise to a rich variety of morphologies. The details will depend on the link between the various physical parameters that control the Marangoni flow (concentration gradients, surface tension, surface diffusion, dynamic surface tension, viscosity) and the bacterial characteristics, which enter the picture predominantly as producers of biosurfactants. How the motility of the individual bacteria intervenes is as yet unclear, but the spatial heterogeneity of surfactant concentration may be affected by differences in motility. Further work with motility mutants is clearly required to clarify this link.
To see if the present model can also predict the patterns which arise when the flow becomes unstable requires a full linear and non-linear stability analysis. Whereas a full stability analysis of eqn (2)–(3) lies beyond the scope of the present work, some important remarks can already be made. First, the mathematical structure of the height-concentration evolution equations is very similar to the reaction diffusion models, and hence a similar richness in possible patterns can be expected. Second, the nonlinearity of the equations will be enhanced even when the shear thinning of the viscosity is taken into account. Finally, the number of arms observed in the dendritic structures is in line with predictions for the dominant wavelength using a linear stability analysis,31 although the variability in the experiments is considerable.
Arresting swarming
Having identified the mechanism of swarming in P. aeruginosa we can now devise strategies to arrest swarming behaviour. Inspection of the relevant dimensionless groups reveals that strategies could include an increase of the viscosity of the EPS matrix, which would slow down swarming or even stop the instability. As it has been suggested that biofilm elastic relaxation times are approximately constant,32 the effective viscosities can be expected to vary strongly with drying. Alternatively, increasing surface diffusivity could also play a role as this would lead to a decrease of Pe and make it impossible for concentration gradients to be sustained. Probably the most obvious strategy to arrest the swarming is to provide a countergradient in surfactant concentration. To test this hypothesis, we performed experiments as shown in Fig. 5. Using a sterile ring a pattern was stamped onto the agar substrate. In Fig. 5A this ring was just wetted with water and the colony can be observed to swarm over the imprint. When the ring is wetted with an aqueous solution containing rhamnolipids, exogeneously added biosurfactant is present on the agar substance, starting at a concentration expected to be 1/100 of the in vivo concentration (i.e. concentrations giving rise to equal surface pressures). As the concentration of rhamnolipids is increased by a factor 100 from panel B to D it can be observed that in panel D, the colony's swarming comes to a stop. Time lapse movies available as supporting information (Supporting information S2: suppmovieRL.mov†) reveal even more clearly how the colony is effectively arrested.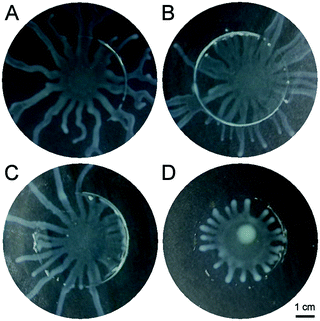 | ||
| Fig. 5 Inhibition of swarming. A–D: swarming can be inhibited by a exogeneously added biosurfactant. A: control; B–D: increasing concentrations of rhamnolipids were added in a circular pattern (concentration relative to the in vivo one: B:1/100, C:1/10; D:1/1). | ||
Conclusions
It has been shown that swarming of an opportunistic pathogen, P. aeruginosa, can be controlled by a balance between gradients in surface tension, capillarity and flow. The rhamnolipids which are part of the quorum sensing systems hence may have a dual role, in the sense that their surface active nature is exploited. The surface tension gradient control is an a-specific mechanism, relying only on surface tension effects, and hence it may play a role in many other bacterial systems. The modeling revealed subtle dependencies on both the wetting conditions and the physical properties of the slime. Understanding the mechanism enables one to arrest swarming gently.Acknowledgements
The authors acknowledge financial support for the Research council of K.U. Leuven through IDO-05-012 and GOA/2003/09 and the FWO (G.0637.06 and G.0554.10 and a fellowship to NV). Luna Imperiali Dafflito and Dr Frank Snijkers are thanked for the rheological measurements and the surface pressure measurements. Marc Peeters (MTM) is thanked for assistance with the interferometry and Dr J. Thaniyavarn, Chulalongkorn University for providing purified P. aeruginosa rhamnolipids.References
- J. W. Costerton, et al., Microbial Biofilms, Annu. Rev. Microbiol., 1987, 41, 435 CrossRef CAS.
- H. C. Berg, The rotary motor of bacterial flagella, Annu. Rev. Biochem., 2003, 72, 19 CrossRef CAS.
- E. M. Purcell, Life at Low Reynolds Number, Am. J. Phys., 1977, 45, 3.
- R. Dreyfus, et al., Microscopic artificial swimmers, Nature, 2005, 437, 862 CrossRef CAS.
- R. M. Harshey, Bacterial motility on a surface: many ways to a common goal, Annu. Rev. Microbiol., 2003, 57, 249 CrossRef CAS.
- N. Verstraeten, et al., Living on a surface: swarming and biofilm formation, Trends Microbiol., 2008, 16, 496 CrossRef CAS.
- R. Daniels, J. Vanderleyden and J. Michiels, Quorum sensing and swarming migration in bacteria, FEMS Microbiol. Rev., 2004, 28, 261 CrossRef CAS.
- D. G. Davies, et al., The involvement of cell-to-cell signals in the development of a bacterial biofilm, Science, 1998, 280, 295 CrossRef CAS.
- M. F. Copeland and D. B. Weibel, Bacterial swarming: a model system for studying dynamic self-assembly, Soft Matter, 2009, 5, 1174 RSC.
- A. Czirok, M. Matsushita and T. Vicsek, Theory of periodic swarming of bacteria: Application to Proteus mirabilis, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2001, 63, 031915 CrossRef CAS.
- H. C. Lai, et al., The RssAB two-component signal transduction system in Serratia marcescens regulates swarming motility and cell envelope architecture in response to exogenous saturated fatty acids, J. Bacteriol., 2005, 187, 3407 CrossRef CAS.
- R. Daniels, et al., Quorum signal molecules as biosurfactants affecting swarming in Rhizobium etli, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 14965 CrossRef CAS.
- N. C. Caiazza, R. M. Q. Shanks and G. A. O'Toole, Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa, J. Bacteriol., 2005, 187, 7351 CrossRef CAS.
- E. Ben-Jacob, I. Cohen and H. Levine, Cooperative self-organization of microorganisms, Adv. Phys., 2000, 49, 395 CrossRef CAS.
- C. Fuqua, M. R. Parsek and E. P. Greenberg, Regulation of gene expression by cell-to-cell communication: Acyl-homoserine lactone quorum sensing, Annu. Rev. Genet., 2001, 35, 439 CrossRef CAS.
- T. E. Angelini, et al., Bacillus subtilis spreads by surfing on waves of surfactant, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 18109 CrossRef CAS.
- J. N. Wilking, et al. Biofilms as complex fluids, MRS Bull., 2011, 36, 385–391 CrossRef CAS.
- M. E. Davey, N. C. Caiazza and G. A. O'Toole, Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1, J. Bacteriol., 2003, 185, 1027 CrossRef CAS.
- G. Soberonchavez, F. Lepine and E. Deziel, Production of rhamnolipids by Pseudomonas aeruginosa, Appl. Microbiol. Biotechnol., 2005, 68, 718 CrossRef CAS.
- T. T. Hoang, et al., Integration-proficient plasmids for Pseudomonas aeruginosa: Site-specific integration and use for engineering of reporter and expression strains, Plasmid, 2000, 43, 59 CrossRef CAS.
- Y. Lequette and E. M. Greenberg, Timing and localization of rhamnolipid synthesis gene expression in Pseudomonas aeruginosa biofilms, J. Bacteriol., 2005, 187, 37–44 CrossRef CAS.
- J. J. Benkoski, et al., Photocurable oil/water interfaces as a universal platform for 2-D self-assembly, Langmuir, 2007, 23, 3530 CrossRef CAS.
- J. Tremblay and E. Deziel, Improving the reproducibility of Pseudomonas aeruginosa swarming motility assays, J. Basic Microbiol., 2008, 48, 509 CrossRef CAS.
- R. D. Deegan, et al., Capillary flow as the cause of ring stains from dried liquid drops, Nature, 1997, 389, 827 CrossRef CAS.
- Y. Wu, B. G. Hosu and H. Berg, Microbubbles reveal chiral fluid flows in bacterial swarms, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 4147–4151 CrossRef CAS.
- J. Tremblay and E. Deziel, Gene expression in Pseudomonas aeruginosa swarming motility, BMC Genomics, 2010, 11, 587 CrossRef.
- L. G. Leal, Advanced Transport Phenomena: Fluid Mechanics and Convective Transport Processes, Cambridge University Press, New York ( 2007) Search PubMed.
- O. K. Matar and S. M. Troian, The development of transient fingering patterns during the spreading of surfactant coated films, Phys. Fluids, 1999, 11, 3232 CrossRef CAS.
- M. R. E. Warner, R. V. Craster and O. K. Matar, Fingering phenomena associated with insoluble surfactant spreading on thin liquid films, J. Fluid Mech., 2004, 510, 169 Search PubMed.
- J. B. furnier and A. M. Cazabat, Tears of Wine, Europhys. Lett., 1992, 20, 517 CAS.
- B. J. Fischer and S. M. Troian, Growth and decay of localized disturbances on a surfactant-coated spreading film, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2003, 67, 016309 CrossRef.
- T. Shaw, et al., Commonality of elastic relaxation times in biofilms, Phys. Rev. Lett., 2004, 93, 098102 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: suppmovie1a.mov and suppmovieRL.mov. See DOI: 10.1039/c1sm06002c |
| This journal is © The Royal Society of Chemistry 2012 |
