Ethenolysis of ricinoleic acid methyl ester – an efficient way to the oleochemical key substance methyl dec-9-enoate
A.
Behr
*,
S.
Krema
and
A.
Kämper
Department of Biochemical und Chemical Engineering, Technical University Dortmund, Emil-Figge Str. 66, Dortmund, 44227, Germany. E-mail: behr@bci.tu-dortmund.de; Fax: +49 231/755-2311; Tel: +49 231/755-2310
First published on 2nd November 2012
Abstract
In the ethenolysis of the renewable raw material ricinoleic acid methyl ester the valuable oleochemical key substance methyl dec-9-enoate is produced. Detailed optimizations lead to a high conversion and yield of the desired product. Another interesting product, the dec-1-ene-4-ol, is built by this reaction as coproduct. The products are accessible under mild reaction conditions and with the use of only small amounts of a commercial available homogeneous ruthenium catalyst. The results obtained are also achievable in the ethenolysis of ricinoleic acid and castor oil, under the same mild reaction conditions.
Motivation
The world's fossil fuel sources are becoming more and more depleted, but technological progress and the need for more energy continue to increase. As well, in the petrochemical industry, the demand for products from natural gas and oil is very high. It is therefore of great interest to put the spotlight on alternative products that can be produced on the basis of renewables, for instance oleochemicals. The chemical utilization of natural fats and oils has great potential, but also represents a challenge in its industrial application.As a promising type of reaction, here olefin metathesis, specifically the ethenolysis of unsaturated oleochemical substances should be pointed out. By ethenolysis with fats, fatty acids or fatty acid esters, the synthesis of short-chain terminal olefins can be carried out, which can be used particularly in the field of pharmaceuticals, cosmetics and in the manufacture of technical polymers and lubricants.1–4
A not yet sufficiently studied type of ethenolysis is the use of castor oil and its derivatives as starting materials. Castor oil contains a high amount of triglycerides of the fatty acid ricinoleic acid. This acid is one of the exceptional natural fatty acids with the specificity of a hydroxyl group at the C12 position. It is therefore of interest to examine the influence of this functional group on the chemistry of this fatty acid. The hydroxyl group changes important properties of this oleochemical compound, especially boiling point, density and viscosity.5–8
These different chemical properties may have different reactivity in terms of ethenolysis. That is why in this article a systematic optimization of the reaction parameters in the ethenolysis of ricinoleic acid methyl ester was carried out.
Castor oil derivatives
The castor oil is extracted from the seeds of the castor plant, which is a frost-sensitive but fast-growing plant found mainly in the tropics and subtropics. The current world production of castor seeds (in 2010) is about 1.53 million tons, the main producing countries are India (1.1 million tons), China (0.2 million tons) and Brazil (0.09 million tons).9Castor oil is not suitable as a food material and is therefore an ideal raw material for chemical use and derivatisation. This is a further important advantage as opposed to other fatty substrates.5,10,11
Detailed information of the regeneration of the described oil was reviewed in 2010 by Meier et al.5
The focus of our investigations was the ethenolysis of ricinoleic acid methyl ester 2, which was synthesized through the transesterification of triricinolein 1 with methanol (Fig. 1). Glycerol 3 was formed as a byproduct, which has many applications, for example as a starting material for industrially important basic chemicals.12a–c
 | ||
| Fig. 1 Synthesis of ricinoleic acid methyl ester (2). | ||
Castor oil already plays a certain role in chemical processing, e.g. in polymer manufacturing.5,12–16 However, to the best of our knowledge till now no systematic investigation of the ethenolysis of ricinoleic acid methyl ester 2 is described in the literature.
Ethenolysis
Ethenolysis generally describes the metathesis between ethene and another unsaturated compound; it is thus a special form of cross metathesis. With ethenolysis, it is possible to convert long-chain alkenes into shorter terminal olefins. By shifting the equilibrium it is possible to predominantly produce the desired short-chain terminal alkenes.In the oleochemical industry, it is currently of great interest to use natural fats or fatty acids to produce substances that can replace petrochemicals.12a Ethenolysis is one of the most intensively studied oleochemical metathesis processes. This reaction is used to produce ω-unsaturated fatty substrates and short-chain 1-alkenes.17 Alpha-olefins are interesting substrates, because they have a variety of applications in the manufacture of industrial important chemicals.18 Probably the most studied ethenolysis is that of methyl oleate, which reacts to dec-1-ene and methyl dec-9-enoate. This reaction has been studied for example in room temperature ionic liquids,14 in low pressure microchemical systems15 and in more recent times many studies on various catalyst systems have been carried out.19
Ethenolysis of rincinoleic acid methyl ester (2)
The ethenolysis of ricinoleic acid methyl ester 2 is a pressure- and temperature-dependent equilibrium reaction according to the pathway in Fig. 2. In parallel, however, the self metathesis to the long chain products 6 and 7 can take place.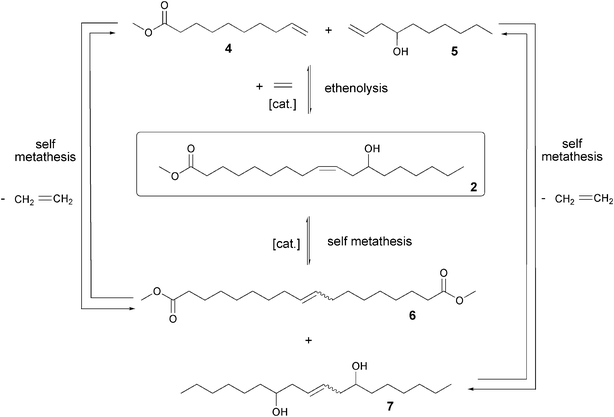 | ||
| Fig. 2 Ethenolysis and self metathesis of ricinoleic acid methyl ester (2). | ||
The ethenolysis of ricinoleic acid methyl ester 2 forms the terminal, short-chain products, methyl dec-9-enoate 4 and dec-1-ene-4-ol 5. Key factors to prefer this pathway are using a high pressure excess of ethene and simultaneously high temperatures. Furthermore, the catalyst used and its proportionate quantity to the substrate used is crucial, because otherwise self metathesis occurs, resulting in long chain, high boiling products. In this parallel reaction the two product molecules dimethyl octadec-9-enedioate 6 and octadec-9-ene-7,12-diol 7 are formed. Also the ethenolysis products 4 and 5 can react in a self metathesis to form these products 6 and 7.
Ethenolysis products have versatile application possibilities because of their terminal double bonds. Methyl dec-9-enoate 4 may preferably be further processed to odors and flavors. It may also be used as an intermediate product for lubricants, as well as prostaglandins and polymers (polyesters, polyamides) or as co-polymers. A particularly interesting possibility is the synthesis of 10-aminodecanoic acid to form nylon-10 (Fig. 3).
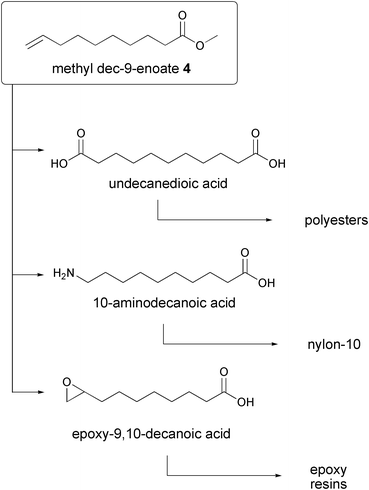 | ||
| Fig. 3 Chemical conversions of methyl dec-9-enoate (4). | ||
This assembly of the use of 4 demonstrates the importance of the described molecule. Because of these molecular properties the methyl dec-9-enoate 4 is viewed as the potential new “oleochemical key substance”.
The second valuable product dec-1-ene-4-ol 5 has an additional functionality, due to the hydroxyl group, compared to dec-1-ene, which results from the well-studied ethenolysis of methyl oleate. This allows various syntheses of natural substances and lubricants as well as polyolefins.1–3
The by-products 6 and 7 that result from the self metathesis can also be processed to value chemicals. The most important way to use dimethyl octadec-9-enedioate 6 consists of the condensation to civetone, an important raw material in the fragrance industry.20 Another application of the diester 6 may be in the manufacture of polyesters, polyamide resins, high performance lubricants and plasticizers. The octadec-9-ene-7,12-diol 7 has the advantage of a wide application field. It can serve as an intermediate in oleoindustry, for instance, as a starting material for the production of polyols.19
Results and discussion
The catalyst screening is the first important step in optimizing the ethenolysis of ricinoleic acid methyl ester 2. In Fig. 4 the investigated catalysts are shown.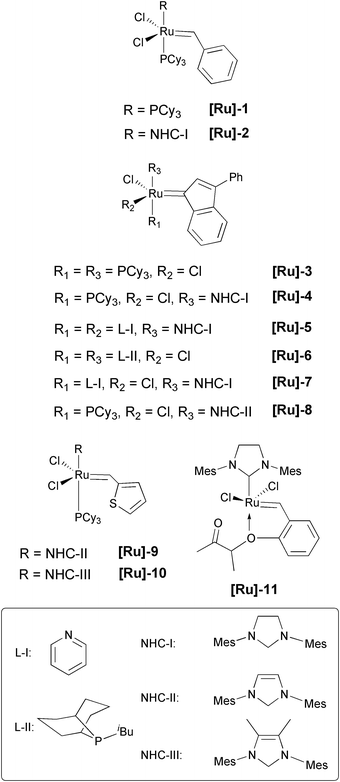 | ||
| Fig. 4 Investigated metathesis catalysts. | ||
In this study, the homogeneous ruthenium complexes [Ru]-1 to [Ru]-11 were investigated. The ricinoleic acid methyl ester 2 as substrate was dissolved in toluene (ratio substrate/solvent: 1/11) with 1 mol% (based on 2) of catalyst. At the beginning of our investigations the standard reaction conditions were an ethene pressure of 20 bar, a reaction temperature of 50 °C and a stirrer speed of 900 rpm for a reaction time of 8 h. Under these reaction conditions the results shown in Table 1 could be obtained.
| Catalyst | Conversion [%] | Yields [%] | |||
|---|---|---|---|---|---|
| CM | SM | ||||
| (2) | (4) | (5) | (6) | (7) | |
| a Reaction conditions: catalyst = 1 mol%, 92 wt% toluene, 20 bar ethene, 50 °C, 8 h, 900 rpm | |||||
| [Ru]-1 | 26 | 16 | 19 | >0 | 1 |
| [Ru]-2 | 98 | 54 | 76 | 8 | 2 |
| [Ru]-3 | 26 | 19 | 22 | >0 | >0 |
| [Ru]-4 | 98 | 63 | 78 | 4 | 1 |
| [Ru]-5 | 98 | 33 | 79 | 20 | 2 |
| [Ru]-6 | 42 | 30 | 34 | 0 | >0 |
| [Ru]-7 | 99 | 36 | 81 | 18 | 1 |
| [Ru]-8 | 84 | 52 | 48 | 6 | 10 |
| [Ru]-9 | 93 | 66 | 66 | 3 | 7 |
| [Ru]-10 | 91 | 62 | 58 | 3 | 8 |
| [Ru]-11 | 89 | 36 | 81 | 18 | 1 |
For both products of the ethenolysis 4 and 5 yields of 100% are possible, because of the high excess of ethene, which shifts the equilibrium of the metathesis. The products 4 and 5 must not be detected in the same yields, because they have other activities in successive metathesis reactions (compare Fig. 2).
Particularly striking in this screening are the significantly lower yields by the use of the catalysts [Ru]-1, [Ru]-3 and [Ru]-6, which have no N-heterocyclic carbene (NHC) ligand. The best results are achieved using catalyst [Ru]-4: The yields of the products are at 63% (4) and 78% (5), which are higher than with the Grubbs II-catalyst [Ru]-2. As compared to [Ru]-2 catalyst [Ru]-4 contains a phenylindenylidene complex, which were found to have a greater functional group tolerance than their benzylidene counterpart.21 Therefore, catalyst [Ru]-4 was used for detailed further investigation of the catalyst concentration. The aim of this study was on one hand to achieve the highest possible yield of ethenolysis products, and on the other hand to use the lowest possible catalyst concentration. The variation in the catalyst concentration was performed in the range 0.05–1.5 mol% (Table 2) with a reaction time of 3 h.
It is remarkable that over the investigated concentration range, high conversions and yields were achieved. Moreover, a steady decrease in the desired ethenolysis products was observed with increasing catalyst concentration. The highest yields of 70% (4) and 65% (5) are obtained at a catalyst concentration of 0.1 mol%.
In the following the influence of different solvents was investigated with regard to improve the mass transport. Non-polar aprotic, protic and polar aprotic solvents were examined for their influence on ethenolysis (Table 3).
| Solvent | Conversion [%] | Yields [%] | |||
|---|---|---|---|---|---|
| CM | SM | ||||
| (2) | (4) | (5) | (6) | (7) | |
| a Reaction conditions: 0.1 mol% [Ru]-4, 92 wt% toluene, 20 bar ethene, 50 °C, 3 h, 900 rpm | |||||
| cyclohexane | 80 | 58 | 35 | 7 | 13 |
| hexadecane | 84 | 49 | 35 | 5 | 14 |
| toluene | 92 | 70 | 65 | 3 | 6 |
| isopropanol | 84 | 51 | 54 | 11 | 5 |
| methanol | 85 | 58 | 59 | 4 | 7 |
| ethyl acetate | 86 | 70 | 55 | 5 | 9 |
| tetrahydrofuran | 84 | 66 | 56 | 5 | 8 |
Non-polar solvents (cyclohexane, hexadecane) resulted in comparably low yields, which can be explained by the presence of the hydroxyl group and the associated slight polarity of ricinoleic acid methyl ester 2. Between the protic (isopropanol, methanol) and the aprotic polar (ethyl acetate, tetrahydrofuran) solvents no significant difference can be found. However, isopropanol allows a lower solubility of ethene, which results in lower yields.22 The particular benefit of toluene compared to the other tested solvents is its relatively low cost production.4 Therefore toluene was used as a solvent for all further optimization studies.
In another series of experiments, the ratio between toluene and substrate was varied in order to achieve similar yields at higher space–time yields. For this purpose a variation of the substrate ratio (toluene/ricinoleic acid methyl ester 2) in the area of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 11
1 to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was examined. A clear trend shows the strong influence of an increased amount of solvent. Even at a ratio of 3
1 was examined. A clear trend shows the strong influence of an increased amount of solvent. Even at a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the yields are significantly lower than at a high dilution of 92 wt% toluene (11
1, the yields are significantly lower than at a high dilution of 92 wt% toluene (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The reason lies again in the improved solubility of ethene. The relatively high viscosity of ricinoleic acid methyl ester 2 is lowered by dilution with toluene which simplifies the transport of the starting compounds to the catalyst. In addition also the ratio between the catalyst and the substrate remained constant with the less amount of solvent, but the absolute amount of catalyst increased in the overall solution. These results in lower yields comparable with the results of the variation of catalyst concentration (see Table 2).
1). The reason lies again in the improved solubility of ethene. The relatively high viscosity of ricinoleic acid methyl ester 2 is lowered by dilution with toluene which simplifies the transport of the starting compounds to the catalyst. In addition also the ratio between the catalyst and the substrate remained constant with the less amount of solvent, but the absolute amount of catalyst increased in the overall solution. These results in lower yields comparable with the results of the variation of catalyst concentration (see Table 2).
Based on the results obtained previously, a 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of solvent to substrate was therefore retained. Variation in ethene pressure was carried out to investigate the influence of changing excess of ethene. The pressure of the ethene was varied for this purpose in the range 5–50 bar. Generally good yields were achievable all over the tested area. However, the maximum yield of methyl dec-9-enoate 4 and dec-1-ene-4-ol 5 was detected at a pressure of 20–30 bar. Lower pressures of about 5–10 bar meant that the excess of ethene was not high enough to dissolve the required amount of ethene in toluene. Over a pressure of 30 bar the opposite effect was seen. Up to this pressure the ethenolysis runs through a maximum of desired yields. Hence, a higher pressure is not necessary. To keep the consumption of ethene to a minimum, a pressure of 20 bar was considered for all further experiments.
1 ratio of solvent to substrate was therefore retained. Variation in ethene pressure was carried out to investigate the influence of changing excess of ethene. The pressure of the ethene was varied for this purpose in the range 5–50 bar. Generally good yields were achievable all over the tested area. However, the maximum yield of methyl dec-9-enoate 4 and dec-1-ene-4-ol 5 was detected at a pressure of 20–30 bar. Lower pressures of about 5–10 bar meant that the excess of ethene was not high enough to dissolve the required amount of ethene in toluene. Over a pressure of 30 bar the opposite effect was seen. Up to this pressure the ethenolysis runs through a maximum of desired yields. Hence, a higher pressure is not necessary. To keep the consumption of ethene to a minimum, a pressure of 20 bar was considered for all further experiments.
To more accurately assess the course of the reaction, various investigations were carried out at different reaction temperatures. These experiments showed a significant increase in yields with increasing temperature. The variation of the reaction temperature between 25 °C and 90 °C is shown in Fig. 5.
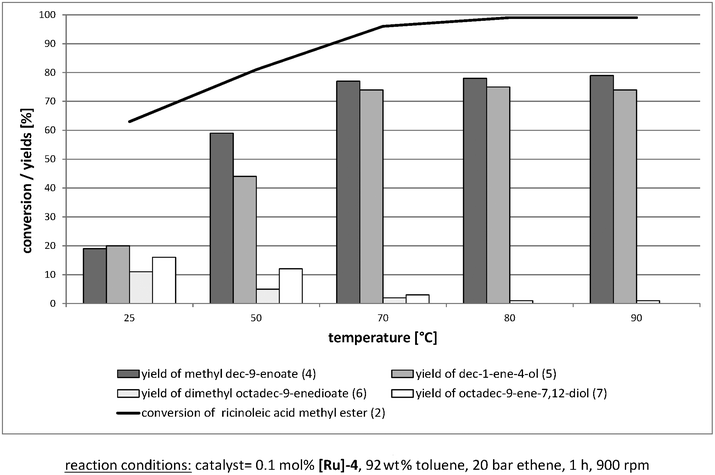 | ||
| Fig. 5 Variation of reaction temperature. | ||
High yields of methyl dec-9-enoate 4 and dec-1-ene-4-ol 5 were determined at temperatures between 70 °C and 80 °C. Further increase to 90 °C shows high conversions up to 99% of 2 and yields of 79% (4) and 75% (5). Furthermore, in the comparison of gradients of 80 °C and 90 °C, although no significant changes were seen in conversion and yields, the reaction rate could be increased such that the reaction equilibrium was reached at 90 °C after about 20 min. The reason for this positive effect was probably the decrease in substrate viscosity due to the increased temperature, thus enabling a better mass transport. In all the following experiments a reaction temperature of 80 °C was used, because the probability of side reactions should be lower at this temperature.
Even if the reaction after one hour is in equilibrium, it is of particular interest to carry out the reaction over a longer period to observe any side effects. The results of some long time experiments with a reaction time of 18 h are shown in Table 4.
The conversion of 2 is as high as in the previous test series (96–98%) but the maximum yield of products is evidently higher compared to the one-hour tests. This series of experiments was carried out with different catalyst concentrations based on the assumption that the amount of the catalyst after a certain time should have no more influence on the yields of the products. These results show that a low catalyst concentration (entry 3/1) is the better choice when using long reaction times. Higher concentrations (entry 3/3) result in lower yields of the desired products 4 and 5. This effect was probably due to increasing interactions between the catalyst molecules. Over the whole reaction time no side products could be detected, only the primary ethenolysis products have been provided.
Ethenolysis of ricinoleic acid (8) and castor oil (10)
With regard to industrial applications the ethenolysis of ricinoleic acid 8 or castor oil 10 in particular are cheap alternatives to the hitherto used model substrate ricinoleic acid methyl ester 2. Therefore, the previously determined optimal reaction parameters were applied to the ethenolysis of ricinoleic acid 8 and castor oil 10 so that the three substrates could be compared. The performed metathesis reactions are shown in Fig. 6.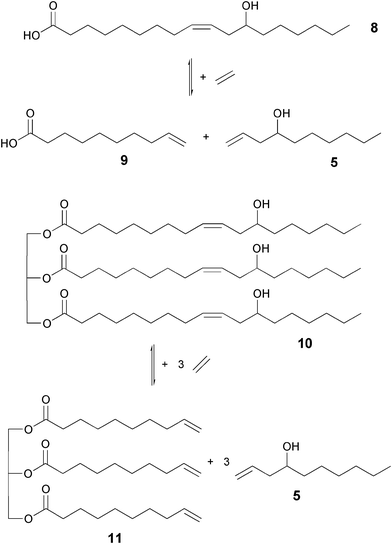 | ||
| Fig. 6 Ethenolysis of ricinoleic acid (8) and castor oil (10). | ||
When evaluating the results, it must be mentioned, that the ricinoleic acid 8 used had only a purity of 80%. In addition, the conversion of the castor oil 10 and the yield of the metathesis product 11 could not be analyzed directly by gas chromatography, but only via the analysis of the low boiling metathesis product 5 (Table 5).
At a reaction time of three hours a conversion of ricinoleic acid methyl ester 2 of 99% and yields of the metathesis product 4 of 83% and 5 of 77% were observed. This corresponds to a TON of 900 and a TOF of 0.091 s−1. The use of these optimized conditions for the reaction with ricinoleic acid 8 showed similar positive results. At a conversion of 97%, yields of 87% for 5 and of 74% for 9 are achieved. Even when using castor oil 10 a high yield of the ethenolysis product dec-1-ene-4-ol 5 of 87% is achievable. This proves that the optimized reaction conditions could successfully be applied both to ricinoleic acid 8 and castor oil 10 without the need for previous purification.
Conclusion
In the present contribution a systematic optimization of the ethenolysis of ricinoleic acid methyl ester 2 was successfully carried out. With a low amount of the commercial available complex [Ru]-4 (ccat = 0.1 mol%) and under mild reaction conditions (p = 20 bar ethene, T = 80 °C) it was possible to perform the ethenolysis of ricinoleic acid methyl ester 2 with nearly quantitative conversions and with high yields till 80% of the target products in just one hour. The described reaction offers access to well-known products with an alternative interesting raw material. Similar results were observed with the use of ricinoleic acid 8 and castor oil 10 as starting substrates, without previous purification of the substrates.Overall, castor oil derivatives are promising substrates for ethenolysis as an alternative to other fats and fatty derivatives, especially methyl oleate. Due to the presence of the hydroxyl group in the products, this type of homogeneous metathesis has great potential for further use in the chemical industry.
Experimental
Analytical equipment and methods
Gas chromatography (GC) analysis of the reaction solutions were carried out on a Hewlett-Packard gas chromatograph Series 6890 equipped with a HP5 capillary column (coating: 5% diphenyl–95% dimethoxy-polysiloxane; length 30 m; diameter 0.25 mm, thickness 0.25 μm). For the detection of individual components, a flame ionization detector (FID) connected to an autosampler was used. The oven temperature program was as follows: initial temperature 130 °C, hold for 6 min, increase by 25 °C min−1 up to 320 °C, hold for 4 min. Measurements were performed in split-split mode (ratio 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) using nitrogen as carrier gas. The qualitative assignment of the chromatographically determined retention times of the individual components was carried out by comparing to the respective pure substances. The quantitative determinations were made by the method with an internal standard.23
1) using nitrogen as carrier gas. The qualitative assignment of the chromatographically determined retention times of the individual components was carried out by comparing to the respective pure substances. The quantitative determinations were made by the method with an internal standard.23
The mass spectra were recorded by GC-MS. The mass spectrometer is a Hewlett Packard 5973 with an electron energy of 70 eV and a scan range [m/z] of 50–700. The oven temperature program, the split-split mode and the specification of the carrier gas were similar to those in the GC-FID method.
Experimental procedure
When using 0.4 g of the substance 4.6 g solvent and an appropriate amount of catalyst were added. Next the desired amount of ethene was introduced into the reactor and the reaction was heated to the desired temperature via an oil bath. After the reaction, the reactors are initially placed in an ice bath to stop the reaction. Subsequently, the excess of ethene was drained or burned and the reactors were opened. Finally, the GC samples were weighed and analyzed accordingly.Synthesis of the ricinoleic acid methyl ester (2)
For the production of 2 pure castor oil was used as starting material. 300 g of castor oil was mixed with 37 g of methanol and 6 g 30% sodium methoxide solution and stirred for 2 h at 70 °C. After the reaction, the aqueous glycerol phase was discharged and another 6 g of methanol was added to the organic phase. Once again, this was stirred for 2 h at 70 °C. The excess methanol was evaporated and the glycerol was separated from the organic phase. The reaction mixture was washed twice with 150 ml of hot, bidestilled water and dried with magnesium sulfate. Finally, a fractional distillation (155 °C, 10−3 mbar) was carried out to isolate the ricinoleic acid methyl ester 2 (purity about 98%).Acknowledgements
This work was financially supported by the German Federal Ministry of Food, Agriculture and Consumer Protection (represented by the Fachagentur Nachwachsende Rohstoffe) and the Emery Oleochemicals GmbH. The authors would like to thank Dr Alfred Westfechtel from Emery Oleochmicals as well as Umicore AG & Co. KG and Evonik Industries for the donation of ruthenium metathesis catalysts.References
- P. B. van Dam, M. C. Mittelmeijer and C. Boelhouwer, Fette, Seifen, Anstrichm., 1974, 76, 264 CrossRef CAS.
- S. Warwel, H.-G. Jägers and S. Thomas, Fett/Lipid, 1992, 94, 323 CrossRef CAS.
- J. C. Mol, Top. Catal., 2004, 27, 97 CrossRef CAS.
- (a) A. Behr, Angewandte homogene Katalyse, Wiley-VCH, Weinheim, 2008 Search PubMed; (b) A. Behr and P. Neubert, Applied homogeneous catalysis, Wiley-VCH, Weinheim, 2012 Search PubMed.
- H. Mutlu and M. A. R. Meier, Eur. J. Lipid Sci. Technol., 2010, 112, 10 CrossRef CAS.
- H. Bertsch, H. Reinheckel and G. Czichocki, Fette, Seifen, Anstrichm., 1965, 67, 780 CrossRef CAS.
- Carlo Erba Reagents, Safety Data Sheet for Ricinoleic Acid: CAS: 141-22-0, version 2011.
- Carl Roth GmbH + Co. KG: Sicherheitsdatenblatt des Ölsäuremethylesters, CAS: 112-62-9, version 2011.
- http://faostat.fao.org (18.04.2012) .
- H. Ebata, M. Yasuda, K. Toshima and S. Matsumura, J. Oleo Sci., 2008, 57, 315 CrossRef CAS.
- S. M. Plentz Meneghetti, M. R. Meneghetti, T. M. Serra, D. C. Barbosa and C. R. Wolf, Energy Fuels, 2007, 21, 3746 CrossRef.
- (a) A. Behr and J. Pérez Gomes, Eur. J. Lipid Sci. Technol., 2010, 112, 31 CrossRef CAS; (b) A. Behr, J. Eilting, K. Irawadi, J. Leschinski and F. Lindner, Green Chem., 2008, 10, 13 RSC; (c) M. A. R. Meier, J. O. Metzger and U. S. Schubert, Chem. Soc. Rev., 2007, 36, 1788 RSC.
- S. Kishino, J. Ogawa, A. Ando, Y. Omura and S. Shimizu, Biosci., Biotechnol., Biochem., 2002, 66, 2283 CrossRef CAS.
- C. Thurier, C. Fischmeister, C. Bruneau, H. Olivier-Bourbigou and P. H. Dixneuf, ChemSusChem, 2008, 1, 118 CrossRef CAS.
- C. P. Park, M. M. Van Wingerden, S.-Y. Han, D.-P. Kim and R. H. Grubbs, Org. Lett., 2011, 13, 2398 CrossRef CAS.
- R. M. Thomas, B. K. Keitz, T. M. Champagne and R. H. Grubbs, J. Am. Chem. Soc., 2011, 133, 7490 CrossRef CAS.
- S. Warwel, Fat Sci. Technol., 1992, 94, 512 CAS.
- B. B. Marvey, Int. J. Mol. Sci., 2008, 9, 1393 CrossRef CAS.
- K. A. Burdett, L. D. Harris, P. Margl, B. R. Maughon, T. Mokhtar-Zadeh, P. C. Saucier and E. P. Wasserman, Organometallics, 2004, 23, 2027 CrossRef CAS.
- J. Tsuji and S. Hashiguchi, J. Organomet. Chem., 1981, 218, 69 CrossRef CAS.
- H. Clavier and S. P. Nolan, Chem.–Eur. J., 2007, 13, 8029 CrossRef CAS.
- A. Sahgal, H. M. La and W. Hayduk, Can. J. Chem. Eng., 1978, 56, 354 CrossRef CAS.
- W. Gottwald, GC für Anwender, Wiley-VCH, Weinheim, 1995 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |
