MCM-48 nanorods: a self-assembled isotropic cubic mesostructure with anisotropic morphology†
Albert
Chang
,
Nien-Chu
Lai
and
Chia-Min
Yang
*
Department of Chemistry, National Tsing Hua University, Hsinchu 30013, Taiwan. E-mail: cmyang@mx.nthu.edu.tw; Fax: +886 3 5165521; Tel: +886 3 5731282
First published on 9th October 2012
Abstract
Mesoporous silica MCM-48 nanorods with oriented domains of a Ia3d mesophase have been synthesized; the material is the first example of a self-assembled isotropic mesostructure with anisotropic morphology.
The past years have been witness to a revolution in materials synthesis, leading to a remarkable variety of nanomaterials with anisotropic shapes, owing to which they may possess peculiar chemical and physical properties.1 Intensive research has also been focused on morphology control of surfactant-templated mesoporous materials2–7 in order to get a better understanding of cooperative self-assemblies2–7 and to realize their promising applications. Two-dimensional (2D)-hexagonal mesoporous silicas are the most intensively studied materials, and MCM-41,2,3 SBA-34 and SBA-155,6 silicas with various shapes have been synthesized.8–11 However, materials simultaneously exhibiting isotropic mesostructure and anisotropic morphology have not yet been reported, and the morphology control of isotropic cubic mesostructures with three-dimensional (3D)-interconnected mesopores,2–7 for example MCM-48 silica with interpenetrating networks of chiral channels,2,3,12,13 is extremely challenging.
We herein report a facile synthesis of MCM-48 nanorods as the first example of a self-assembled isotropic mesostructure with anisotropic morphology. The synthetic strategy is originated from our discovery of a synthesis system leading to silica mesostructures (designated as MMT-1) correlated to the ribbon phase with 2D-rectangular c2mm symmetry.14–16 Since ribbon phases and the Ia3d phase are present between 2D-hexagonal and lamellar liquid crystal phases,4,7 it might be possible to form the Ia3d mesophase by modifying the synthesis of MMT-1. Indeed, we found that MCM-48 nanospheres and, more interestingly, MCM-48 nanorods could be prepared by adding a suitable amount of isopropanol (IPA). We further studied the structure–morphology correlation and the formation of MCM-48 nanorods. The discovery not only demonstrates a new route toward MCM-48 nanostructures but also shows prospects in morphology control of self-assembled mesostructures.
The synthesis was performed by adding tetraethoxysilane (TEOS) into a mixture of cetyltrimethylammonium bromide (CTAB), tetraethylene glycol dodecyl ether (C12EO4), IPA and sodium hydroxide (NaOH) with a molar composition of 1.0 TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.17 (CTAB + C12EO4)
0.17 (CTAB + C12EO4)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x IPA
x IPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 NaOH
0.4 NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1300 H2O. The molar fraction of C12EO4 in the surfactant mixture is denoted by fn. Experimental details are given in the ESI.†Fig. 1 shows the X-ray diffraction (XRD) patterns of the materials synthesized with varied x and fn. In the absence of IPA, the synthesis with fn = 0.25 resulted in MMT-114 with unit cell parameters a and b of 11.6 nm and 4.6 nm, respectively. The mesostructure already changed with x = 20, and the 20 and 11 reflections shifted towards a higher angle and became broader, closer to each other and partially overlapped. As x = 27, the sample transformed to MCM-48 with a unit cell parameter of 9.5 nm. The XRD pattern of the calcined sample contains at least six peaks that can be indexed as reflections of Ia3d structure (cf. Fig. S1 in ESI†). A further increase of x to 47 resulted in a sample exhibiting sharper and more intense reflections of Ia3d structure, but too much IPA (x ≥ 60) produced lamellar or disordered materials. On the other hand, the nonionic surfactant was also found to be indispensable for forming the Ia3d phase, and the C12EO4-free synthesis (i.e. fn = 0) with x = 27 produced MCM-41 silica (cf.Fig. 1).
1300 H2O. The molar fraction of C12EO4 in the surfactant mixture is denoted by fn. Experimental details are given in the ESI.†Fig. 1 shows the X-ray diffraction (XRD) patterns of the materials synthesized with varied x and fn. In the absence of IPA, the synthesis with fn = 0.25 resulted in MMT-114 with unit cell parameters a and b of 11.6 nm and 4.6 nm, respectively. The mesostructure already changed with x = 20, and the 20 and 11 reflections shifted towards a higher angle and became broader, closer to each other and partially overlapped. As x = 27, the sample transformed to MCM-48 with a unit cell parameter of 9.5 nm. The XRD pattern of the calcined sample contains at least six peaks that can be indexed as reflections of Ia3d structure (cf. Fig. S1 in ESI†). A further increase of x to 47 resulted in a sample exhibiting sharper and more intense reflections of Ia3d structure, but too much IPA (x ≥ 60) produced lamellar or disordered materials. On the other hand, the nonionic surfactant was also found to be indispensable for forming the Ia3d phase, and the C12EO4-free synthesis (i.e. fn = 0) with x = 27 produced MCM-41 silica (cf.Fig. 1).
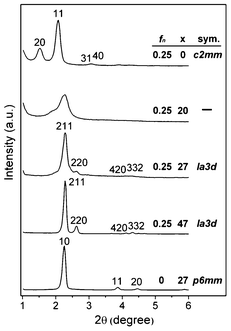 | ||
| Fig. 1 XRD patterns of as-synthesized samples with varied fn and x. | ||
The two MCM-48 samples were characterized by scanning electron microscopy (SEM). The sample with x = 47 consists of 500–800 nm particles. Most of the particles exhibit truncated facets (cf. Fig. S2 in ESI), a feature for highly crystalline MCM-48 materials.12 The other sample with x = 27, to our surprise, comprises almost exclusively (over 90%) L-shaped nanorods with two arms that are roughly equal in length (cf.Fig. 2). The angle between the arms is around 90–150°, and each arm has a hexagonal cross section (cf. inset of Fig. 2), a diameter of 400–600 nm and a length of 1.5–2.0 μm.
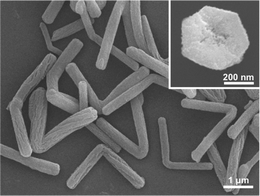 | ||
| Fig. 2 SEM image of the sample with fn = 0.25 and x = 27. | ||
The calcined and platinum-infiltrated MCM-48 nanorods were oriented and ultramicrotomed to study the structure-morphology correlation by transmission electron microscopy (TEM). Interestingly, the images viewed parallel to the faces of hexagonal rods, both in the middle and at rod ends, are almost exclusively the projections along [110] direction of Ia3d mesostructure (cf.Fig. 3A and 3B). Besides, the [111] projection is observed along the rod axis, both in pure-silica (cf.Fig. 3C-1) and Pt-infiltrated samples (cf.Fig. 3C-2). The observations could be comprehended by considering that the six {110} planes of a cubic structure belong to the zone with [111] as the zone axis and they intersect to form a hexagon. Nearly all nanorods contain large and oriented domains of Ia3d mesostructure that extend along the rods. Nevertheless, there exist structural defects in the rods and voids at rod center (cf. insets in Fig. 2 and 3), and the ordered mesostructure discontinues at the joint of two arms (cf. Fig. S3 in ESI†).
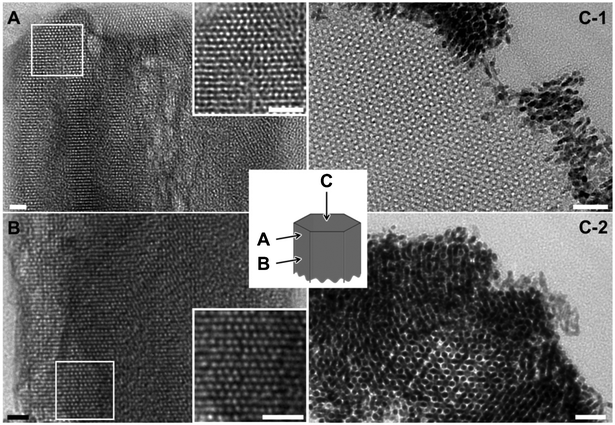 | ||
| Fig. 3 TEM image of the ultramicrotomed pure-silica (A and B) and Pt-infiltrated (C) MCM-48 nanorods. Scale bars indicate 20 nm. | ||
The nanorod sample exhibits a type IV nitrogen physisorption isotherm (cf. Fig. S4 in ESI†) with a steep step at a relative pressure (p/p0) of ∼0.38 corresponding to capillary condensation in channel-type mesopores with a diameter of 3.0 nm. The H2-type-like hysteresis loop with the desorption branch closed at p/p0 ∼0.49 is likely to be attributed to the voids observed at the rod center that exhibit ink-bottle mesopore-like sorption behavior.17,18 The sample has a surface area of 1115 m2g−1 and a pore volume of 1.03 cm3g−1, typical for MCM-48 and CTAB-templated mesoporous silicas.2,3,12
Small-angle X-ray scattering (SAXS) measurements on the synthesis mixture of MCM-48 nanorods and SEM on the solid products taken at different reaction times t (cf. ESI for experimental details) were further performed to get insights into the development of rod morphology and the oriented cubic mesostructure. As shown in Fig. 4, one could observe some tiny and structureless aggregates, presumably composed of silicatropic micelles and silicate species, at t = 15 min. At t = 18 min, a Bragg peak (q = 0.148 Å−1) corresponding to a d-spacing of 4.24 nm appeared, and micrometer-long and bent rods with helical stripes similar to those on MMT-1 nanorods15 were already formed. Within the following two minutes, the bent rods transformed to angled rods. Besides, the Bragg peak became sharper and slightly shifted (q = 0.150 Å−1, d = 4.20 nm), and a new peak at q = 0.297 Å−1 (d = 2.11 nm) appeared. The SAXS pattern is more likely to be correlated to a 2D-hexagonal than lamellar structure, considering the one-dimensional morphology of the rods and the fact that a transformation from the 2D-hexagonal mesophase was previously observed during the IPA-free synthesis of MMT-1 under similar conditions.14,15 At t = 23 min, the growth of L-shaped nanorods was almost complete and the SAXS pattern contained Bragg peaks that can be indexed as 211, 220, 420 and 332 reflections of the Ia3d structure. We noticed that the formation of MCM-48 nanorods was very sensitive to the conditions of mixing and stirring. That was the reason why we failed to perform in situ SAXS measurements by using a continuous flow-type setup14–16 and why additional peaks that were weak in intensity also appeared in the SAXS pattern. For the formation of MCM-48 nanospheres (with x = 47), a similar but slightly faster structural transformation was observed (cf. Fig. S5 in ESI†).
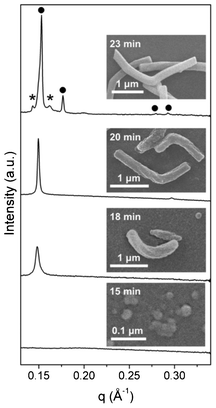 | ||
| Fig. 4 SAXS patterns of the synthesis mixture of MCM-48 nanorods and SEM images of the solid products taken at different reaction times. The peaks marked by filled circles and asterisks are attributed to the Ia3d structure and unknown mesophase, respectively. | ||
The IPA-induced formation of MCM-48 nanospheres and nanorods may be correlated with the fact that short-chain alcohols penetrate slightly into the palisade layer of surfactant micelles.19 During the IPA-free synthesis of helical MMT-1, the preferential adsorption of anionic silicate species (produced from TEOS hydrolysis) onto cetyltrimethylammonium (CTA) cations induces a segregation of CTAB and C12EO4, resulting in deformed and bent silicatropic micellar rods.14–16 When IPA is added, the penetrating IPA molecules may reduce the repulsion between surfactants' head groups and, after subsequent introduction of silicate species, lead to a uniform decrease of interfacial curvature to form the thermodynamically favored Ia3d mesostructure. As for MCM-48 nanorods, a proposed formation pathway based on the results of SAXS and SEM is shown in Scheme 1. It seems that self-assembled helical rods, probably with p6mm structure, are formed before the transformation to Ia3d structure takes place. The p6mm-to-Ia3d transformation, probably via the cylinder-branching mechanism,20 may then result in the oriented cubic phase with [111] direction parallel to the rod axis. The development of the two-armed shape of the nanorods might be likely related to the release of bending energy of the helical rods during structural transformation. Although the proposed mechanism needs to be further examined, the successful synthesis of MCM-48 nanorods offers prospects for preparation of self-assembled materials simultaneously having isotropic mesostructure and anisotropic morphology.
 | ||
| Scheme 1 Schematic representation of the formation of MCM-48 nanorods. | ||
Acknowledgements
The authors thank U. S. Jeng and C. H. Su (NSRRC) for their help with SAXS measurements, and the National Science Council of the Republic of China for financial support under the contract no. NSC98-2113-M-007-020-MY3.References
- S. C. Glotzer and M. J. Solomon, Nat. Mater., 2007, 6, 557 CrossRef
.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olson, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834 CrossRef CAS
.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS
.
- Q. S. Huo, D. I. Margolese, U. Ciesla, P. Y. Feng, T. E. Gier, P. Sieger, R. Leon, P. M. Petroff, F. Schüth and G. D. Stucky, Nature, 1994, 368, 317 CrossRef CAS
.
- D. Y. Zhao, Q. S. Huo, J. L. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024 CrossRef CAS
.
- D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS
.
- U. Ciesla and F. Schüth, Microporous Mesoporous Mater., 1999, 27, 131 CrossRef CAS
.
- Q. S. Huo, D. Y. Zhao, J. L. Feng, K. Weston, S. K. Buratto, G. D. Stucky, S. Schacht and F. Schüth, Adv. Mater., 1997, 9, 974 CrossRef CAS
.
- H. Yang, N. Coombs and G. A. Ozin, Nature, 1997, 386, 692 CrossRef CAS
.
- Q. S. Huo, J. L. Feng, F. Schüth and G. D. Stucky, Chem. Mater., 1997, 9, 14 CrossRef CAS
.
- C. Z. Yu, J. Fan, B. Z. Tian, D. Y. Zhao and G. D. Stucky, Adv. Mater., 2002, 14, 1742 CrossRef CAS
.
- J. M. Kim, S. K. Kim and R. Ryoo, Chem. Commun., 1998, 259 RSC
.
- T. W. Kim, P. W. Chung and V. S. Y. Lin, Chem. Mater., 2010, 22, 5093 CrossRef CAS
.
- C. M. Yang, C. Y. Lin, Y. Sakamoto, W. C. Huang and L. L. Chang, Chem. Commun., 2008, 5969 RSC
.
- W. C. Huang, L. L. Chang, Y. Sakamoto, C. Y. Lin, N. C. Lai and C. M. Yang, RSC Adv., 2011, 1, 229 RSC
.
- W. C. Huang, N. C. Lai, L.-L. Chang and C. M. Yang, Microporous Mesoporous Mater., 2012, 151, 411 CrossRef CAS
.
- Y. C. Hsu, Y. H. Chang and C. M. Yang, Adv. Funct. Mater., 2008, 18, 1799 CrossRef CAS
.
- C. M. Yang, W. Schmidt and F. Kleitz, J. Mater. Chem., 2005, 15, 5112 RSC
.
- R. Zana, Adv. Colloid Interface Sci., 1995, 57, 1 CrossRef CAS
.
- C. C. Landry, S. H. Tolbert, K. W. Gallis, A. Monnier, G. D. Stucky, F. Norby and J. C. Hanson, Chem. Mater., 2001, 13, 1600 CrossRef CAS
.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental details, XRD patterns, SEM and TEM images, nitrogen physisorption isotherm and SAXS patterns. See DOI: 10.1039/c2ra22444e |
| This journal is © The Royal Society of Chemistry 2012 |
