Pd(OAc)2 without added ligand as an active catalyst for Mizoroki–Heck reaction in aqueous media†
Mojtaba
Amini
*a,
Mojtaba
Bagherzadeh
*b,
Zeinab
Moradi-Shoeili
b and
Davar M.
Boghaei
b
aDepartment of Chemistry, Faculty of Science, University of Maragheh, Golshahr, P.O. Box: 55181-83111731, Maragheh, Iran. E-mail: mamini@maragheh.ac.ir; Tel: +98 421 2278900; Fax: +98 421 2276066
bChemistry Department, Sharif University of Technology, PO Box 11155-3516, Tehran, Iran
First published on 4th October 2012
Abstract
Conditions for an efficient ligand-free Heck C–C coupling reaction of aryl iodides and bromides with terminal olefins under aerobic conditions have been developed. Critical to the success of this new protocol is the use of palladium acetate as an extremely active catalyst for the Heck reaction in water and aqueous media. Both the base and the solvent were found to have a fundamental influence on the efficiency of the reaction, with K2CO3 and a mixture of (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) H2O/DMSO being the optimal base and solvent, respectively.
1) H2O/DMSO being the optimal base and solvent, respectively.
Introduction
In recent years, removing toxic, unsafe and non-environmentally friendly reagents and the use of water as an attractive solvent in transition metal catalyzed reactions have rigorously been studied from the standpoint of green chemistry.1In the class of palladium-catalyzed C–C coupling reactions, the Mizoroki–Heck reaction is an important transformation in organic synthesis and the resulting products have been widely used in the syntheses of natural products, pharmaceuticals and advanced functional materials.2 Generally, the efficiency of the Pd-based catalyst is strongly dependent on the nature of the coordinated ligands, which leads to toxicity, high cost and problems of waste disposal on the large scale.3 Therefore, based on the concerns of economic and green chemistry, the development of mild, rapid, and aerobic ligandless-based procedures using low catalyst loading, cheaper, environmentally friendly catalysts and solvent systems is still a topic of considerable interest to organic synthesis and industry.4
Recently, different methods for the palladium-catalyzed Mizoroki–Heck reaction without the aid of any ligands have been reported, but their major drawbacks are the use of unsafe and toxic solvents and the need for a high temperature and long reaction time.5 To the best of our knowledge, ligand-free one-pot consecutive Heck couplings in aqueous media under aerobic conditions have not yet been reported. Encouraged by these findings, we intend to find an efficient ligand-free catalytic system for Heck reactions in water or water–cosolvent under aerobic conditions. We herein report that ligand-free Palladium-catalyzed arylation of olefins by the Heck reaction selectively provide E-internal olefin derivatives with yields ranging from good to excellent. An overall investigation of the effects of different bases, solvents and atmospheres on the Heck reaction is described.
Results and discussion
The model coupling reaction between iodobenzene and n-butyl acrylate was conducted to screen the optimal reaction conditions, including catalysts, bases, solvents and atmospheres. Initially, considering that the catalyst always plays important roles in metal-catalyzed chemistry, a series of copper, nickel and palladium salts such as CuI, CuBr, Cu(OAc)2, Ni(OAc)2, NiCl2·2H2O, and Pd(OAc)2 were examined to study the effects of different precatalysts on the synthesis of coupling product n-butyl (E)-cinnamate. The results are illustrated in Fig. 1. To our delight, the coupling reaction proceeded smoothly and generated the desired product n-butyl (E)-cinnamate in 100% yield when 0.5 mol% Pd(OAc)2 was used as ligand-free catalyst, representing the best result of all salts tested in DMSO under an air atmosphere at 80 °C. To improve the efficiency of the reaction, further screening with 5 mol% of copper and nickel salts was carried out, but none of them provided the product in a yield as high as that obtained with Pd(OAc)2.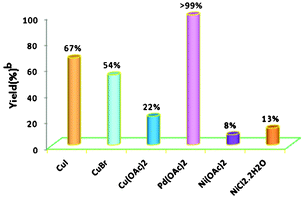 | ||
| Fig. 1 Optimization of various catalytic salts for the Heck reaction. Reaction conditions: 1.0 mmol iodobenzene, 1.2 mmol n-butyl acrylate, 2 mmol K2CO3, 0.5 mol% Pd(OAc)2 or 5.0 mol% Cu and Ni salts, 80 °C, 4 h, 3 mL DMSO. b GC yield. | ||
The choice of the solvent was found to be crucial to the success of the catalytic reaction. First the Heck reaction of n-butyl acrylate with iodobenzene at 80 °C in a number of solvents such as DMSO, iPrOH, H2O and (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixtures of H2O/DMSO and H2O/iPrOH was studied (Fig. 2). H2O, DMSO and the (2
1) mixtures of H2O/DMSO and H2O/iPrOH was studied (Fig. 2). H2O, DMSO and the (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture of H2O/DMSO appeared to be the best choices of the solvents used by providing maximum yields for the product n-butyl (E)-cinnamate. iPrOH and the (2
1) mixture of H2O/DMSO appeared to be the best choices of the solvents used by providing maximum yields for the product n-butyl (E)-cinnamate. iPrOH and the (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture of H2O/iPrOH were not good choices here and the yields were comparatively lower. In addition, for a better comparison and understanding of the influence of the solvent nature on the yields, the coupling of iodobenzene with styrene under similar reaction conditions was also studied (Fig. 2). It has been found that DMSO and the (2
1) mixture of H2O/iPrOH were not good choices here and the yields were comparatively lower. In addition, for a better comparison and understanding of the influence of the solvent nature on the yields, the coupling of iodobenzene with styrene under similar reaction conditions was also studied (Fig. 2). It has been found that DMSO and the (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture of H2O/DMSO performed most efficiently as solvent systems among the various solvent mixtures screened and gave the coupling product (E)-stilbene in maximum yield. The reduction of reaction yields in solvents other than DMSO suggests several mechanistic possibilities, including the prospect that DMSO itself participates in redox chemistry. The essential role of DMSO as a solvent therefore appears to be associated with its palladium-coordination ability.6 Access to both hard (O-) and soft (S-) ligand donor atoms perhaps facilitate the reoxidation of Pd(0) to Pd(II) (presumably by air) should the Pd(II) ever be reduced to Pd(0).7 Moreover, it is shown that the Pd(0) cluster can be synthesized from Pd(II) salt precursors in DMSO solution both as a reducing agent and as a stabilizer.8
1) mixture of H2O/DMSO performed most efficiently as solvent systems among the various solvent mixtures screened and gave the coupling product (E)-stilbene in maximum yield. The reduction of reaction yields in solvents other than DMSO suggests several mechanistic possibilities, including the prospect that DMSO itself participates in redox chemistry. The essential role of DMSO as a solvent therefore appears to be associated with its palladium-coordination ability.6 Access to both hard (O-) and soft (S-) ligand donor atoms perhaps facilitate the reoxidation of Pd(0) to Pd(II) (presumably by air) should the Pd(II) ever be reduced to Pd(0).7 Moreover, it is shown that the Pd(0) cluster can be synthesized from Pd(II) salt precursors in DMSO solution both as a reducing agent and as a stabilizer.8
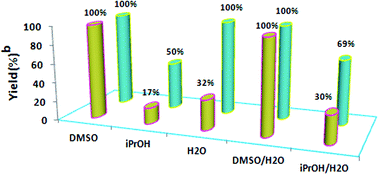 | ||
| Fig. 2 The effect of solvent on the Heck reaction. Reaction conditions: 1.0 mmol iodobenzene, 1.2 mmol n-butyl acrylate or styrene, 2 mmol K2CO3, 0.5 mol% Pd(OAc)2, 80 °C, 4 h, 3 mL solvent. b GC yield. | ||
In order to find a suitable base that would effect the desired reaction, we also screened several bases such as K2CO3, Na3PO4, NEt3, and NaOAc. All the reactions examined led to some conversion, albeit with quite different efficiencies (Table 1, entries 1–4), with K2CO3 being the most reactive, allowing the reaction to be completed in 2 h.
| Entry | Base | Atmosphere | Yield (%)b |
|---|---|---|---|
a Reaction conditions: 1.0 mmol iodobenzene, 1.2 mmol n-butyl acrylate, 2 mmol of base, 0.5 mol% Pd(OAc)2, 80 °C, 4 h, 3 mL (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture of H2O/DMSO.
b GC yield.
c deoxygenated solvent ((2 1) mixture of H2O/DMSO.
b GC yield.
c deoxygenated solvent ((2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture of H2O/DMSO). 1) mixture of H2O/DMSO).
|
|||
| 1 | K2CO3 | Air | >99 |
| 2 | Na3PO4 | Air | 73 |
| 3 | NEt3 | Air | 23 |
| 4 | NaOAc | Air | 59 |
| 5 | K2CO3 | O2 | >99 |
| 6 | K2CO3 | N2 | 85 |
| 7 | K2CO3 | Air | 78c |
As reported in Table 1, we next studied the impacts of different atmospheres, namely, open air, oxygen and nitrogen, on the Heck coupling reaction between iodobenzene and n-butyl acrylate.
We observed that the couplings gave higher yields under air and oxygen than in nitrogen and the reaction was quantitative in both open air and oxygen and reached 85% yield in nitrogen (Table 1, entries 1, 5–6). However, a yield of 78% was obtained under similar conditions, when the reaction proceeded in deoxygenated solvent, (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture of H2O/DMSO, under open air (Table 1, entry 7).
1) mixture of H2O/DMSO, under open air (Table 1, entry 7).
These results disclosed that the oxygen played a promoting role in the palladium-catalyzed ligand-free Heck reaction as Jung et al. reported earlier.9 Based on these results we propose the catalytic cycle depicted in Scheme 1. The first Pd(II) is reduced in situ to Pd(0) and then following the oxidative addition, the iodobenzene chelates to the Pd(0) centre and migratory insertion of the olefin is followed by β-hydride elimination to produce a Pd(II) species (H–Pd–X), which undergoes reductive elimination to form the Pd(0) species and the desired product. The Pd(0) species is subject to two competing processes. Pd(0) can be either oxidized to a Pd(II) species by molecular oxygen, possibly via a peroxopalladium(II) complex,10 and enters the catalytic cycle, or it can aggregate to form insoluble palladium black.
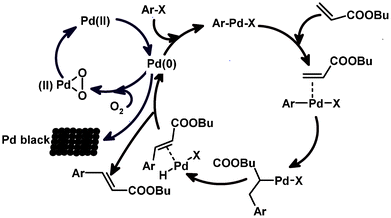 | ||
| Scheme 1 Proposed Mechanism for the Heck Reaction Catalyzed by Pd(OAc)2. | ||
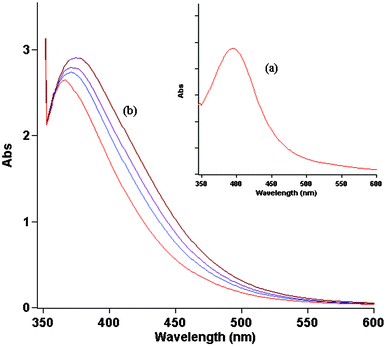
The aerobic redox cycling between Pd(II) and Pd(0) in DMSO was confirmed by a UV-visible spectroscopy study of Pd(OAc)2 solution (Fig. 3). The expected characteristic strong absorption of Pd(OAc)2 near 400 nm (Fig. 3a) is not observed in the initial absorption spectrum. The absorbance at around 360 nm (Fig. 3b) can be assigned to a Pd(0) species.11 A downfield shift of CT band towards 400 nm in the presence of dioxygen (Fig. 3a) was not observed in N2 saturated DMSO solvent (not shown). Hence, it is supposed that the DMSO exerts an efficient stabilizing effect on the Pd(0) species obtained in situ by reduction of the Pd(II) catalyst precursor. The dependence of the reaction yield on O2 presence supports a mechanism in which oxidation of Pd(0) by dioxygen is associated with the reaction sequence. Although we obtained no direct evidence for the intermediate formation of peroxopalladium(II) (Scheme 1), such reactivity has substantial precedent.12 These results are in agreement with the values reported by Gaikwad and Rothenberg.13
To study additional insights into the catalytic mechanism, reaction progress was monitored using UV-visible spectrometry. The Pd(OAc)2 (0.5 mol%) was dissolved in DMSO solution containing aryl halide (1.0 mmol), olefins (1.2 mmol), K2CO3 (2 mmol) and the initial yellow solution was left to react at 353 K for a certain amount of time under aerobic conditions. The samples of the reaction mixture after filtration (used for removal of solids) were placed into a quartz cell without additional dilution. Fig. 4 demonstrates the absorption spectral changes in the region from 350 to 600 nm associated with the catalytic reaction mixture. The gradual decrease of the absorption band at 360 nm is accompanied by a growth in the spectra baseline, possibly related to Pd(0) colloid formation. It is noteworthy that a shoulder at 440 nm was observed as well when the Heck reaction was performed and can be assigned to an oxidative arylpalladium complex.14 The reaction proceeded smoothly in DMSO in which the precipitation of Pd black gradually occurs after approximately 100 min. According to experimental results,15 Pd aggregates do not show any signals in the UV-visible region. The mechanistic studies outlined above clearly define the challenge in developing a more active aerobic oxidation catalyst system, namely, enhancing the rate of Pd(0) oxidation, particularly with respect to competing Pd(0) aggregation.
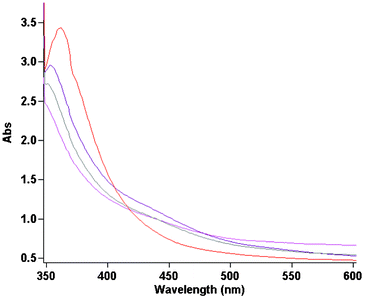 | ||
| Fig. 4 Spectral changes during Heck reaction performance in DMSO at 353 K (with 30 min time intervals). | ||
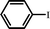
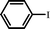
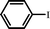
With optimal conditions determined, the scope of Heck coupling reactions of iodobenzene, bromobenzene and 4-acetylbromobenzene with various olefins was investigated and the results are summarized in Table 2.
| Yield (%)b | Olefin | ArX | Entry |
|---|---|---|---|
a Reaction conditions: 1.0 mmol aryl halide, 1.2 mmol olefin, 2 mmol K2CO3, 0.5 mol% Pd(OAc)2, 80 °C, 2 h, 3 mL (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture of H2O/DMSO as solvent.
b Isolated yield.
c Ratio (Z 1) mixture of H2O/DMSO as solvent.
b Isolated yield.
c Ratio (Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E) is 23 E) is 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77. 77.
|
|||
| 97 |

|

|
1 |
| 96 |
|
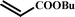
|
2 |
| 95 |
|
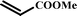
|
3 |
| 97 |

|
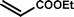
|
4 |
| 96c |

|
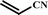
|
5 |
| 94 |
|

|
6 |
| 95 |

|
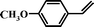
|
7 |
| 91 |
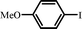
|
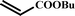
|
8 |
| 88 |
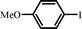
|
|
9 |
| 69 |
|

|
10 |
| 71 |
|
|
11 |
| 79 |

|
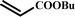
|
12 |
| 57 |
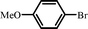
|
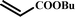
|
13 |
| 46 |
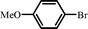
|
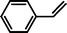
|
14 |
| 39 |

|
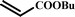
|
15 |
| 41 |
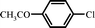
|
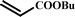
|
16 |
| 33 |
|

|
17 |
| 11 |

|
|
18 |
| trace |
|
|
19 |
The coupling of iodobenzene was superior and afforded the desired product in quantitative yields (Table 2, entries 1–9). The electronic nature of substituents on the olefins was initially investigated. Various olefins bearing either electron-donating groups or electron-withdrawing groups such as methoxy, methyl and cyano groups, provided the corresponding products in excellent yields and short reaction times. A mixture of the two stereoisomers [(Z) and (E)] was obtained for the reaction of acrylonitrile (Table 2, entry 5). Due to the crowding effect, aryl halides substituted with a methoxy group at the ortho position were less reactive to the coupling reaction than the para derivatives (Table 2, entries 8, 10, 13, 15). It is well known that electron-poor aryl halides undergo Heck reactions more easily than electron-rich substrates and this general trend has been observed for both aryl bromides and chlorides (Table 2, entries 11–18). It is worth noting that the most electron-poor aryl bromide and chloride, 4-acetylbromobenzene and 4-acetylchlorobenzene, showed good reactivity and were successfully coupled with styrene and n-butylacrylate in good to excellent yields (Table 2, entries 11, 12, 17, 18). However, the Heck coupling of electron-rich aryl bromide and bromobenzene did not proceed as well under the same reaction conditions and trace amounts of coupling product were observed (Table 2, entries 18, 19).
In conclusion, a ligand-free palladium-catalyzed Heck reaction has been developed for the selective synthesis of E-internal olefins in good to excellent yields in aqueous phase in a short reaction time under aerobic conditions. The high efficiency and use of only cosolvent without other additives and ligands make the method useful and attractive for the Heck reaction. This procedure is more reliable than previous methods using higher concentrations of palladium catalysts.
Experimental section
General remarks
All the reactions were carried out under air. Chemical materials and solvents were purchased from the Fluka and Merck Chemical companies and used without purification. The elemental analyses (carbon, hydrogen, and nitrogen) of compounds were obtained from a Carlo ERBA Model EA 1108 analyzer. UV-vis analyses were performed by a CARY 100 Bio VARIAN spectrophotometer using quartz cells having a 1.0 cm path length. The 1H and 13C NMR spectra were recorded on a Bruker FT-NMR 500 MHZ spectrometer. Chemical shifts are reported in ppm relative to TMS. The reaction products of the Heck reaction were determined and analyzed by a HP Agilent 6890 gas chromatograph equipped with a HP-5 capillary column (phenyl methyl siloxane 30 m 320 μm 0.25 μm) and flame-ionization detector. All products were isolated by short chromatography on a silica gel 60 (0.063–0.20 mesh ASTM) column using hexane/ethyl acetate as eluent.General procedure for the Heck reactions
All Heck reactions were carried out under air. A mixture of aryl halide (1.0 mmol), olefins (1.2 mmol), K2CO3 (2 mmol) and Pd(OAc)2 (0.5 mol%) in a (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture of H2O/DMSO (3 mL) was allowed to react in a sealed tube at 80 °C. The reaction mixture was added to brine (15 mL) and extracted three times with diethyl ether (3 × 15 mL). The aqueous solution was separated from the organic layer. The diethyl ether solution was washed with water (3 × 15 mL), dried over MgSO4, and evaporated to dryness under reduced pressure to afford the desired product, which was then washed with hexane (3 × 5 mL). The further purification of the product was achieved by flash chromatography on a silica gel column using hexane/ethyl acetate (5
1) mixture of H2O/DMSO (3 mL) was allowed to react in a sealed tube at 80 °C. The reaction mixture was added to brine (15 mL) and extracted three times with diethyl ether (3 × 15 mL). The aqueous solution was separated from the organic layer. The diethyl ether solution was washed with water (3 × 15 mL), dried over MgSO4, and evaporated to dryness under reduced pressure to afford the desired product, which was then washed with hexane (3 × 5 mL). The further purification of the product was achieved by flash chromatography on a silica gel column using hexane/ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Acknowledgements
M.A. thanks the Research Council of University of Maragheh for financial support of this work and M.B. thanks the Research Council of Sharif University of Technology for funding of this work.References
- (a) D. Dallinger and C. O. Kappe, Chem. Rev., 2007, 107, 2563 CrossRef CAS; (b) K. G. Thakur, D. Ganapathy and G. Sekar, Chem. Commun., 2011, 47, 5076 RSC; (c) R. A. Sheldon, Green Chem., 2005, 7, 267 RSC.
- (a) J. Tsuji, Palladium Reagents and Catalysts, John Wiley & Sons, Chichester, 1995 Search PubMed; (b) F. Diederich and P. J. Stang, Metal-Catalyzed Cross-Coupling Reactions, Wiley-VCH, Weinheim, 1998 Search PubMed; (c) M. T. Reetz, Transition Metal Catalysed Reactions (ed.: S. G. Davies, S.-I. Murahashi), Blackwell Science, Oxford, 1999p 303 Search PubMed; (d) L. S. Søbjerg, D. Gauthier, A. T. Lindhardt, M. Bunge, K. Finster, R. L. Meyer and T. Skrydstrup, Green Chem., 2009, 11, 2041 RSC; (e) E. M. Beccalli, E. Borsini, S. Brenna, S. Galli, M. Rigamonti and G. Broggini, Chem.–Eur. J., 2010, 16, 1670 CrossRef CAS; (f) V. Farina, Adv. Synth. Catal., 2004, 346, 1553 CrossRef CAS; (g) W. Cabri and I. Candiani, Acc. Chem. Res., 1995, 28, 2 CrossRef CAS; (h) E. M. Beccalli, G. Broggini, M. Martinelli, N. Masciocchi and S. Sottocornola, Org. Lett., 2006, 8, 4521 CrossRef CAS; (i) B. Clique, C. Fabritius, C. Couturier, N. Monteiro and G. Balme, Chem. Commun., 2003, 272 RSC; (j) S. Yahiaoui, A. Fardost, A. Trejos and M. Larhed, J. Org. Chem., 2011, 76, 2433 CrossRef CAS.
- (a) M. Bagherzadeh, M. Amini, A. Ellern and L. K. Woo, Inorg. Chim. Acta, 2012, 383, 46 CrossRef CAS; (b) H. Yang, X. Han, G. Li and Y. Wang, Green Chem., 2009, 11, 1184 RSC; (c) X. Q. Zhang, Y. P. Qiu, B. Rao and M. M Luo, Organometallics, 2009, 28, 3093 CrossRef CAS; (d) B. P. Morgan, G. A. Galdamez, R. J. Gilliard, Jr. and R. C. Smith, Dalton Trans., 2009, 2020 RSC; (e) Y. B. Zhou, Z. X. Xi, W. Z. Chen and D. Q. Wang, Organometallics, 2008, 27, 5911 CrossRef CAS; (f) J. Broggi, H. Clavier and S. P. Nolan, Organometallics, 2008, 27, 5525 CrossRef CAS; (g) O. Diebolt, P. Braunstein, S. P. Nolan and C. S. J. Cazin, Chem. Commun., 2008, 3190 RSC.
- (a) Y. Peng, J. Chen, J. Ding, M. Liu, W. Gao and H. Wu, Synthesis, 2011, 213 Search PubMed; (b) A. Del Zotto, F. Amoroso, W. Baratta and P. Rigo, Eur. J. Org. Chem., 2009, 110 CAS; (c) B. P. Carrow and J.. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 79 CrossRef CAS; (d) G. Palmisano, W. Bonrath, L. Boffa, D. Garella, A. Barge and G. Cravotto, Adv. Synth. Catal., 2007, 349, 2338 CrossRef CAS.
- (a) T. Jeffery, Tetrahedron, 1996, 52, 10113 CrossRef CAS; (b) T. Jeffery and M. David, Tetrahedron Lett., 1998, 39, 5751 CrossRef CAS; (c) A. H. M. de Vries, J. M. C. A. Mulders, J. H. M. Mommers, H. J. W. Henderickx and J. G. de Vries, Org. Lett., 2003, 5, 3285 CrossRef CAS; (d) C. Liu, Q. Ni, P. Hu and J. Qiu, Org. Biomol. Chem., 2011, 9, 1054 RSC; (e) X. Du, M. Suguro, K. Hirabayashi and A. Mori, Org. Lett., 2001, 3, 3313 CrossRef CAS; (f) M. T. Reetz and E. Westermann, Angew. Chem., Int. Ed., 2000, 39, 165 CrossRef CAS; (g) A. H. M. de Vries, F. J. Parlevliet, L. S. de Vondervoort, J. H. M. Mommers, H. J. W. Henderickx, M. A. M. Walet and J. G. de Vries, Adv. Synth. Catal., 2002, 344, 996 CrossRef CAS.
- J. A. Davies and F. R. Hartley, Chem. Rev., 1981, 81, 79 CrossRef CAS.
- (a) B. A. Steinhoff, S. R. Fix and S. S. Stahl, J. Am. Chem. Soc., 2002, 124, 766 CrossRef CAS; (b) C. Zhou and R. C. Larock, J. Am. Chem. Soc., 2004, 126, 2302 CrossRef CAS; (c) R. A. T. M. van Benthem, H. Hiemstra, P. W. N. M. van Leeuwen, J. W Geus and W. N. Speckamp, Angew. Chem., Int. Ed. Engl., 1995, 34, 457 CrossRef CAS.
- R. Redon, S. K. Rendón-Lara, A. L. Fernández-Osorio and V. M. Ugalde-Saldivar, Rev. Adv. Mater. Sci., 2011, 27, 31 CAS.
- (a) Y. C. Jung, R. K. Mishra, C. H. Yoon and K. W. Jung, Org. Lett., 2003, 5, 2231 CrossRef CAS; (b) J. P. Parrish, Y. C. Jung, S. I. Shin and K. W. Jung, J. Org. Chem., 2002, 67, 7127 CrossRef CAS; (c) J. P. Parrish, Y. C. Jung, R. J. Floyd and K. W. Jung, Tetrahedron Lett., 2002, 43, 7899 CrossRef CAS.
- (a) S. S. Stahl, J. L. Thorman, N. de Silva, I. A. Guzei and R. W. Clark, J. Am. Chem. Soc., 2003, 125, 12 CrossRef CAS; (b) G. Broggini, E. M. Beccalli, E. Borsini, A. Fasana and G. Zecchi, Synlett, 2011, 227 CrossRef CAS; (c) K. Yip and D. Yang, Org. Lett., 2011, 13, 2134 CrossRef CAS; (d) J. W. Wrigglesworth, B. Cox, G. C. Lloyd-Jones and K. I. Booker-Milburn, Org. Lett., 2011, 13, 5326 CrossRef CAS.
- (a) J. Athilakshmi, S. Ramanathan and D. K. Chand, Tetrahedron Lett., 2008, 49, 5286 CrossRef CAS; (b) J. Athilakshmi and D. K. Chand, J. Chem. Sci., 2011, 123, 875 CrossRef CAS.
- (a) Sh. S. Stahl, Angew. Chem., Int. Ed., 2004, 43, 3400 CrossRef CAS; (b) J. M. Keith and W. A. Goddard, III, Organometallics, 2012, 31, 545 CrossRef CAS; (c) S. S. Stahl, J. L. Thorman, R. C. Nelson and M. A. Kozee, J. Am. Chem. Soc., 2001, 123, 7188 CrossRef CAS; (d) A. N. Campbell and S. S. Stahl, Acc. Chem. Res., 2012, 45, 851 CrossRef CAS.
- A. V. Gaikwad and G. Rothenberg, Phys. Chem. Chem. Phys., 2006, 8, 3669 RSC.
- A. V. Gaikwad, A. Holuigue, M. B. Thathagar, J. E. ten Elshof and G. Rothenberg, Chem.–Eur. J., 2007, 13, 6908 CrossRef CAS.
- (a) N. Karousis, G. E. Tsotsou, F. Evangelista, P. Rudolf, N. Ragoussis and N. Tagmatarchis, J. Phys. Chem. C, 2008, 112, 13463 CrossRef CAS; (b) W. Han, Ch. Liu and Z. Jin, Adv. Synth. Catal., 2008, 350, 501 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of coupling products. See DOI: 10.1039/c2ra21459h |
| This journal is © The Royal Society of Chemistry 2012 |