The recognition of 1,5-naphthalenedisulfonate by a protonated azamacrocyclic ligand†‡
Gao-Yi
Xie
,
Long
Jiang
and
Tong-Bu
Lu
*
MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, State Key Laboratory of Optoelectronic Materials and Technologies, School of Chemistry and Chemical Engineering, Sun Yat-Sen University, Guangzhou 510275, China. E-mail: lutongbu@mail.sysu.edu.cn; Fax: +86-20-84112921
First published on 8th November 2012
Abstract
A protonated azamacrocyclic chemosensor containing two anthracene fragments can recognize 1,5-naphthalenedisulfonate (1,5-NDS) over halides and other oxoanions, 1H NMR spectra and crystal structure analysis give further insight into the binding interactions.
Anion recognition and sensing continue to attract much attention due to their wide applications in chemistry,1 biology,2 medicine,3 and environment science.4 In general, to encapsulate an anion, the receptor should provide a cavity which contains functional groups capable of interacting with the guest, and the selectivity depends both on the energy terms (related to the intensity of the receptor–substrate interaction) and on geometrical factors (size and shape matching between the receptor and substrate).1e Custelcean and Moyer5 also suggested that the selectivity would be affected by the organizational rigidity of the receptor, including both the rigidity and structural uniqueness. In the past few decades, many polyaza ligands (including cryptands) have been designed and synthesized for the recognition and sensing of anions.6 Among them, fluorescent chemosensors draw particular attention due to the simple instrumentation required and usually low detection limit reached.7
In the present paper, we focused our attention on the polyaza macrocyclic ligand L1 (Scheme 1), which was previously shown as a fluorescent molecular switch driven by the addition sequence of metal cations (Zn2+ and Cd2+).8 We noted that L1 contains two polyamine binding units and two fluorescent anthracene fragments. Upon protonation, L1 can recognize anions through both energy terms (electrostatic, hydrogen-bonding and anion–π/π–π interactions) and geometrical factors (size matching effect). So the protonated L1 should be a good receptor and fluorescent chemosensor for anions which contain aromatic groups. Indeed, the 1,5-naphthalenedisulfonate (1,5-NDS) anion, which is a pollutant in industrial dying wastewater,9 was found to be recognized selectively by the protonated L1. In acidic conditions, the fluorescence of the protonated L1 is quenched significantly upon the addition of 50 equiv of 1,5-NDS, and crystal structure analysis provided further insight into the binding interactions between the host and guest. For comparison, a similar azamacrocyclic ligand L2 was also synthesized, and its recognizing property for 1,5-NDS has also been investigated.
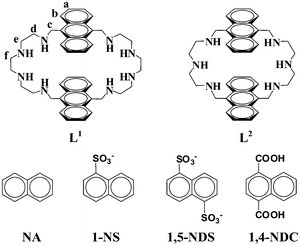 | ||
| Scheme 1 The structures of the receptors and substrates. | ||
Protonated receptors H8L1Cl8 and H6L2Cl6 (Scheme 1) were synthesized according to literature methods.8,10 Both receptors display strong fluorescence at low pH values, as the electron transfer process is prohibited by the protonation of amines, as observed in previous studies.8,11 Fluorescent spectra of [H8L1]8+ with various anions in aqueous solution (Britton–Robinson buffer, pH 1.81) were investigated. As shown in Fig. 1, the fluorescent intensities of [H8L1]8+ do not change even when 50 equiv of F−, Cl−, Br−, HCO3−, NO3−, ClO4− were added, and only slightly decrease in the presence of 50 equiv of I−, HSO4−, and C2O42−. However, it is very interesting to note that the fluorescence of [H8L1]8+ was significantly quenched when 50 equiv of 1,5-NDS was added, indicating [H8L1]8+ can recognize 1,5-NDS over other anions. The Job’s plot of [H8L1]8+ with 1,5-NDS in 1H NMR measurements displays a maximum chemical shift of Hf in [H8L1]8+ at [H8L1]/{[H8L1] + [1,5-NDS]} = 0.5 ([H8L1] is the concentration of [H8L1]8+), indicating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion compound was formed (see Fig. S1, ESI†).12 In addition, the fluorescent titration of [H8L1]8+ with 1,5-NDS (Fig. 2) gave an association constant (Ka) of 4.0 ± 0.3 × 104 M−1 (R2 = 0.9963).13 We postulate that the fluorescence quenching effect by 1,5-NDS is caused by π–π stacking interactions between the two anthracene fragments of [H8L1]8+ and the naphthalene fragment of 1,5-NDS, through an energy transfer process,14 and this was confirmed by the results of the UV–vis titration of [H8L1]8+ with 1,5-NDS. As shown in Fig. S2, ESI,† the absorption bands at 356, 375 and 395 nm for [H8L1]8+ are red shifted upon addition of 1,5-NDS, and the red shift is attributed to the π–π stacking interactions between the two anthracene fragments of [H8L1]8+ and the naphthalene fragment of 1,5-NDS.14b To reveal the binding mechanism, fluorescent spectra of [H8L1]8+ with naphthalene (NA), 1-naphthalenesulfonate (1-NS), and 1,4-naphthalenedicarboxylic acid (1,4-NDC) were also investigated (see Fig. S3–S5, ESI†). However, the fluorescent intensities of [H8L1]8+ do not change or slightly decrease upon adding NA, 1-NS and 1,4-NDC, indicating NA, 1-NS and 1,4-NDC can not be encapsulated into the cavity of [H8L1]8+. This implies that electrostatic and hydrogen-bonding interactions between [H8L1]8+ and 1,5-NDS play a pivotal role during the association.
1 inclusion compound was formed (see Fig. S1, ESI†).12 In addition, the fluorescent titration of [H8L1]8+ with 1,5-NDS (Fig. 2) gave an association constant (Ka) of 4.0 ± 0.3 × 104 M−1 (R2 = 0.9963).13 We postulate that the fluorescence quenching effect by 1,5-NDS is caused by π–π stacking interactions between the two anthracene fragments of [H8L1]8+ and the naphthalene fragment of 1,5-NDS, through an energy transfer process,14 and this was confirmed by the results of the UV–vis titration of [H8L1]8+ with 1,5-NDS. As shown in Fig. S2, ESI,† the absorption bands at 356, 375 and 395 nm for [H8L1]8+ are red shifted upon addition of 1,5-NDS, and the red shift is attributed to the π–π stacking interactions between the two anthracene fragments of [H8L1]8+ and the naphthalene fragment of 1,5-NDS.14b To reveal the binding mechanism, fluorescent spectra of [H8L1]8+ with naphthalene (NA), 1-naphthalenesulfonate (1-NS), and 1,4-naphthalenedicarboxylic acid (1,4-NDC) were also investigated (see Fig. S3–S5, ESI†). However, the fluorescent intensities of [H8L1]8+ do not change or slightly decrease upon adding NA, 1-NS and 1,4-NDC, indicating NA, 1-NS and 1,4-NDC can not be encapsulated into the cavity of [H8L1]8+. This implies that electrostatic and hydrogen-bonding interactions between [H8L1]8+ and 1,5-NDS play a pivotal role during the association.
![Fluorescent spectra of [H8L1]8+ (5 μM) upon additions of 50 equiv of different anions at pH 1.81 (Britton–Robinson buffer) (excitation at 368 nm).](/image/article/2012/RA/c2ra22423b/c2ra22423b-f1.gif) | ||
| Fig. 1 Fluorescent spectra of [H8L1]8+ (5 μM) upon additions of 50 equiv of different anions at pH 1.81 (Britton–Robinson buffer) (excitation at 368 nm). | ||
![Fluorescent titration of [H8L1]8+ (5 μM) upon additions of 1,5-NDS at pH 1.81 (Britton–Robinson buffer) (excitation at 368 nm). Inset: curve-fitting analysis of the fluorescence emission change at 425 nm.](/image/article/2012/RA/c2ra22423b/c2ra22423b-f2.gif) | ||
| Fig. 2 Fluorescent titration of [H8L1]8+ (5 μM) upon additions of 1,5-NDS at pH 1.81 (Britton–Robinson buffer) (excitation at 368 nm). Inset: curve-fitting analysis of the fluorescence emission change at 425 nm. | ||
To confirm that 1,5-NDS was encapsulated by [H8L1]8+, 1H NMR experiments were carried out in DMSO-d6–D2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH 1.81). As shown in Fig. 3, the addition of 2 equiv of 1,5-NDS resulted in significant upfield shifts of the anthracene protons Ha and Hb, demonstrating the presence of π–π stacking interactions between the two anthracene fragments of [H8L1]8+ and the naphthalene fragment of 1,5-NDS. On the other hand, the signals of the methylene protons (Hd, He, Hf) in [H8L1]8+ moved downfield, implying strong interactions between the protonated polyamine binding units in [H8L1]8+ and the sulfonate groups of 1,5-NDS.15 In contrast to 1,5-NDS, the addition of NA or 1-NS caused no change or a very small change (Δδ < 0.05 ppm) in the chemical shifts of the protons in [H8L1]8+ (Fig. 3), implying neither NA nor 1-NS was encapsulated by [H8L1]8+. The Ka value of [H8L1]8+ with 1,5-NDS was calculated by monitoring the chemical shifts of the proton Hf in [H8L1]8+ with 1
1 v/v, pH 1.81). As shown in Fig. 3, the addition of 2 equiv of 1,5-NDS resulted in significant upfield shifts of the anthracene protons Ha and Hb, demonstrating the presence of π–π stacking interactions between the two anthracene fragments of [H8L1]8+ and the naphthalene fragment of 1,5-NDS. On the other hand, the signals of the methylene protons (Hd, He, Hf) in [H8L1]8+ moved downfield, implying strong interactions between the protonated polyamine binding units in [H8L1]8+ and the sulfonate groups of 1,5-NDS.15 In contrast to 1,5-NDS, the addition of NA or 1-NS caused no change or a very small change (Δδ < 0.05 ppm) in the chemical shifts of the protons in [H8L1]8+ (Fig. 3), implying neither NA nor 1-NS was encapsulated by [H8L1]8+. The Ka value of [H8L1]8+ with 1,5-NDS was calculated by monitoring the chemical shifts of the proton Hf in [H8L1]8+ with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry, giving the association constant (Ka) 1.6 ± 0.3 × 104 M−1 (Fig. S6 and S7, ESI†).16 The different values of the binding constants (Ka) obtained by the two different methods are attributed to the different measuring solvents.17
1 binding stoichiometry, giving the association constant (Ka) 1.6 ± 0.3 × 104 M−1 (Fig. S6 and S7, ESI†).16 The different values of the binding constants (Ka) obtained by the two different methods are attributed to the different measuring solvents.17
![Partial 1H NMR spectra for (a) [H8L1]8+ (2 mM), (b) [H8L1]8+ + NA (2 equiv), (c) [H8L1]8+ + 1-NS (2 equiv), (d) [H8L1]8+ + 1,5-NDS (2 equiv) in DMSO-d6–D2O (1 : 1 v/v, pH 1.81, adjusted by DCl).](/image/article/2012/RA/c2ra22423b/c2ra22423b-f3.gif) | ||
Fig. 3 Partial 1H NMR spectra for (a) [H8L1]8+ (2 mM), (b) [H8L1]8+ + NA (2 equiv), (c) [H8L1]8+ + 1-NS (2 equiv), (d) [H8L1]8+ + 1,5-NDS (2 equiv) in DMSO-d6–D2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH 1.81, adjusted by DCl). 1 v/v, pH 1.81, adjusted by DCl). | ||
To further understand the encapsulating nature of [H8L1]8+ towards 1,5-NDS, the crystal structure of [(H8L1)(1,5-NDS)][1,5-NDS]2[HPO4](H2O)16 (1·16H2O) was investigated. Single crystals of 1·16H2O were obtained by diffusing an aqueous solution of H8L1Cl8 with a methanol solution of 1,5-NDS. As shown in Fig. 4 and S8, ESI,† one divalent 1,5-NDS anion is encapsulated into the cavity of [H8L1]8+, forming a sandwich like structure. The naphthalene ring of the guest and the two anthracene rings of the host are subparallel, with dihedral angles of 7.863° and 10.504°, respectively. The shortest C⋯C contacts between the naphthalene ring of 1,5-NDS and the two anthracene rings of [H8L1]8+ are 3.331(7) Å (C(30)⋯C(46)) and 3.454(8) Å (C(23)⋯C(49)), respectively, demonstrating there are π–π stacking interactions between the host and the guest.18 In addition, the encapsulated 1,5-NDS anion forms four hydrogen bonds with four protonated nitrogen atoms of [H8L1]8+, with O⋯N hydrogen bonding distances of 2.721(5)–2.885(5) Å (Table S1†). The other two 1,5-NDS and one HPO42− counter anions locate outside the cavity of [H8L1]8+ for charge balance (see Fig. S9, ESI†). No complex crystals could be obtained when 1,5-NDS was replaced by NA or 1-NS under the same conditions, due to the weaker interactions between the receptor and the two substrates.
![The structure of the [(H8L1)(1,5-NDS)]6+ cation in 1.](/image/article/2012/RA/c2ra22423b/c2ra22423b-f4.gif) | ||
| Fig. 4 The structure of the [(H8L1)(1,5-NDS)]6+ cation in 1. | ||
To see if the similar [H6L2]6+ receptor can also encapsulate 1,5-NDS or not, the fluorescent titration of [H6L2]6+ with 1,5-NDS was performed (see Fig. S10, ESI†), which showed less fluorescence decrease in comparison with the fluorescent titration of [H8L1]8+ with 1,5-NDS, indicating 1,5-NDS may not be encapsulated into the cavity of [H6L2]6+, and this was confirmed by the result of the crystal structure analysis. The crystal structure of [H6L2][1,5-NDS]3(H2O)6 (2·6H2O) demonstrates that [H6L2]6+ does not encapsulate 1,5-NDS due to the size mismatch between the host and guest. As shown in Fig. 5, 1,5-NDS anions locate outside the cavity of [H6L2]6+, and interact with [H6L2]6+ through intermolecular hydrogen bonds. Two oxygen atoms O(8) and O(9) in one 1,5-NDS anion form two hydrogen bonds with two hydrogen atoms of protonated N(2) and N(2A), resulting in the N(2)⋯N(2A) distance (5.22 Å) being much shorter than the distance between two anthracene rings (10.13 Å). In addition, each [H6L2]6+ forms twelve hydrogen bonds with six 1,5-NDS, and each 1,5-NDS forms four hydrogen bonds with two [H6L2]6+, with O⋯N hydrogen bonding distances of 2.679(4)–3.003(4) Å (see Fig. S11 and Table S1, ESI†).
![The structure of the [(H6L2)(1,5-NDS)2]2+ cation in 2.](/image/article/2012/RA/c2ra22423b/c2ra22423b-f5.gif) | ||
| Fig. 5 The structure of the [(H6L2)(1,5-NDS)2]2+ cation in 2. | ||
In summary, we have shown here the highly selective recognition of 1,5-NDS over halide anions and other oxoanions by [H8L1]8+ in water. The 1,5-NDS anion is encapsulated in the cavity of [H8L1]8+, causing the fluorescence quenching of [H8L1]8+ through π–π stacking interactions. The high affinity of [H8L1]8+ toward 1,5-NDS is attributed to electrostatic, hydrogen-bonding, and π–π stacking cooperative interactions, as well as the size matching effect between the host and guest.
Acknowledgements
This work was supported by the 973 Program of China (2012CB821705) and NSFC (Grant Nos. 20831005, 21121061 and 91127002).References
- (a) M. Wenzel, J. R. Hiscock and P. A. Gale, Chem. Soc. Rev., 2012, 41, 480 RSC; (b) I. Ravikumar and P. Ghosh, Chem. Soc. Rev., 2012, 41, 3077 RSC; (c) E. A. Katayev, G. D. Pantos, M. D. Reshetova, V. N. Khrustalev, V. M. Lynch, Y. A. Ustynyuk and J. L. Sessler, Angew. Chem., Int. Ed., 2005, 44, 7386 CrossRef CAS; (d) M. Bru, I. Alfonso, M. I. Burguete and S. V. Luis, Angew. Chem., Int. Ed., 2006, 45, 6155 CrossRef CAS; (e) V. Amendola, M. Boiocchi, L. Fabbrizzi and A. Palchetti, Chem.–Eur. J., 2005, 11, 5648 CrossRef CAS.
- (a) I. Ravikumar and P. Ghosh, Inorg. Chem., 2011, 50, 4229 CrossRef CAS; (b) A. Ojida, I. Takashima, T. Kohira, H. Nonaka and I. Hamachi, J. Am. Chem. Soc., 2008, 130, 12095 CrossRef CAS; (c) H. Wu, C. He, Z. Lin, Y. Liu and C. Duan, Inorg. Chem., 2008, 48, 408 CrossRef; (d) D. Wang, X. Zhang, C. He and C. Duan, Org. Biomol. Chem., 2010, 8, 2923 RSC.
- (a) X. Li, Y.-D. Wu and D. Yang, Acc. Chem. Res., 2008, 41, 1428 CrossRef CAS; (b) K. Gloe, H. Stephan and M. Grotjahn, Chem. Eng. Technol., 2003, 26, 1107 CrossRef CAS.
- (a) Y. Kim and F. P. Gabbai, J. Am. Chem. Soc., 2009, 131, 3363 CrossRef CAS; (b) L. Z. Yang, Y. Li, L. Jiang, X. L. Feng and T. B. Lu, CrystEngComm, 2009, 11, 2375 RSC; (c) Z.-H. Lin, Y.-G. Zhao, C.-Y. Duan, B.-G. Zhang and Z.-P. Bai, Dalton Trans., 2006, 3678 RSC; (d) Z.-H. Lin, S.-J. Ou, C.-Y. Duan, B.-G. Zhang and Z.-P. Bai, Chem. Commun., 2006, 624 RSC.
- R. Custelcean and B. A. Moyer, Eur. J. Inorg. Chem., 2007, 1321 CrossRef CAS.
- (a) S. O. Kang, J. M. Llinares, V. W. Day and K. Bowman-James, Chem. Soc. Rev., 2010, 39, 3980 RSC; (b) C. Bazzicalupi, S. Biagini, A. Bencini, E. Faggi, C. Giorgi, I. Matera and B. Valtancoli, Chem. Commun., 2006, 4087 RSC; (c) V. Martí-Centelles, M. I. Burguete, F. Galindo, M. A. Izquierdo, D. K. Kumar, A. J. P. White, S. V. Luis and R. Vilar, J. Org. Chem., 2012, 77, 490 CrossRef; (d) Z. Xu, N. J. Singh, J. Lim, J. Pan, H. N. Kim, S. Park, K. S. Kim and J. Yoon, J. Am. Chem. Soc., 2009, 131, 15528 CrossRef CAS; (e) H. J. Kim, S. Bhuniya, R. K. Mahajan, R. Puri, H. Liu, K. C. Ko, J. Y. Lee and J. S. Kim, Chem. Commun., 2009, 7128 RSC; (f) L. Rodriguez, J. C. Lima, A. J. Parola, F. Pina, R. Meitz, R. Aucejo, E. Garcia-Espana, J. M. Llinares, C. Soriano and J. Alarcon, Inorg. Chem., 2008, 47, 6173 CrossRef CAS.
- M. E. Moragues, R. Martinez-Manez and F. Sancenon, Chem. Soc. Rev., 2011, 40, 2593 RSC.
- G. Nishimura, H. Maehara, Y. Shiraishi and T. Hirai, Chem.–Eur. J., 2008, 14, 259 CrossRef CAS.
- E. Szabó-Bárdos, Z. Zailák, G. Lendvay, O. Horváth, O. Markovics, A. Hoffer and N. Törő, J. Phys. Chem. B, 2008, 112, 14500 CrossRef.
- L. Fabbrizzi, M. Licchelli, N. Marcotte, F. Stomeo and A. Taglietti, Supramol. Chem., 2002, 14, 127 CrossRef CAS.
- H. G. Hao, X. D. Zheng and T. B. Lu, Angew. Chem., Int. Ed., 2010, 49, 8148 CrossRef CAS.
- G. W. Bates, P. A. Gale and M. E. Light, Chem. Commun., 2007, 2121 RSC.
- Y. Shiraishi, S. Sumiya and T. Hirai, Chem. Commun., 2011, 47, 4953 RSC.
- (a) J. J. Gassensmith, E. Arunkumar, L. Barr, J. M. Baumes, K. M. DiVittorio, J. R. Johnson, B. C. Noll and B. D. Smith, J. Am. Chem. Soc., 2007, 129, 15054 CrossRef CAS; (b) P. P. Neelakandan, M. Hariharan and D. Ramaiah, J. Am. Chem. Soc., 2006, 128, 11334 CrossRef CAS.
- A. Petitjean, R. G. Khoury, N. Kyritsakas and J. M. Lehn, J. Am. Chem. Soc., 2004, 126, 6637 CrossRef CAS.
- A. Sygula, F. R. Fronczek, R. Sygula, P. W. Rabideau and M. M. Olmstead, J. Am. Chem. Soc., 2007, 129, 3842 CrossRef CAS.
- Y. Shiraishi, M. Itoh and T. Hirai, Tetrahedron, 2011, 67, 891 CrossRef CAS.
- H. M. Colquhoun, Z. Zhu and D. J. Williams, Org. Lett., 2003, 5, 4353 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available: experimental methods, NMR spectra and fluorescence data, calculation details, supplementary figures and crystallographic data in CIF (PDF). See DOI: 10.1039/c2ra22423b |
‡ Crystal data for 1·16H2O: C74H115N8O38PS6, M = 1948.07, triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 12.9514(4), b = 14.2719(5), c = 25.9721(9) Å, α = 87.967(3)°, β = 86.878(3)°, γ = 67.322(3)°, V = 4422.4(3) Å3, Z = 2, μ = 2.413 mm−1, Dc = 1.463 Mg m−3, F(000) = 2060. 14678 unique (Rint = 0.0367), R1 = 0.0641, wR2 = 0.1802 [I > 2σ(I)], GOF = 1.032. For 2·6H2O: C70H82N6O24S6, M = 1583.78, monoclinic, P21/c, a = 13.3839(7), b = 22.0863(13), c = 11.7496(5) Å, β = 95.793(4)°, V = 3455.5(3) Å3, Z = 2, μ = 2.576 mm−1, Dc = 1.522 Mg m−3, F(000) = 1664. 5792 unique (Rint = 0.0493), R1 = 0.0616, wR2 = 0.1648 [I > 2σ(I)], GOF = 1.036. CCDC 874373(1) and 874374(2). , a = 12.9514(4), b = 14.2719(5), c = 25.9721(9) Å, α = 87.967(3)°, β = 86.878(3)°, γ = 67.322(3)°, V = 4422.4(3) Å3, Z = 2, μ = 2.413 mm−1, Dc = 1.463 Mg m−3, F(000) = 2060. 14678 unique (Rint = 0.0367), R1 = 0.0641, wR2 = 0.1802 [I > 2σ(I)], GOF = 1.032. For 2·6H2O: C70H82N6O24S6, M = 1583.78, monoclinic, P21/c, a = 13.3839(7), b = 22.0863(13), c = 11.7496(5) Å, β = 95.793(4)°, V = 3455.5(3) Å3, Z = 2, μ = 2.576 mm−1, Dc = 1.522 Mg m−3, F(000) = 1664. 5792 unique (Rint = 0.0493), R1 = 0.0616, wR2 = 0.1648 [I > 2σ(I)], GOF = 1.036. CCDC 874373(1) and 874374(2). |
| This journal is © The Royal Society of Chemistry 2012 |
