Recognition of poly(dimethylsiloxane) with phage displayed peptides†
Swathi
Swaminathan
and
Yue
Cui
*
Utah State University, Department of Biological Engineering, Logan, UT 84322. E-mail: yue.cui@usu.edu; Fax: +1-435-797-1248; Tel: +1-435-797-9276
First published on 30th October 2012
Abstract
We demonstrate for the first time a powerful yet benign approach for identification of binding motifs to poly(dimethylsiloxane) (PDMS) via comprehensively screened phage displayed peptides. Our results show that PDMS can be selectively recognized with peptide-displaying phages and bifunctional peptides. Further, along with the development of PDMS-based microstructures, recognition of PDMS with phage displayed peptides can be specifically localized in these microstructures.
Poly(dimethylsiloxane) (PDMS) is a silicon-based elastomer, which has been widely used for a variety of in vitro and in vivo applications, including microfluidic devices,1 micro-nanostructures,2 surface and interface,3 construction of hybrid materials4 and cell biology.5 PDMS has shown excellent thermal stability, optical, electrical, mechanical, and biocompatible properties.5–7 A variety of surface functionalization and recognition of PDMS has been studied for its biological,8 chemical,8 mechanical9 and electrical applications.10 To date, the developed methods are based on nonspecific functionalization, including polymer and copolymer coatings11 or covalent binding with silanes.12 These modification strategies may be limited in the scope of their applicability. A general method for chemical and biological functionalization of PDMS with specific binding motifs is thus highly desired.
Oligopeptides are robust biorecognition molecules, displaying broad chemical diversity (acidity, hydrophobicity, etc.), and can be chemically engineered to bind specific targets.13 Further, peptides can form complex, self-assembled hybrid conjugates with a variety of materials or assemble specific materials on patterned or microstructured surfaces, with their ability to be linked into multifunctional networks.14–16 Phage display has emerged as a powerful method for identifying peptide motifs with enhanced binding affinities toward specific targets. In phage display, a library of approximately a billion (109) peptide variants is displayed on the phage as a fusion with the surface coat protein of the bacteriophage, which allows for rapid, combinatorial screening of sequences displaying high affinities toward specific targets. Phage display has been investigated to identify specific binding peptides for a wide range of target analytes, including metals,17,18 semiconductors,19–21 polymers22,23 and small molecules.24 Recently, we have also identified phage displayed peptides for binding to graphene16 and small molecule ink.25
In this communication, we demonstrate for the first time the identification of a specific peptide binding motif for PDMS via phage display technology. A dominant peptide can be obtained by screening from the phage display peptide library. The screened peptide is shown to recognize and bind to PDMS surface. These advances allow for the development of designer bifunctional peptides for the binding of other materials (such as streptavidin) to PDMS. Further, we show the recognition of PDMS microdevices and microstructures with peptide-displaying phages and bifunctional peptides.
The PDMS prepolymer and its curing agent (Sylgard 184 silicone elastomer kit, Dow Corning, Midland, MI) were thoroughly mixed in a ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, followed by degassing in a vacuum for 30 min. The mixture was then poured over a petri dish and microstructured masters, and cured at 80 °C for 2 h, followed by peeling off to obtain a flat PDMS surface, PDMS microdots and PDMS microchannels. The PDMS slabs were then used for incubation with phages or treated by plasma oxidation. The masters for the PDMS microdots are TEM grids (Electron Microscopy Sciences, Hatfield, PA; Ted Pella Inc, Redding, CA). The masters for the PDMS microchannels were prepared according to a reported procedure,26 and briefly, the channel structures were printed on shrink-dink plastics by a laser printer, and after curing and drilling holes, the PDMS microchannels with an inlet and an outlet were sealed to a glass slide. The solutions were delivered into the microfluidic channels with a syringe pump (New Era Pump systems, Inc., Farmingdale, NY) through Teflon tubing. Plasma oxidation (15 W, 30 s) was performed with a plasma etcher (Plasma Etch, Carson city, Nv) for changing the PDMS surface from being hydrophobic to hydrophilic, and for bonding the PDMS slab with microchannels to a glass substrate.
1, followed by degassing in a vacuum for 30 min. The mixture was then poured over a petri dish and microstructured masters, and cured at 80 °C for 2 h, followed by peeling off to obtain a flat PDMS surface, PDMS microdots and PDMS microchannels. The PDMS slabs were then used for incubation with phages or treated by plasma oxidation. The masters for the PDMS microdots are TEM grids (Electron Microscopy Sciences, Hatfield, PA; Ted Pella Inc, Redding, CA). The masters for the PDMS microchannels were prepared according to a reported procedure,26 and briefly, the channel structures were printed on shrink-dink plastics by a laser printer, and after curing and drilling holes, the PDMS microchannels with an inlet and an outlet were sealed to a glass slide. The solutions were delivered into the microfluidic channels with a syringe pump (New Era Pump systems, Inc., Farmingdale, NY) through Teflon tubing. Plasma oxidation (15 W, 30 s) was performed with a plasma etcher (Plasma Etch, Carson city, Nv) for changing the PDMS surface from being hydrophobic to hydrophilic, and for bonding the PDMS slab with microchannels to a glass substrate.
An aliquot of phage display library (New England BioLabs, pIII system, Ph.D. 7, Beverly, MA) was incubated with a PDMS slab (non-oxidized) in Tris buffered saline containing 0.1–0.5% Tween-20 (TBST) for 1 h at room temperature. The PDMS slab was then washed several times with TBST buffer. The phages were eluted from the PDMS by addition of glycine-HCl (pH 2.2) for 15 min, neutralized with Tris-HCl, pH 9.1, amplified, and subjected to additional pannings. Eluted phages were then amplified in E. coli, and the panning was repeated for up to three rounds. This was conducted under increasingly stringent conditions with Tween-20 concentrations increased from 0.1% to 0.5%, to obtain phage clones expressing peptides having the highest binding affinities to the PDMS samples. After the final round of panning, DNA sequence analysis of the isolated phage clones yielded heptameric PDMS-binding peptides. As shown in Fig. 1a, after performing biocombinatorial screening from a phage display library, specific peptides binding to PDMS can be identified. Fig. 1b shows the characteristics of the peptide sequences screened from three rounds of biopanning, and LSNNNLR appears to show the highest frequency from the biopanning process with a frequency of 19/20. A comparative analysis of the observed frequency of the amino acids binding to PDMS and the recorded frequency in the NEB library has also been recorded in Fig. 1c. A plot analysis of these peptide sequences shows that asparagine and leucine are frequently the binding amino acid residues.
 | ||
| Fig. 1 Identification of phage displayed peptides binding to PDMS. (a) Schematic illustration of the generalized screening protocol for identifying phage displayed peptides which recognize PDMS. (b) Summary of consensus PDMS binding peptides. (c) Amino acid frequencies in the PDMS-binding peptide sequences, as compared to the observed frequencies in the phage library from New England Biolabs (NEB). | ||
The dominant phage displayed peptide (LSNNNLR) was also isolated as a single colony and selected for further investigation. First, the fluorescent characterization for the binding of LSNNNLR to a flat PDMS surface was investigated, as shown in Fig. 2a. This was accomplished by incubating the substrates sequentially with (1) the phage displayed peptides or M13 phages with the same concentrations, (2) blocking buffer 0.1 M NaHCO3, 1% BSA, (3) biotin conjugated anti-M13 antibody (1 mg ml−1), and (4) avidin-FITC (1 mg ml−1), with TBS buffer washing steps in between to remove non-specific binding. Significantly, the PDMS surface with peptide-displaying phages (LSNNNLR) shows much higher fluorescent intensity relative to the PDMS incubated with M13 phages (without phage displayed peptides on the coat; isolated from the phage display library). The absence of phage displayed peptide on the coat of M13 phage can be correlated with the absence of fluorescence on the surface of PDMS. Thus, the phage displayed peptide is essential for binding to PDMS. In addition, by using a plasma-oxidized PDMS slab (15 w, 30 s) which shows hydrophilic surface behavior, the fluorescent characterization exhibits similar behavior as the non-oxidized hydrophobic PDMS (see supplementary information Fig. S1, ESI†). Similarly, the low frequency peptide (LQPRANF) can also bind to both non-oxidized and slightly oxidized PDMS slabs (see supplementary information Fig. S2, ESI†). The results suggest that the phage displayed peptides can bind to PDMS with different surface behaviours. Recent studies showed that the PDMS surface did not suffer from considerable chemical modifications with a mild plasma oxidation, and the chemical groups on the PDMS surface remained largely unaltered.27–31 Thus, the PDMS-binding phage displayed peptides can bind to both non-oxidized PDMS and slightly oxidized PDMS. While for PDMS that has suffered from a strong plasma oxidation, the chemical groups on the PDMS surface were changed significantly, and the fluorescent characterization showed that the phage displayed peptides did not bind to the strongly oxidized PDMS (see supplementary information Fig. S3, ESI†).
 | ||
| Fig. 2 Recognition of PDMS surface with phage displayed peptides. (a) Fluorescent characterization of the bindings of peptide-displaying phages (left) and M13 phages (right) to PDMS. (b) Water contact angle measurement for PDMS and PDMS bound with phages. (c) Fluorescent characterization of the binding of bifunctional peptide (left) and no peptide (right) to PDMS. Insets of (a) and (c): schematics of bindings of peptide-displaying phages and bifunctional peptides. Green is PDMS. Streptavidin is blue. All scale bars: 50 μm. | ||
The water contact angles of PDMS before and after incubation with peptide-displaying phages were also investigated using a goniometer (Sindatek Model 100 SB, Sindatek Instruments Co, Ltd, Taipei city, Taiwan). As shown in Fig. 2b, the water contact angle for a plain PDMS (without plasma oxidation) was found to be hydrophobic with a contact angle of 100°, while that of the PDMS with the peptide-displaying phages showed a hydrophilic contact angle of 17°. The results suggest that the change in the surface behavior of PDMS is due to the strong binding of phage displayed peptides to PDMS.
We explored a synthetic peptide for binding to the PDMS. A synthetic bifunctional peptide (LSNNNLRGGGGHPQ) (Peptide 2.0, Chantilly, VA) was designed and synthesized, with a PDMS-binding motif LSNNNLR, a linker GGGG, and a streptavidin binding motif HPQ.32 Here, we use streptavidin-FITC as the analyte, which is expected to be bound to the PDMS surface via the HPQ binding motif. The fluorescent characterization of the binding of bifunctional peptides was accomplished by incubating the substrates sequentially with (1) the bifunctional peptide (50 μg ml−1 TBS), (2) blocking buffer 0.1 M NaHCO3, 1% BSA, and (3) streptavidin-FITC (1 mg ml−1), with TBS buffer washing steps in between to remove non-specific binding. The control experiment for the PDMS slab without the bifunctional peptide was accomplished by incubating the substrates sequentially to (1) blocking buffer 0.1 M NaHCO3, 1% BSA, and (2) streptavidin-FITC (1 mg ml−1), with TBS buffer washing steps in between. The fluorescent intensity due to FITC, is a measure of the bifunctional peptide bound to PDMS. As shown in Fig. 2c left image, the PDMS slab incubated with the bifunctional peptides shows much higher fluorescent intensity compared to the PDMS slab without the bifunctional peptide (Fig. 2c right image), indicating the strong binding of the bifunctional peptide to both PDMS and streptavidin-FITC. In this work, we used HPQ to perform the initial study. Further, via design of bifunctional peptides for PDMS and other materials, a variety of materials can be explored for binding to the PDMS surface to form hybrid materials, such as Au nanoparticles on PDMS, carbon nanotubes on PDMS, etc.
Further, we investigated the localized binding of phage displayed peptide (LSNNNLR) to microstructured PDMS, including that of peptide-displaying phages and bifunctional peptides to PDMS microfluidic channels and micropatterns, as shown in Fig. 3. Fig. 3a shows the binding of the peptide-displaying phages and the bifunctional peptides to PDMS microfluidic channels. The microfluidic channels exhibit strong fluorescent signals with the binding of both peptide-displaying phages (Fig. 3a middle) and bifunctional peptides (Fig. 3a right). Next, we investigated the binding of phage displayed peptides to PDMS micropatterned stamp, as shown in Fig. 3b. The fluorescent characterization shows that the peptide-displaying phages (Fig. 3b middle image) and bifunctional peptides (Fig. 3b right image) bind to the micropatterned PDMS effectively, and both of them show strong patterned fluorescent signals. Blank experiments with M13 phages binding to microfluidic channels and micropatterns were investigated for fluorescent characterization, and no fluorescent signal was observed (see supplementary information Fig. S4, ESI†). This result clearly indicates that microstructures, including microfluidic channels and micropatterns, can map the binding of phage displayed peptides to PDMS.
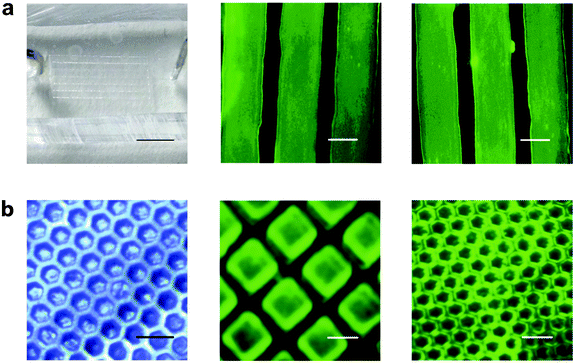 | ||
| Fig. 3 Recognition of PDMS microstructures with phage displayed peptides. (a) Microfluidic channel based binding. Left: camera image of microfluidic channel. Scale bar: 1.0 cm. Middle: Fluorescent characterization of peptide-displaying phage binding to microfluidic channels. Scale bar: 50 μm. Right: Fluorescent characterization of bifunctional peptide binding to microfluidic channels. Scale bar: 50 μm. (b) Micropatterns based binding. Left: camera image of the PDMS micropatterns (replicated from a 200 mesh TEM grid). Scale bar: 150 μm. Middle: Fluorescent characterization of peptide-displaying phages binding to PDMS micropatterns (replicated from a 400 mesh TEM grid). Scale bar: 50 μm. Right: Bifunctional peptides binding to PDMS micropatterns (replicated from a 600 mesh TEM grid). Scale bar: 50 μm. | ||
In summary, we have demonstrated for the first time the recognition of PDMS with specific peptides identified from a combinatorial phage display library. The approach we describe here may open new avenues for a variety of PDMS-based fundamental studies and practical applications, including biological analytical devices, self-assembly of PDMS-based hybrid materials, surface and interface, cell biology, etc. Although these results are promising, further studies are needed to help elucidate the mechanism of the peptides in recognizing PDMS, and the properties of the peptide functionalized PDMS.
Acknowledgements
The authors acknowledge Utah State University and Utah Water Research Laboratory for the financial support.References
- W. F. Zheng, Z. Wang, W. Zhang and X. Y. Jiang, Lab Chip, 2010, 10, 2906–2910 RSC.
- C. Y. Xue, W. Zhang, W. H. S. Choo and K. L. Yang, Langmuir, 2011, 27, 13410–13414 CrossRef CAS.
- M. H. Wu, Surf. Interface Anal., 2009, 41, 11–16 CrossRef CAS.
- A. Misra, J. R. Raney, L. De Nardo, A. E. Craig and C. Daraio, ACS Nano, 2011, 5, 7713–7721 CrossRef CAS.
- C. H. Hsieh, C. J. C. Huang and Y. Y. Huang, Biomed. Microdevices, 2010, 12, 897–905 CrossRef CAS.
- M. Liu, J. R. Sun, Y. Sun, C. Bock and Q. F. Chen, J. Micromech. Microeng., 2009, 19 Search PubMed.
- J. Lee and J. Kim, J. Micromech. Microeng., 2011, 21 CAS.
- R. Wang, Y. L. Yang, M. Qin, L. K. Wang, L. Yu, B. Shao, M. Q. Qiao, C. Wang and X. Z. Feng, Chem. Mater., 2007, 19, 3227–3231 CrossRef CAS.
- K. L. Mills, X. Y. Zhu, S. C. Takayama and M. D. Thouless, J. Mater. Res., 2008, 23, 37–48 CrossRef CAS.
- X. Z. Niu, S. L. Peng, L. Y. Liu, W. J. Wen and P. Sheng, Adv. Mater., 2007, 19, 2682–2686 CrossRef CAS.
- J. Fang, A. Kelarakis, D. Y. Wang, E. P. Giannelis, J. A. Finlay, M. E. Callow and J. A. Callow, Polymer, 2010, 51, 2636–2642 CrossRef CAS.
- V. Sharma, M. Dhayal, Govind, S. M. Shivaprasad and S. C. Jain, Vacuum, 2007, 81, 1094–1100 CrossRef CAS.
- L. Stryer, Biochemistry, W. H. Freeman & Company, New York, 1995 Search PubMed.
- S. N. Kim, Z. F. Kuang, J. M. Slocik, S. E. Jones, Y. Cui, B. L. Farmer, M. C. McAlpine and R. R. Naik, J. Am. Chem. Soc., 2011, 133, 14480–14483 CrossRef CAS.
- M. J. Pender, L. A. Sowards, J. D. Hartgerink, M. O. Stone and R. R. Naik, Nano Lett., 2006, 6, 40–44 CrossRef CAS.
- Y. Cui, S. N. Kim, S. E. Jones, L. L. Wissler, R. R. Naik and M. C. McAlpine, Nano Lett., 2010, 10, 4559–4565 CrossRef CAS.
- R. R. Naik, S. J. Stringer, G. Agarwal, S. E. Jones and M. O. Stone, Nat. Mater., 2002, 1, 169–172 CrossRef CAS.
- R. J. Zuo, D. Ornek and T. K. Wood, Appl. Microbiol. Biotechnol., 2005, 68, 505–509 CrossRef CAS.
- C. B. Mao, D. J. Solis, B. D. Reiss, S. T. Kottmann, R. Y. Sweeney, A. Hayhurst, G. Georgiou, B. Iverson and A. M. Belcher, Science, 2004, 303, 213–217 CrossRef CAS.
- E. Estephan, M. B. Saab, C. Larroque, M. Martin, F. Olsson, S. Lourdudoss and C. Gergely, J. Colloid Interface Sci., 2009, 337, 358–363 CrossRef CAS.
- E. Estephan, M. B. Saab, M. Martin, C. Larroque, F. J. G. Cuisinier, O. Briot, S. Ruffenach, M. Moret and C. Gergely, J. Pept. Sci., 2011, 17, 143–147 CrossRef CAS.
- A. B. Sanghvi, K. P. H. Miller, A. M. Belcher and C. E. Schmidt, Nat. Mater., 2005, 4, 496–502 CrossRef CAS.
- M. Vodnik, B. Strukelj and M. Lunder, Anal. Biochem., 2012, 424, 83–86 CrossRef CAS.
- F. Sugawara, J. Pestic. Sci., 2004, 29, 279–283 Search PubMed.
- Y. Cui, A. Pattabiraman, B. Lisko, S. C. Collins and M. C. McAlpine, J. Am. Chem. Soc., 2010, 132, 1204–1205 CrossRef CAS.
- A. Grimes, D. N. Breslauer, M. Long, J. Pegan, L. P. Lee and M. Khine, Lab Chip, 2008, 8, 170–172 RSC.
- K. Efimenko, W. E. Wallace and J. Genzer, J. Colloid Interface Sci., 2002, 254, 306–315 CrossRef CAS.
- J. R. Hollahan and G. L. Carlson, J. Appl. Polym. Sci., 1970, 14, 2499–2508 CrossRef CAS.
- M. Morra, E. Occhiello, R. Marola, F. Garbassi, P. Humphrey and D. Johnson, J. Colloid Interface Sci., 1990, 137, 11–24 CrossRef CAS.
- M. J. Owen and P. J. Smith, J. Adhes. Sci. Technol., 1994, 8, 1063–1075 CrossRef CAS.
- E. A. Waddell, S. Shreeves, H. Carrell, C. Perry, B. A. Reid and J. McKee, Appl. Surf. Sci., 2008, 254, 5314–5318 CrossRef CAS.
- D. S. Wilson, A. D. Keefe and J. W. Szostak, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 3750–3755 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra22137c |
| This journal is © The Royal Society of Chemistry 2012 |
