A novel poly[(N-vinylimidazole)-co-(1-pyrenylmethyl methacrylate)] ferric complex with fluorescence and superparamagnetism†
Jian
Cui
,
Shaobei
Yang
,
Jinfang
Zhang
,
Shuai
Zhao
and
Yehai
Yan
*
Key Laboratory of Rubber-Plastics, Ministry of Education/Shandong Provincial Key Laboratory of Rubber-Plastics, College of Polymer Science and Engineering, Qingdao University of Science and Technology, Qingdao 266042, China. E-mail: yhyan@qust.edu.cn; Fax: +86-532-84023977; Tel: +86-532-84022416
First published on 9th October 2012
Abstract
Due to its unique physical and chemical properties, the polymer N-vinylimidazole (VI), either as a homopolymer or copolymer, has a large number of important applications. Currently, technological advances have identified further uses for it and there are practical demands for multiply functional forms of the polymer; e.g., fluorescence and magnetism in one VI-polymer material. Toward this end, a novel poly[(N-vinylimidazole)-co-(1-pyrenylmethyl methacrylate)] (VI-co-PyMMA) ferric complex prepared by complexing a VI-co-PyMMA copolymer with 1-chlorobutane and FeCl3 in succession is presented in this work. The complexed FeCl3, which predominately exists as the FeCl4− anion, endows the resultant material with superparamagnetism; while the presence of pyrene moieties allows the complex to exhibit additional fluorescence. Both the fluorescence and magnetism exhibit a strong dependence on the content of the complexed FeCl3 but in opposite manners. The preferred complex, S4, shows a balance of the properties, with a fluorescence quantum yield of 0.13 and a magnetic susceptibility of 16.1 × 10−6 emu g−1. Along with re-dispersibility, this single-component fluorescent–magnetic material may replace some corresponding multi-component nanomaterials, where the polymer component acts only as a protective coating and/or an organic fluorophore and the magnetic performance is usually provided by magnetic nanoparticles (MNPs). It may find potential applications in the booming biotechnology field.
Introduction
Imidazole and its derivatives occupy an important place in the field of modern chemistry, including polymer chemistry.1,2 A simple exemplification is that both the homopolymer and copolymer of N-vinylimidazole (VI) have persistently evoked great interest over the past century. The driving force of this interest is mainly due to its unique physical and chemical properties, which endow these VI-polymers with a wide scope of applications, ranging from binders to biomaterials. For instance, poly[(N-isopropylacrylamide)-co-(N-vinylimidazole)], a so-called protein-like copolymer, was found to possess selective affinity and has been successfully used in a bioseparation process for the coprecipitation of a soybean trypsin inhibitor.3 In another typical example, its high proton conductivity of 10−1 S cm−1 at 30 °C has enabled poly[(diisopropyl-p-vinylbenzyl phosphonate)-co-(N-vinylimidazole)] to be used in proton exchange membranes in fuel cells.4 Recently, Iván and coworkers reported an amphiphilic co-network of poly(N-vinylimidazole)-l-poly(tetrahydrofuran) (“l” stands for “linked by”), which behaves as either a hydrogel or a hydrophobic gel depending on the medium nature, with promising applications ranging from the medical fields to novel nanomaterials.5,6 These representative examples clearly demonstrate that the VI-polymer is a versatile material capable of conducting specific function and executing increasingly complex task; whereas copolymerization of the VI monomer with a certain co-monomer is a convenient way to acquire such a material. In other words, it is still an interesting open research topic, at least currently, to develop VI-polymer materials with novel structures and desirable properties.Pyrene is a commonly used fluorescent compound and has an excellent quantum yield of 0.65 in ethanol at 293 K with a long fluorescent lifetime of 410 ns.7 Through incorporation into a polymer chain8–10 and even onto the surface of fullerenes11 and carbon nanotubes,12 the pyrene moiety has equipped various target materials with a strong blue fluorescence. This strongly implies that copolymerization of VI with a vinyl monomer bearing a pyrene moiety, such as 1-pyrenylmethyl methacrylate (PyMMA), may produce a new fluorescent VI-polymer. Such accessible fluorescence, together with the biocompatibility of the imidazole moiety, which is present in many important biomolecules, such as histidine and histamine and has already been incorporated in large numbers of synthetic drugs,2 may allow this new VI-polymer to develop additional potential applications, e.g., as a component in biosensors and chemical sensors.13,14 An application of rather high possibility is to utilize the copolymer as a carrier agent for protein separation, as has been realized with other VI-polymers3,15 and characterization of the separation process can be monitored in an online manner by a spectrofluorometer or a fluorescence microscope.
On the other hand, imidazoles are also well-known for their very strong complexing ability, which has been widely used to prepare ionic liquids (ILs).16 An impressive work has been reported related to 1-butyl-3-methylimidazolium tetrachloroferrate(III) ([bmim]FeCl4), a compound reported as early as 2001.17 A recent re-examination discovered its distinct magnetic properties18 and has accordingly inspired the new research area of magnetic ILs. The efficient complexing ability was also noticed in VI-polymers. Actually, by reaction of VI-polymer with different metallic ions, such as Zn(II),19,20 Os(IV),21,22 Co(II),23,24 Cu(II),25,26 Pd(II),27,28 and Ni(II),26 numerous polymer–metal complexes have been synthesized and utilized for gas separation, biochemical sensing, antifouling coating, catalysis, gene delivery, and so forth. Among them, VI-polymer ferric/ferrous complexes have been one of the earliest synthesized complexes.29 Inspired by [bmim]FeCl4, it is highly probable that this polymeric complex will exhibit magnetic responsive behavior. According to our best knowledge, however, this physical performance has not been explored to date. Once the magnetic properties are established, the applications of VI-polymers may be further extended to magnetically-assisted chemical/biomolecule sorting and separation, targeted delivery of drugs/biomolecules, etc.13 Therefore, evaluating the magnetic performance of VI-polymer ferric/ferrous complexes is also a necessary and meaningful work.
To address the above-mentioned subjects, we here present the design, synthesis and characterization of a novel poly[(N-vinylimidazole)-co-(1-pyrenemethyl methacrylate)] (VI-co-PyMMA) ferric complex. VI-co-PyMMA was first synthesized through a convenient free-radical copolymerization with azobisisobutyronitrile (AIBN) as an initiator. Subsequently, the ferric complex was prepared by reaction of VI-co-PyMMA with 1-chlorobutane (BuCl) and anhydrous FeCl3, sequentially, following a strategy that has been previously used for the synthesis of imidazole-based magnetic ILs.17,18,30,31 Property measurements revealed that the complex has the desired fluorescence and superparamagnetic properties. This work thus represents a first attempt to develop a single-component fluorescent–magnetic polymer material. Despite reports of a large number of multi-component nanomaterials,32–35 often, the polymer component acts only as a protective coating and/or an organic fluorophore and the magnetic-responsive behavior is usually provided by magnetic nanoparticles (MNPs). The fluorescent–magnetic ferric complex described here, which was synthesized by a simple method using economical starting chemicals, has potential to replace some fluorescent–magnetic nanomaterials for applications in the expanding biotechnology field.
Experimental section
Materials
PyMMA was synthesized by reaction of 1-pyrenylmethanol (Aldrich) with methacryloyl chloride (Fluka) in dry tetrahydrofuran (THF) according to the previously reported method (Scheme 1).36 VI, BuCl and anhydrous FeCl3 were obtained from Aladdin and used without further purification. AIBN was recrystallized twice from methanol prior to use. All organic solvents used in this work were dried according to the standard procedures and stored over 5A molecular sieves before use. | ||
| Scheme 1 The synthesis of VI-co-PyMMA and its derivatives. | ||
Synthesis of VI-co-PyMMA
For the synthesis of VI-co-PyMMA (Scheme 1), VI (947 mg, 10 mmol), PyMMA (30 mg, 0.1 mmol), AIBN (20 mg, 2 wt% of the total monomer weight) and dimethylformamide (DMF, 10 ml) were added to a 150 ml two-neck flask and then heated to 70 °C under an N2 atmosphere. After the reaction was sustained for 20 h, the liquid solution was dropped into 40 ml ethyl acetate. The precipitated solid was washed with THF (3 × 20 ml) and dried in the vacuum oven at 60 °C for 12 h. The final product was a light yellow solid with a yield of 82%. Its viscosity-average molecular weight ( ) was measured to be 12.5 kDa by dilute solution viscometry using ethanol as a solvent. Elementary analysis (%): C 64.52 ± 1.02, H 6.34 ± 0.26 and N 28.77 ± 0.86.
) was measured to be 12.5 kDa by dilute solution viscometry using ethanol as a solvent. Elementary analysis (%): C 64.52 ± 1.02, H 6.34 ± 0.26 and N 28.77 ± 0.86.
Complexing VI-co-PyMMA with BuCl
981 mg (10.6 mmol) of BuCl was added dropwise into a solution of VI-co-PyMMA (500 mg, 5.3 mmol VI unit, which was determined based on the assumption that VI-co-PyMMA consists only of the VI unit) in 20 ml ethanol at room temperature under an N2 atmosphere. The reaction was subsequently performed for 24 h at 70 °C. After cooling to room temperature, the clear solution was poured into 80 ml of stirred ethyl acetate. The precipitate was separated by filtration through a 0.2 μm poly(tetrafluoroethylene) (PTFE) membrane, washed thoroughly with ethyl acetate and vacuum-dried at 60 °C for 12 h to yield 689 mg of the final orange product designated as S0 (Scheme 1). Elementary analysis (%): C 59.11 ± 1.14, H 7.78 ± 0.15, N 16.52 ± 0.91 and Cl 16.33 ± 0.37.Complexing S0 with FeCl3
Into a solution of S0 (50 mg) in ethanol (5 ml), the desired volume of FeCl3 in an ethanol solution (22 mg ml−1) was added dropwise at room temperature under an N2 atmosphere (Table 1). After being stirred for a further 30 min, the reaction mixture was heated to 70 °C and the reaction was allowed to continue for 6 h. Ethanol was evaporated under reduced pressure. The collected solid material was ground and repeatedly washed with ethyl ether (a good solvent for FeCl3) to remove free FeCl3. The final product, designated as SN, (Scheme 1) was obtained by vacuum-drying the material at 60 °C.| Sample No. | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| S0/mg | 50 | 50 | 50 | 50 | 50 | 50 |
| FeCl3/mg | 2.2 | 4.3 | 8.7 | 21.7 | 32.6 | 87.0 |
| Content of complexed FeCl3 (μg per milligram of S0) | 25 | 73 | 140 | 259 | 296 | 384 |
| [Fe3+] in the sample for the fluorescence tests/μg ml−1 | 0.04 | 0.13 | 0.24 | 0.45 | 0.51 | 0.66 |
Characterization
FTIR, 1H-NMR (500 MHz, DMSO-d6), ultraviolet–visible (UV–vis) and Raman spectra were recorded on a Bruker VERTEX 70 spectrometer, a Bruker AV 500 spectrometer, a TU-1800PC spectrometer and a Renishaw inVia micro-Raman spectrometer, respectively. Elemental analysis was carried out using an Elementar Vario el Cube analyzer. Each reported data point is an average of three parallel measurements. The content of complexed FeCl3 in SN was determined by the inductively coupled plasma-optical emission spectrometric (ICP-OES) technique using a Prodigy XP ICP-OES instrument. Fluorescence spectra were collected on a HITACHI F-4600 spectrophotometer. The magnetic properties were measured using a Quantum Design MPMS XL-5 SQUID magnetometer. Powder X-ray diffraction (XRD) data for S4 were collected using a Rigaku D-MAX2500-PC (Japan) X-ray diffractometer with Cu-Kα radiation (λ = 0.1541 nm) at 200 mA and 40 kV. Transmission electron microscopy (TEM) images were recorded on a JEOL JEM-2100 with an accelerating voltage of 200 kV. The sample was obtained by placing a drop of the S4 solution in a mixed solvent of acetonitrile and water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) onto a carbon-covered copper grid and dried in air at room temperature.
1, v/v) onto a carbon-covered copper grid and dried in air at room temperature.
Results and discussion
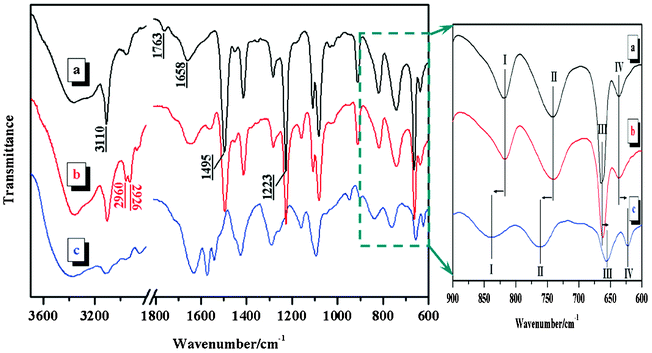
VI-co-PyMMA and its complexes were synthesized according to the procedures summarized in Scheme 1. The structures were characterized using multiple spectroscopic techniques. As indicated by Fig. 1a, the moieties of the imidazole ring (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H, 3110 cm−1; C
C–H, 3110 cm−1; C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, 1658 cm−1; C–N, 1495 cm−1) and the ester (C
N, 1658 cm−1; C–N, 1495 cm−1) and the ester (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 1763 cm−1; C–O–C, 1223 cm−1) were found to be included in the VI-co-PyMMA sample. In the NMR spectrum, a multiplet at 7.84–8.40 ppm (Fig. 2) indicates the presence of a pyrene moiety in the sample. Further analysis of the 1H-NMR spectrum showed that the integration ratio of the imidazole protons (6.55–7.70 ppm) to the pyrene protons (7.84–8.40 ppm) was around 29.3, suggesting that the molar ratio of the VI-unit to the PyMMA-unit in VI-co-PyMMA is 87.9. This value is consistent with 90.3, which was determined via elemental analysis. Furthermore, under UV-light irradiation at 365 nm, the clear colorless VI-co-PyMMA solution (5 μg ml−1) in ethanol emitted bright blue fluorescence. Upon excitation at a maximum absorption wavelength of 347 nm (vide infra), the same solution displayed characteristic fluorescence of pyrene with distinct peaks at 376, 397 and 415 nm (Fig. S1, ESI†). These observations clearly demonstrate that a fluorescent VI copolymer has been successfully synthesized.
O, 1763 cm−1; C–O–C, 1223 cm−1) were found to be included in the VI-co-PyMMA sample. In the NMR spectrum, a multiplet at 7.84–8.40 ppm (Fig. 2) indicates the presence of a pyrene moiety in the sample. Further analysis of the 1H-NMR spectrum showed that the integration ratio of the imidazole protons (6.55–7.70 ppm) to the pyrene protons (7.84–8.40 ppm) was around 29.3, suggesting that the molar ratio of the VI-unit to the PyMMA-unit in VI-co-PyMMA is 87.9. This value is consistent with 90.3, which was determined via elemental analysis. Furthermore, under UV-light irradiation at 365 nm, the clear colorless VI-co-PyMMA solution (5 μg ml−1) in ethanol emitted bright blue fluorescence. Upon excitation at a maximum absorption wavelength of 347 nm (vide infra), the same solution displayed characteristic fluorescence of pyrene with distinct peaks at 376, 397 and 415 nm (Fig. S1, ESI†). These observations clearly demonstrate that a fluorescent VI copolymer has been successfully synthesized.
 | ||
| Fig. 1 FTIR spectra of a) VI-co-PyMMA, b) S0, and c) S4. | ||
After reaction of VI-co-PyMMA (a light yellow solid) with BuCl (a colorless liquid), an orange solid product S0 was obtained. By taking the 3110 cm−1 peak as a reference, the intensity of the C–H stretching peaks of S0 (2960 cm−1 for –CH3 and 2926 cm−1 for –CH2, Fig. 1b) was noted to be much stronger than that of VI-co-PyMMA (Fig. 1a), indicating that butyl moieties have been incorporated into S0. The 1H-NMR spectral features (Fig. S2, ESI†) provide additional evidence to support the successful synthesis of S0. From the elementary analysis data, it is suggested that 78% of the imidazole moieties in S0 have reacted with BuCl.
Final ferric complexes SN (N = 1–6), with color ranging from orange to dark brown, were prepared by reacting S0 with various amount of anhydrous FeCl3. Differing significantly from VI-co-PyMMA and S0, which are both soluble in ethanol, the ferric complexes were completely insoluble in ethanol but dissolved well in water, DMF and acetonitrile. Fig. 1c shows the FTIR spectrum of a typical ferric complex, S4. As compared with that of VI-co-PyMMA (Fig. 1a) and S0 (Fig. 1b), this spectrum obviously shows different spectral features. The shape of the absorption peaks becomes less sharp and more broad and apeak shift takes place for nearly all absorption bands. Typically, as shown in the fingerprint spectral region of 600–900 cm−1, bands I and II shift to their respective higher wavenumbers in the S4 spectrum; meanwhile, bands III and IV shift to lower wavenumbers. Since free FeCl3 has been removed by thorough washing of the ground product with ethyl ether (a good solvent for FeCl3), the spectral differences observed between S4 and VI-co-PyMMA or S0 may hence be attributed to the introduction of complexed FeCl3.
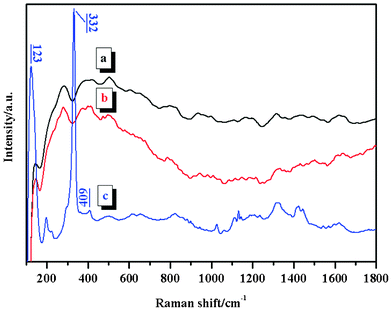
To confirm our assertion that ferric complexes have been succesfully synthesized, UV–vis and Raman spectroscopy were used to make a more definitive characterization. Fig. 3 shows the UV–vis spectra of VI-co-PyMMA, S0 and S4 thin films cast from the diluted ethanol (for the first two samples) or acetonitrile (for the third) solutions on glass substrates. The characteristic pyrene absorption peaks at 332 and 347 nm demonstrate, again, that the pyrene moieties are involved in the copolymer and its derivatives. In the visible wavelength region (Fig. 3, inset), however, only the S4 sample showed absorption peaks at 528, 619 and 684 nm. These peaks are absent from FeCl3 and have already been assigned to the characteristic absorption of FeCl4− species.18,37 Such an assignment was strengthened by Raman analysis. By using an excitation laser wavelength of 785 nm, resonance Raman spectra were collected and are displayed in Fig. 4. The strong bands at 123 and 332 cm−1 in the spectrum of S4, which have also been observed in ILs containing FeCl4−,17,18,31 correspond to the totally symmetric Fe–Cl stretch vibration of discrete FeCl4− anions, whereas the weak band at 409 cm−1 is due to small amounts of Fe2Cl7−.17 Combining the UV–vis, Raman and FTIR analyses, it is reasonable to conclude that ferric complexes have been successfully synthesized through the S0 intermediate and the introduced FeCl3 exists predominantly as FeCl4−. The definite contents of complexed FeCl3 in SN (N = 1–6) were determined by an ICP-OES and the results are summarized in Table 1.
 | ||
| Fig. 3 UV–vis spectra of a) VI-co-PyMMA, b) S0 and c) S4. | ||
 | ||
| Fig. 4 Raman spectra of a) VI-co-PyMMA, b) S0 and c) S4. | ||
As shown in Fig. 5a, the blue fluorescence of VI-co-PyMMA was evident in S0 and SN. Identical to that of VI-co-PyMMA, three characteristic emission peaks of pyrene at 376, 397 and 415 nm were also present in the fluorescence spectra of S0 and SN. From these spectra, it is interesting to note that the fluorescence intensities of SN decrease with an increasing content of complexed FeCl3. Since all the tested samples have a constant concentration of 5 μg ml−1 of S0 or S0-counterparts (for SN) in the same mixed solvent of acetonitrile and water (acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water = 9
water = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume), the gradually attenuated fluorescence of SN relative to that of S0 can not be ascribed to collision quenching (self-quenching), which normally occurs in solid pyrene-containing samples or concentrated solutions.38 This is likely to be due to energy transfer between the pyrene moieties and the FeCl4− anions, as has been suggested for a similar quenching effect observed in other materials consisting of organic fluorophores and inorganic metal nanoparticals or cations.39–43 Moreover, the excitation light absorbed by complexed FeCl3 (indicated by curve c in Fig. 3) may also play a certain role in the decrease of the fluorescence intensity.
1 by volume), the gradually attenuated fluorescence of SN relative to that of S0 can not be ascribed to collision quenching (self-quenching), which normally occurs in solid pyrene-containing samples or concentrated solutions.38 This is likely to be due to energy transfer between the pyrene moieties and the FeCl4− anions, as has been suggested for a similar quenching effect observed in other materials consisting of organic fluorophores and inorganic metal nanoparticals or cations.39–43 Moreover, the excitation light absorbed by complexed FeCl3 (indicated by curve c in Fig. 3) may also play a certain role in the decrease of the fluorescence intensity.
![a) Fluorescence spectra of S0 and SN (N = 1–6) (the inset is a digital photo of the samples irradiated by 365 nm UV light) and b) dependence of the fluorescence intensity at 397 nm of SN (N = 1–6) on [Fe3+].](/image/article/2012/RA/c2ra22395c/c2ra22395c-f5.gif) | ||
| Fig. 5 a) Fluorescence spectra of S0 and SN (N = 1–6) (the inset is a digital photo of the samples irradiated by 365 nm UV light) and b) dependence of the fluorescence intensity at 397 nm of SN (N = 1–6) on [Fe3+]. | ||
To describe quantitatively the quenching effect, the fluorescence intensity at 397 nm was plotted against the content of complexed FeCl3. For the sake of convenience, the latter parameter was converted to [Fe3+] (Table 1). As shown in Fig. 5b, the fluorescence intensity was linearly dependent on [Fe3+] within the detected range of 0.04–0.66 μg ml−1. The linear fit was written as F = 1135 – 844 × [Fe3+] (μg ml−1) with a R2 of 0.998. Such linear dependence indicates that the VI-polymer/ferric complex can be controllably synthesized with desirable fluorescence intensity. On the other hand, it demonstrates that VI-co-PyMMA has potential for applications in the detection of ferric ions. The limit of detection (LOD) was estimated to be 18 ng ml−1 from the equation: LOD = 3SD/k, where SD is the standard deviation of the blank signals and k is the slope of the linear calibration curve.
The magnetic properties of the ferric complexes were measured using a SQUID magnetometer with fields up to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe. Fig. 6 shows the hysteresis loops of three typical samples of S2, S4 and S6 at 300 K (Fig. 6a) and S6 at 5 K (Fig. 6b). They all have negligible coercivity, indicating the superparamagnetic characteristics of these samples. Similar to [bmim]FeCl4,18 magnetization of S2, S4 and S6 also exhibits a perfect linear response under the applied fields at 300 K (Fig. 6a). From the slopes of the linear regression curves, the magnetic susceptibilities (χ) were determined to be 9.2 × 10−6, 16.1 × 10−6 and 19.1 × 10−6 emu g−1 for S2, S4 and S6, respectively. As indicated by both the XRD pattern (Fig. S3, ESI†) and the TEM images (Fig. S4, ESI†) of the typical ferric complex, S4, there is no evidence to support the existence of iron oxide nanoparticles within the complex, thus confirming that the magnetic properties observed here arise from intrinsic spin coupling between magnetic centers in the complex rather than extrinsic sources like iron oxide nanoparticles. Taking into account the non-magnetic contributions of the PyMMA-units and VI-units uncomplexed with FeCl3 in the ferric complexes, it is not surprising that their χ values are slightly less than that of [bmim]FeCl4 (40.6 × 10−6 emu g−1).18 Although the higher content of complexed FeCl3 induces a larger χ value, it is also a fact that complexed FeCl3 has a negative impact on the fluorescence intensity (Fig. 5). In general, a material with a quantum yield (φ) of more than 0.1 is considered to be quite fluorescent and to be practically valuable. Therefore, S4, which has a φ of 0.13, as determined by quinine sulfate as a standard (φ = 0.55), was picked out for further magnetic studies (Table S1, ESI†).
000 Oe. Fig. 6 shows the hysteresis loops of three typical samples of S2, S4 and S6 at 300 K (Fig. 6a) and S6 at 5 K (Fig. 6b). They all have negligible coercivity, indicating the superparamagnetic characteristics of these samples. Similar to [bmim]FeCl4,18 magnetization of S2, S4 and S6 also exhibits a perfect linear response under the applied fields at 300 K (Fig. 6a). From the slopes of the linear regression curves, the magnetic susceptibilities (χ) were determined to be 9.2 × 10−6, 16.1 × 10−6 and 19.1 × 10−6 emu g−1 for S2, S4 and S6, respectively. As indicated by both the XRD pattern (Fig. S3, ESI†) and the TEM images (Fig. S4, ESI†) of the typical ferric complex, S4, there is no evidence to support the existence of iron oxide nanoparticles within the complex, thus confirming that the magnetic properties observed here arise from intrinsic spin coupling between magnetic centers in the complex rather than extrinsic sources like iron oxide nanoparticles. Taking into account the non-magnetic contributions of the PyMMA-units and VI-units uncomplexed with FeCl3 in the ferric complexes, it is not surprising that their χ values are slightly less than that of [bmim]FeCl4 (40.6 × 10−6 emu g−1).18 Although the higher content of complexed FeCl3 induces a larger χ value, it is also a fact that complexed FeCl3 has a negative impact on the fluorescence intensity (Fig. 5). In general, a material with a quantum yield (φ) of more than 0.1 is considered to be quite fluorescent and to be practically valuable. Therefore, S4, which has a φ of 0.13, as determined by quinine sulfate as a standard (φ = 0.55), was picked out for further magnetic studies (Table S1, ESI†).
 | ||
| Fig. 6 Hysteresis loop analysis of a) S2, S4 and S6 at 300 K and b) S6 at 5 K. | ||
The temperature dependence of χ for S4 when it was subjected to an applied field of 100 Oe is shown in Fig. 7. A sudden large increase of χ at around 15 K suggested the occurrence of spontaneous magnetization. Over the whole detected temperature range of 2–300 K, the value of 1/χ increased linearly with temperature, showing a good agreement with the Curie–Weiss law with a Weiss constant of −36.7 K. The negative Weiss constant indicated the existence of an antiferromagnetic interaction, originating from the antiferromagnetic coupling of the FeCl4− anion with the butylimidazolium cation in S4. Macroscopically, S4 shows a strong response to an external magnetic field (Fig. 8). Within 1 min, S4, dispersed in ethyl ether, was attracted to the side of a container facing a 0.3 T magnet. By removing the magnet and gently shaking, the collective S4 readily re-dispersed. This re-dispersibility, together with the good response to a magnetic field (two beneficial properties for bioapplications),33 suggest that the ferric complex presented here may find some potential applications in the biotechnological field.
 | ||
| Fig. 7 χ–T and 1/χ–T curves of S4 under an applied magnetic field of 100 Oe. | ||
 | ||
| Fig. 8 Digital photo of S4 dispersed in ethyl ether with (right) and without (left) an external magnetic field of 0.3 T. | ||
Conclusions
A novel polymer–metal complex has been designed and successfully synthesized by reacting VI-co-PyMMA with BuCl and FeCl3 successively. Systematic structural characterization showed that the introduced FeCl3 exists predominantly as FeCl4−, endowing the resultant ferric complex with superparamagnetic characteristics. In addition, the presence of pyrene moieties ensures the ferric complex exhibits characteristic blue fluorescence. Quantitative studies reveal that the fluorescence intensity decreases with an increasing content of complexed FeCl3 over the detected range of 25–384 μg per milligram of S0, while the magnetic susceptibility shows the opposite dependence. From a practical application point of view, S4, with a magnetic susceptibility of 16.1 × 10−6 emu g−1, is preferred as it has a sufficiently high quantum yield of 0.13, it is easily dispersed and it is sensitive to a relatively weak magnetic field. To the best of our knowledge, this is the first report on a single-component fluorescent–magnetic polymer material. As has been well demonstrated using various multi-component fluorescent–magnetic nanomaterials, the ferric complex presented here may also find potential applications in the booming biotechnology field. Toward this goal, it is necessary to introduce a third block with a functional group, such as –OH, to facilitate covalent/noncovalent interactions with biomolecules. Such studies are currently under way in our laboratory.Acknowledgements
Financial support from the NSF of China (No. 51173091) and the Taishan Mountain Scholar Constructive Engineering Foundation are gratefully acknowledged.References
- K. Hofmann, The chemistry of heterocyclic compounds: Imidazole and its derivatives, Interscience Publishers, London, 1953 Search PubMed.
- E. G. Brown, Ring nitrogen and key biomolecules: The biochemistry of N-heterocycles, Kluwer Academic Press, Dordrecht, 1998, pp. 40–59 Search PubMed.
- P.-O. Wahlund, I. Yu. Galaev, S. A. Kazakov, V. I. Lozinsky and B. Mattiasson, Macromol. Biosci., 2002, 2, 33 CrossRef CAS.
- H. T. Pu, Y. J. Qin, D. C. Wan and Z. L. Yang, Macromolecules, 2009, 42, 3000 CrossRef CAS.
- Cs. Fodor, G. Kali and B. Iva′n, Macromolecules, 2011, 44, 4496 CrossRef CAS.
- Cs. Fodor and B. Iván, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 4729 CrossRef CAS.
- J. A. M. Rasmussen and A. Hermetter, Prog. Lipid Res., 2008, 47, 436 CrossRef CAS.
- M. Ertas, R. M. Walczak, R. K. Das, A. G. Rinzler and J. R. Reynolds, Chem. Mater., 2012, 24, 433 CrossRef CAS.
- Y. H. Yan, J. Cui, P. Pötschke and B. Voit, Carbon, 2010, 48, 2603 CrossRef CAS.
- T. Ogoshi, Y. Takashima, H. Yamaguchi and A. Harada, J. Am. Chem. Soc., 2007, 129, 4878 CrossRef CAS.
- D. M. Guldi, E. Menna, M. Maggini, M. Marcaccio, D. Paolucci, F. Paolucci, S. Campidelli, M. Prato, G. M. A. Rahman and S. Schergna, Chem.–Eur. J., 2006, 12, 3975 CrossRef CAS.
- R. B. Martin, L. Qu, Y. Lin, B. A. Harruff, C. E. Bunker, J. R. Gord, L. F. Allard and Y. P. Sun, J. Phys. Chem. B, 2004, 108, 11447 CrossRef CAS.
- P. R. Gil, L. L. del Mercato, P. del Pino, A. Muoñz Javier and W. J. Parak, Nano Today, 2008, 3, 12 CrossRef CAS.
- L. Basabe-Desmonts, D. N. Reinhoudt and M. Crego-Calama, Chem. Soc. Rev., 2007, 36, 993 RSC.
- R. Lemque, C. Vidal-Madjar, M. Racine, J. Piquion and B. Sebille, J. Chromatogr., A, 1991, 553, 165 CrossRef CAS.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123 RSC.
- M. S. Sitze, E. R. Schreiter, E. V. Patterson and R. G. Freeman, Inorg. Chem., 2001, 40, 2298 CrossRef CAS.
- S. Hayashi and H. Hamaguchi, Chem. Lett., 2004, 33, 1590 CrossRef CAS.
- K. Yao, Z. Wang, J. Wang and S. Wang, Chem. Commun., 2012, 48, 1766 RSC.
- S. Asayama, S. Nishinohara and H. Kawakami, Bioconjugate Chem., 2011, 22, 1864 CrossRef CAS.
- V. Coman, T. Gustavsson, A. Finkelsteinas, C. Von Wachenfeldt, C. Hägerhäll and L. Gorton, J. Am. Chem. Soc., 2009, 131, 16171 CrossRef CAS.
- J. W. Gallaway and S. A. Calabrese Barton, J. Am. Chem. Soc., 2008, 130, 8527 CrossRef CAS.
- T. Hyakutake, I. Okura, K. Asaic and H. Nishide, J. Mater. Chem., 2008, 18, 917 RSC.
- N. Pekel, B. Salih and O. Güven, J. Biomater. Sci., Polym. Ed., 2005, 16, 253 CrossRef CAS.
- Y. M. A. Yamada, S. M. Sarkar and Y. Uozumi, J. Am. Chem. Soc., 2012, 134, 9285 CrossRef CAS.
- B. Mattiasson, A. Kumar, A. E. Ivanov and I. Y. Galaev, Nat. Protoc., 2007, 2, 213 CrossRef CAS.
- Y. M. A. Yamada, S. M. Sarkar and Y. Uozumi, J. Am. Chem. Soc., 2012, 134, 3190 CrossRef CAS.
- I. P. Beletskaya, A. R. Khokhlov, E. A. Tarasenko and V. S. Tyurin, J. Organomet. Chem., 2007, 692, 4402 CrossRef CAS.
- E. Tsuchida and K. Honda, Polym. J., 1975, 7, 498 CrossRef CAS.
- B. Mallick, B. Balke, C. Felser and A.-V. Mudring, Angew. Chem., Int. Ed., 2008, 47, 7635 CrossRef CAS.
- R. E. Del Sesto, T. Mark McCleskey, A. K. Burrell, G. A. Baker, J. D. Thompson, B. L. Scott, J. S. Wilkes and P. Williams, Chem. Commun., 2008, 447 RSC.
- X. Lu, R. Jiang, Q. Fan, L. Zhang, H. Zhang, M. Yang, Y. Ma, L. Wang and W. Huang, J. Mater. Chem., 2012, 22, 6965 RSC.
- Q. Li, L. Zhang, L. Bai, Z. Zhang, J. Zhu, N. Zhou, Z. Cheng and X. Zhu, Soft Matter, 2011, 7, 6958 RSC.
- P. Howes, M. Green, A. Bowes, D. Parker, G. Varma, M. Kallumadil, M. Hughes, A. Warley, A. Brain and R. Botnar, J. Am. Chem. Soc., 2010, 132, 9833 CrossRef CAS.
- B. Sun, M.-J. Sun, Z. Gu, Q.-D. Shen, S.-J. Jiang, Y. Xu and Y. Wang, Macromolecules, 2010, 43, 10348 CrossRef CAS.
- Y. H. Yan, S. B. Yang, J. Cui, L. Jakisch, P. Pötschke and B. Voit, Polym. Int., 2011, 60, 1425 CrossRef CAS.
- H. L. Friedman, J. Am. Chem. Soc., 1952, 74, 5 CrossRef CAS.
- J. B. Birks, Photophysics of Aromatic Molecules, Wiley, New York, 1970, pp. 301 Search PubMed.
- Y.-H. Li, T. Song, J.-Q. Liu, S.-J. Zhu and J. Chang, J. Mater. Chem., 2011, 21, 12520 RSC.
- H. Qu, D. Caruntu, H. Liu and C. J. O'Connor, Langmuir, 2011, 27, 2271 CrossRef CAS.
- F. Erogbogbo, K.-T. Yong, R. Hu, W.-C. Law, H. Ding, C.-W. Chang, P. N. Prasad and M. T. Swihart, ACS Nano, 2010, 4, 5131 CrossRef CAS.
- E. J. Cho, S. Jung, K. Lee, H. J. Lee, K. C. Nam and H.-J. Bae, Chem. Commun., 2010, 46, 6557 RSC.
- S. Zhang, H.-Y. Zhu, Z.-B. Hu, L. Liu, S.-F. Chen and S.-H. Yu, Chem. Commun., 2009, 2326 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: fluorescence performance of VI-co-PyMMA; 1H-NMR spectrum of S0; XRD pattern and TEM images of S4; fluorescence quantum yields of S0 and SN (N = 1–6). |
| This journal is © The Royal Society of Chemistry 2012 |

