Heteropolyanion-based polymeric hybrids: highly efficient and recyclable catalysts for oxidation of alcohols with H2O2†
Yan
Leng
*a,
Jian
Liu
a,
Pingping
Jiang
a and
Jun
Wang
b
aThe Key Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, Wuxi, 214122, China. E-mail: lengyan1114@126.com; Fax: +86-510-85917763; Tel: +86-510-85917090
bState Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemistry and Chemical Engineering, Nanjing University of Technology, Nanjing, 210009, China
First published on 3rd October 2012
Abstract
New heteropolyanion-based polymeric hybrids prepared by the anion-exchange of newly task-specific designed ionic copolymers with Keggin heteropolyacids are revealed to be highly efficient, conveniently recoverable, and steadily reusable catalysts for the oxidation of alcohols with H2O2.
Polyoxomolybdates (POMs) are distinctive inorganic transition metal–oxygen clusters that are the source of fascinating architectures and very rich redox chemistry, upon which their catalytic activity in oxidation reactions is based.1 A key topic of interest in organic synthetic chemistry is the development of catalytic methods based on POMs for engendering catalyst recycling systems.2 Although various types of recyclable catalytic systems have been reported, including heterogeneous catalysis employing supported POM catalysts,3 biphasic systems using ionic liquids (ILs) as solvents,4 and phase transfer catalysis involving quaternary ammonium organic cations,5 however, it is difficult to retain or raise their activities relative to those of their homogeneous counterparts.
Recently, much attention has been focused on the modification of POMs with organic species to achieve stimuli-responsive (such as temperature-responsive, solvent-responsive, or reaction-responsive) POM-based hybrid catalysts with improved catalytic activity and convenient catalyst recovery and recycling.6 For example, Ikegami and coworkers7 reported a new temperature-responsive catalyst consisting of poly(N-isopropylacrylamide) polymer with a quaternary ammonium and phosphotungstate anion (PW12O403−) for the oxidation of alcohols, which caused the thermoregulated formation of stable emulsion species at 90 °C in water. Hou and coworkers8 reported that the exchange of the protons of the POM by alkylimidazoles can yield a liquid derivative of POM, and it can be used as a reaction-induced phase-separation catalyst for olefin epoxidation. Previously, we designed and synthesized a family of POM-based acidic hybrids by combining SO3H-functionalized ionic liquid (IL) cations with heteropolyanions, revealing that the resulting hybrids were highly efficient reaction-induced self-separation catalysts in esterifications.9 Clearly, the emergence of stimuli-responsive POM-based materials will endow POMs with huge diversity both in catalytic properties and with more space for organic synthesis.
The oxidation of alcohols with H2O2 has played an important role in organic synthesis, owing to their utility both in academic research and in industry. As a kind of POM, tungsten-containing Keggin-structured heteropolyacids (HPAs) have been revealed to be very effective homogeneous catalysts for this reaction.10 We have recently found HPA-based ionic hybrids prepared by combining amino-functionalized organic cations with heteropolyanions to be highly efficient solid catalysts for the oxidation of alcohols with H2O2.11 Nevertheless, catalytic selectivity and stability still need to be improved.
Considering that polymers with the features of stimuli-responsive behavior can influence the catalyst sterically and electronically and have a dramatic effect on the polarity of the microenvironment surrounding the active site,12 the introduction of catalysts or reagents on functionalized-polymer backbones seems more feasible for applications in recyclable catalysis.13 On the other hand, ionic copolymers with imidazolium IL units are capable of acting the bifunctions of both ILs and polymers.14 Accordingly, we herein report the synthesis of new types of HPA-based polymeric hybrids by coupling task-specific synthesized ionic copolymers and Keggin HPAs (Scheme 1). Characterization results of 1H NMR, TG, FT-IR, UV-vis and CHN elemental analysis (see ESI†), together with catalytic tests, demonstrate that these polymeric hybrids are stimuli-responsive and can be used as highly efficient catalysts for the oxidation of alcohols with H2O2. The catalysts could be easily recycled and steadily reused.
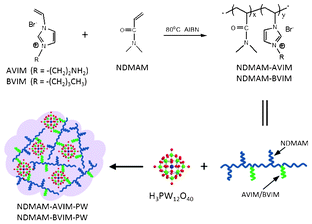 | ||
| Scheme 1 Synthesis of the HPA-based polymeric hybrids. | ||
The oxidation of benzyl alcohol by aqueous H2O2 under solvent-free conditions was first carried out as a model reaction. The reaction conditions and catalytic performances of the polymeric HPA hybrid catalysts and various control catalysts are listed in Table 1. Ionic polymer NDMAM-AVIM was inactive (Table 1, entry 1) in a homogeneous reaction system. Using amino-functionalized polymeric hybrid NDMAM-AVIM-PW as the catalyst, a high conversion of 93% with 99% selectivity was obtained (Table 1, entry 2). The reaction process shown in Fig. 1A revealed that the substrate benzyl alcohol containing catalyst NDMAM-AVIM-PW is solid–liquid biphasic (Fig. 1A (a)). Interestingly, a swelling behavior of the catalyst was clearly observed after the addition of H2O2 (Fig. 1A (b)). After reaction, the catalyst returned to its original solid state at the bottom of the reactor, and the colored product benzaldehyde released from the aqueous upper phase to the lower layer (Fig. 1A (c)), which allows easy separation of not only the solid catalyst but also the insoluble product. Noticeably, this procedure is probably induced and controlled by the reaction temperature and H2O2, because no catalyst swelling was observed when the oxidation was carried out at room temperature or without the addition of H2O2. Moreover, when the NDMAM unit in the polymeric cation of the hybrid catalyst was changed into N-vinyl pyrrolidone (NVPL), the resulting NVPL-AVIM-PW initially exhibited a traditional solid–liquid heterogeneous catalysis in the presence of H2O2. After a while, the catalyst mostly dissolved in the produced benzaldehyde, and the reaction was a liquid–liquid biphasic system, giving low conversion and selectivity (Table 1, entry 3) due to the lower accessibility of the H2O2 molecules to catalysts active sites.
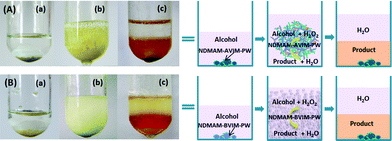 | ||
| Fig. 1 Photographs of the solvent-free oxidation of benzyl alcohol with H2O2 over (A) NAM-AVIM-PW and (B) NAM-BVIM-PW: (a) catalyst (light brown solid at bottom) and alcohol (liquid) before mixing; (b) during the reaction after adding H2O2; (c) at the end of the reaction. | ||
| Entry | Catalyst | Phenomenon | Conb(%) | Selc(%) |
|---|---|---|---|---|
| a Reaction conditions: catalyst (0.1 g), benzyl alcohol (10 mmol), 30% H2O2 (12 mmol), 90 °C, 2 h. b Conversion based on H2O2. c Selectivity for benzaldehyde (byproduct: benzoic acid). d Reaction time 4 h. e Reaction time 0.5 h. | ||||
| 1 | NDMAM-AVIM | homogeneous | — | — |
| 2 | NDMAM-AVIM-PW | swelling | 93/94/94/92 | 99 |
| 3 | NVPL-AVIM-PW | heterogeneous | 53 | 94 |
| 4 | NDMAM-BVIM-PW | emulsion | 75 | 100 |
| 5d | NDMAM-BVIM-PW | emulsion | 96/95/93/92 | 98 |
| 6 | NVPL-BVIM-PW | heterogeneous | 57 | 100 |
| 7e | MimAM(H)-PW | homogeneous | 100 | 83 |
| 8 | [Bmim]3PW | homogeneous | 94 | 92 |
These results reveal that the specific swelling behavior of the present catalytic system has substantial advances for the oxidation of benzyl alcohol with H2O2. It is well known that polymer gels could cause drastic mode transfer at several conditions, due to their characteristic temperature/solvent-responsive intelligence.12a It is thus proposed that in the present system, owing to the absorption of organic substrates by heating, the formed specific hybrid catalyst gel phase holding the flexible solid state provided the efficient thermomorphic catalysis system. The reaction proceeded in the inner hybrid catalyst gel phase, and the formed product in the catalyst gel would be released, thus, the product/H2O/solid catalyst were separated after the reaction.
The attractive properties of NDMAM-AVIM-PW prompted us to test the amino-free polymeric hybrids, NDMAM-BVIM-PW and NVPL-BVIM-PW. In the oxidation of benzyl alcohol with H2O2, NDMAM-BVIM-PW was also insoluble in benzyl alcohol before the reaction (Fig. 1B (a)), and the formation of a stable emulsion was observed at 90 °C after the addition of H2O2 (Fig. 1B (b)). Finally, the product/H2O/solid catalyst were separated by cooling the reaction mixture to room temperature (Fig. 1B (c)). Although this system afforded a lower conversion of 75% (Table 1, entry 4) than the amino-attached NDMAM-AVIM-PW did, a satisfactory conversion of 96% with 98% selectivity could be obtained by prolonging the reaction time to 4 h (Table 1, entry 5). For another amino-free hybrid catalyst NVPL-BVIM-PW (Table 1, entry 6), a switching from solid–liquid to liquid–liquid biphasic catalysis was clearly observed, which is very similar to the phenomenon resulted by NDMAM-AVIM-PW, and a low catalytic activity was observed.
In contrast, both the non-polymeric ionic hybrid control catalysts, MimAM(H)-PW (MimAM: 1-aminoethyl-3-methylimidazolium) and [Bmim]3PW (Bmim: 1-n-butyl-3-methylimidazolium), caused homogeneous reaction systems. Moreover, the amino-functionalized hybrid MimAM(H)-PW exhibited a dramatic increase of conversion up to 100% within a very short reaction time of 0.5 h (Table 1, entry 7). It is much more active than the amino-free [Bmim]3PW (Table 1, entry 8). Therefore, all the above comparisons suggest that the featured polymeric framework of poly(NDMAM) with IL endows the hybrid catalysts with insoluble nature, and plays an important role for the stimuli-response intelligence of the polymeric hybrid catalysts, which accounts for the excellent catalytic performance.
To investigate the promotional effects of the amino functional groups in the polymeric catalyst on the oxidation reaction, the UV-vis profiles of NDMAM-AVIM-PW and NDMAM-BVIM-PW are compared (Fig. S2, ESI†). NDMAM-AVIM-PW showed a broad absorption band in the range 600–800 nm that is assignable to the intramolecular charge transfer from the amino-tethered cations to the PW anions,15 but the band was undetectable for the amino-free NDMAM-BVIM-PW. These observations indicate the effective tuning of the redox properties of the W species by the incorporated amino groups.16
After reaction, the catalysts NDMAM-AVIM-PW and NDMAM-BVIM-PW could be easily recovered by filtration and directly reused for the next run. Entries 2 and 5 in Table 1 revealed quite a steady reusability for these hybrid catalysts without observing significant loss of conversion and selectivity. The TG pattern for the recovered catalysts (Fig. S3B, ESI†) showed that the weight losses for the organic moiety in catalysts were the same as those of the fresh ones. This implies that the contents of PW for the recovered catalysts are not changed. Furthermore, the profiles of the IR spectra for the recovered catalysts (Fig. S4A, ESI†) were consistent with those of the fresh ones, respectively, revealing very durable catalyst structures. Thus, the present concept might be helpful in tailoring other recyclable HPA-based reaction systems.
The recovery of the present stimuli-responsive catalysts is explicitly easier than the previous homogeneous and liquid–liquid biphasic HPA-based catalysts.10 Furthermore, not only can our catalysts be comparable to the previous phase-transfer catalyst [C7H7N(CH3)3]7PW11O39 in conversion, selectivity and reusability, but also the catalyst recovery rate of the present catalysts is higher.17
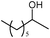
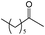
The alcohol substrates other than benzyl alcohol such as cyclohexanol, 1-phenylethyl alcohol, 2-phenylethyl alcohol, and 2-octanol were also investigated over the polymeric catalysts NDMAM-AVIM-PW and NDMAM-BVIM-PW (Table 2). Due to the immiscible property of these substrates with aqueous H2O2, a small amount of mixed solvent of CH3CN–H2O was added into the reaction media to make a monophasic liquid system. For the catalyst NDMAM-AVIM-PW, although a transitory swelling behavior was observed, it was attached onto the bottom surface of the flask reactor as a gelatinous solid, which greatly lowered the mass transfer giving much lowered conversions. When NDMAM-BVIM-PW was used as the catalyst, emulsion reaction systems were well formed in the oxidation of such alcohols, and excellent conversions and selectivities were obtained as well. Furthermore, for the oxidation of α-phenylethyl alcohol in the solvent CH3CN–H2O, a leaching amount of less than 2.5 wt.% W of NDMAM-BVIM-PW was analyzed by inductively coupled plasma-atomic emission spectrometry (ICP-AES) for the reacted filtrate.
| Entry | Substrate | Product | Catalyst | t (h) | Conb(%) | Selc(%) |
|---|---|---|---|---|---|---|
a Reaction conditions: catalyst (0.1 g), substrate (10 mmol), 30% H2O2 (12 mmol), 90 °C, solvent CH3CN–H2O (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 1 v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5 mL) for entries 3–10.
b Conversion of the substrate based on H2O2.
c Selectivity for the aldehyde product (byproduct for entries 5 and 6: phenyl acetic acid). 1, 5 mL) for entries 3–10.
b Conversion of the substrate based on H2O2.
c Selectivity for the aldehyde product (byproduct for entries 5 and 6: phenyl acetic acid).
|
||||||
| 1 |

|
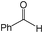
|
A | 2 | 93 | 99 |
| 2 | B | 4 | 96 | 98 | ||
| 3 |
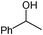
|
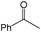
|
A | 4 | 25 | 100 |
| 4 | B | 4 | 95 | 100 | ||
| 5 |

|
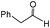
|
A | 6 | 34 | 92 |
| 6 | B | 6 | 87 | 97 | ||
| 7 |
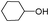
|
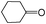
|
A | 4 | 31 | 100 |
| 8 | B | 4 | 94 | 100 | ||
| 9 |
|
|
A | 6 | 8 | 100 |
| 10 | B | 6 | 57 | 100 | ||
Conclusions
We have synthesized novel HPA-based polymeric hybrids by anion-exchange of the task-specifically designed ionic copolymers with the Keggin HPA of phosphotungstic acid. They were proved to be highly efficient catalysts for the oxidation of alcohols with H2O2, presenting the advantages of easy recovery and steady reuse. The featured structure of polymeric framework endows the catalysts with solid nature and stimuli-responsive behavior, which accounts for their excellent performances.Experimental
Preparation of [AVIM]Br·HBr and [BVIM]Br
The IL monomers [3-aminoethyl-1-vinylimidazolium]Br·HBr ([AVIM]Br·HBr) and [3-butyl-1-vinylimidazolium]Br ([BVIM]Br) were prepared according to the literature,18 and the experimental details are in the supplementary material.† [AVIM]Br·HBr: 1H NMR (300 MHz, D2O, TMS) (Fig. S1A, ESI†) δ (ppm) = 3.47 (m, 2H, –CH2), 4.58 (m, 2H, –CH2), 5.40 (d, 1H, –CH), 5.81 (d, 1H, –CH), 7.13 (m, 1H, –CH), 7.66 (s, 1H, –CH), 7.81 (s, 1H, –CH), 9.18 (s, 1H, –CH). [BVIM]Br: 1H NMR (300 MHz, D2O, TMS) (Fig. S1B, ESI†) δ (ppm) = 0.89 (t, 2H, –CH3), 1.29 (m, 2H, –CH2), 1.82 (m, 2H, –CH2), 4.24 (t, 2H, –CH2), 5.41 (d, 1H, –CH), 6.01 (d, 1H, –CH), 7.35 (m, 1H, –CH), 8.00 (s, 1H, –CH), 8.28 (s, 1H, –CH), 9.76 (s, 1H, –CH).Preparation of ionic copolymers
Amino-functionalized ionic copolymer (NDMAM-AVIM) was prepared according to the literature.19 The obtained [AVIM]Br·HBr (1.0 g), N,N-dimethyl acrylamide (NDMAM) (1.0 g), and azobisisobutyronitrile (AIBN) (0.05 g) were dissolved in ethanol–water (50 mL, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) under nitrogen. The mixture was refluxed at 80 °C with stirring. After 24 h, solvent was removed in vacuum to give the product as a solid, which was washed with ethyl ether. KOH was added into the aqueous solution of the above solid for neutralization (pH = 9–10), followed by evaporation under vacuum. Methanol was added into the resulting mixture with the appearance of precipitated salts. After filtration, the filtrate was evaporated to give the NDMAM-AVIM product as a yellow oil. Silver nitrate titration was used to detect the content of bromine ions in the copolymer, so as to determine the molar ratio of NDMAM to [AVIM]Br. The ionic copolymers NVPL-AVIM, NDMAM-BVIM, and NVPL-BVIM (NVPL: N-vinyl pyrrolidone) were prepared accordingly, by the free radical copolymerization of NDMAM (or NVPL) and AVIM (or BVIM) using AIBN as the initiator.
1 v/v) under nitrogen. The mixture was refluxed at 80 °C with stirring. After 24 h, solvent was removed in vacuum to give the product as a solid, which was washed with ethyl ether. KOH was added into the aqueous solution of the above solid for neutralization (pH = 9–10), followed by evaporation under vacuum. Methanol was added into the resulting mixture with the appearance of precipitated salts. After filtration, the filtrate was evaporated to give the NDMAM-AVIM product as a yellow oil. Silver nitrate titration was used to detect the content of bromine ions in the copolymer, so as to determine the molar ratio of NDMAM to [AVIM]Br. The ionic copolymers NVPL-AVIM, NDMAM-BVIM, and NVPL-BVIM (NVPL: N-vinyl pyrrolidone) were prepared accordingly, by the free radical copolymerization of NDMAM (or NVPL) and AVIM (or BVIM) using AIBN as the initiator.
Preparation of the HPA-based ionic hybrids
NDMAM-AVIM (0.7 g) was dissolved in 30 mL deionized water with stirring. The aqueous solution containing 2 g of H3PW12O40 (PW) was added to the above solution, and the resulting mixture was stirred at room temperature for 24 h. The thus obtained solid catalyst (NDMAM-AVIM-PW) was filtered and washed with water three times, followed by drying in a vacuum. Elemental analysis, found: C: 15.21%, N: 5.32% and H: 2.08%. NDMAM-BVIM-PW (elemental analysis, found: C: 16.03%, N: 3.91% and H: 2.20%), NVPL-AVIM-PW (elemental analysis, found: C: 17.70%, N: 5.42% and H: 2.34%) and NVPL-BVIM-PW (elemental analysis, found: C: 18.63%, N: 4.21% and H: 2.33%) were prepared accordingly. The synthesis of the control hybrids [Bmim]3PW (Bmim: 1-n-butyl-3-methylimidazolium) and MimAM(H)-PW (MimAM: 1-aminoethyl-3-methylimidazolium), and the corresponding characterizations are described in detail in our recently published literature.20Typical procedure for the oxidation of alcohols with H2O2
Benzyl alcohol (10 mmol) and catalyst NDMAM-AVIM-PW (0.1 g) were added to a 25 mL flask. Under conditions of reflux, vigorous stirring, and a heating temperature of 90 °C, the aqueous H2O2 (30 wt.%, 12 mmol) was added into the above mixture within 5 min, then the reaction mixture was stirred for another 2 h. After reaction, the reaction mixture was centrifuged to remove the solid catalyst, and the liquid was analyzed using a gas chromatograph (GC SP-6890) equipped with a FID detector and a capillary column (SE-54; 30 m × 0.32 mm × 0.25 μm). The isolated yield was obtained using column chromatography, and the product was characterized by 1H NMR (Fig. S1C, ESI†). Due to the adsorption of the product benzaldehyde on the silica gel and the weight loss in the operation, a low isolated yield of about 60% of benzaldehyde was obtained. The concentration of H2O2 in the reacted mixture was determined by titration with sodium thiosulfate (starch as indicator) in the presence of potassium iodide, sulfuric acid and ammonium molybdate. No remaining H2O2 was detected due to the self-decomposition of H2O2 at 90 °C accompanying the oxidation of alcohol. The recovered catalyst for the recycling tests was obtained by centrifugation, washing with ethanol and vacuum drying.Acknowledgements
The authors thank the financial support from the National Natural Science Foundation of China (Nos. 21206052 and 21136005), the National “Twelfth Five-Year” Plan for Science & Technology (2012BAD32B03).References
- (a) D. Long, E. Burkholder and L. Cronin, Chem. Soc. Rev., 2007, 36, 105 RSC; (b) A. Dolbecq, E. Dumas, C. R. Mayer and P. Mialane, Chem. Rev., 2010, 110, 6009 CrossRef CAS.
- Y. Qiao and Z. Hou, Curr. Org. Chem., 2009, 13, 1347 CrossRef CAS.
- K. Inumaru, T. Ishihara, Y. Kamiya, T. Okuhara and S. Yamanaka, Angew. Chem., Int. Ed., 2007, 46, 7625 CrossRef CAS.
- (a) L. Gharnati, O. Walter, U. Arnold and M. Döring, Eur. J. Inorg. Chem., 2011, 2756 CrossRef CAS; (b) A. Bordoloi, S. Sahoo, F. Lefebvre and S. B. Halligudi, J. Catal., 2008, 259, 232 CrossRef CAS.
- Z. Xi, N. Zhou, Y. Sun and K. Li, Science, 2001, 292, 1139 CrossRef CAS.
- (a) R. N. Biboum, F. Doungmene, B. Keita, P. de Oliveira, L. Nadjo, B. Lepoittevin, P. Roger, F. Brisset, P. Mialane, A. Dolbecq, I. M. Mbomekalle, C. Pichon, P. Yin, T. Liu and R. Contant, J. Mater. Chem., 2012, 22, 319 RSC; (b) H. Lu, J. Gao, Z. Jiang, Y. Yang, B. Song and C. Li, Chem. Commun., 2007, 150 Search PubMed.
- H. Hamamoto, Y. Suzuki, Y. M. A. Yamada, H. Tabata, H. Takahashi and S. Ikegami, Angew. Chem., Int. Ed., 2005, 44, 4536 CrossRef CAS.
- Y. Qiao, Z. Hou, H. Li, Y. Hu, B. Feng, X. Wang, L. Hua and Q. Huang, Green Chem., 2009, 11, 1955 RSC.
- (a) Y. Leng, J. Wang, D. Zhu, X. Ren, H. Ge and L. Shen, Angew. Chem., Int. Ed., 2009, 48, 168 CrossRef CAS; (b) Y. Leng, J. Wang, D. Zhu, Y. Wu and P. Zhao, J. Mol. Catal. A: Chem., 2009, 313, 1 CrossRef CAS.
- (a) P. Tundo, G. P. Romanelli, P. G. Vázquez and F. Aricò, Catal. Commun., 2010, 11, 1181 CrossRef CAS; (b) D. Sloboda-Rozner, P. L. Alsters and R. Neumann, J. Am. Chem. Soc., 2003, 125, 5280 CrossRef.
- Y. Leng, P. Zhao, M. Zhang and J. Wang, J. Mol. Catal. A: Chem., 2012, 358, 67 CrossRef CAS.
- (a) R. Akiyama and S. Kobayashi, Chem. Rev., 2009, 109, 594 CrossRef CAS; (b) J. Lu and P. H. Toy, Chem. Rev., 2009, 109, 815 CrossRef CAS.
- (a) S. Ikegami and H. Hamamoto, Chem. Rev., 2009, 109, 583 CrossRef CAS; (b) D. E. Bergbreiter, J. Tian and C. Hongfa, Chem. Rev., 2009, 109, 530 CrossRef CAS; (c) D. E. Bergbreiter, Immobilized Catalysts, 2004, 242, 113 CrossRef CAS; (d) M. Benaglia, A. Puglisi and F. Cozzi, Chem. Rev., 2003, 103, 3401 CrossRef CAS; (e) D. E. Bergbreiter and S. D. Sung, Adv. Synth. Catal., 2006, 348, 1352 CrossRef CAS.
- J. Yuan, Polymer, 2011, 52, 1469 CrossRef CAS.
- T. Yamase, Chem. Rev., 1998, 98, 307 CrossRef CAS.
- (a) J. C. Duhacek and D. C. Duncan, Inorg. Chem., 2007, 46, 7253 CrossRef CAS; (b) C. Rocchiccioli-Deltcheff, R. Thouvenot and R. Franck, Spectrochim. Acta., 1976, 32A, 587 CrossRef CAS; (c) S. Bareyt, S. Piligkos, B. Hasenknopf, P. Gouzerh, E. Lacote, S. Thorimbert and M. Malacria, J. Am. Chem. Soc., 2005, 127, 6788 CrossRef CAS.
- Z. Weng, J. Wang and X. Jian, Catal. Commun., 2008, 9, 1688 CrossRef CAS.
- (a) E. D. Bates, R. D. Mayton, I. Ntai and J. H. Davis Jr, J. Am. Chem. Soc., 2002, 124, 926 CrossRef CAS; (b) T. Payagala, J. Huang, Z. S. Breitbach, P. S. Sharma and D. W. Armstrong, Chem. Mater., 2007, 19, 5848 CrossRef CAS.
- G. Liu, M. Hou, J. Song, T. Jiang, H. Fan, Z. Zhang and B. Han, Green Chem., 2010, 12, 65 RSC.
- Y. Leng, J. Wang, D. Zhu, M. Zhang, P. Zhao, Z. Long and J. Huang, Green Chem., 2011, 13, 1636 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental details and catalyst characterizations. See DOI: 10.1039/c2ra22348a |
| This journal is © The Royal Society of Chemistry 2012 |
