Fluorescent Zn-based hetero-MOFs design via single metal site substitution†‡
Qi-Bing
Bo
*a,
Hong-Yan
Wang
a,
Jin-Ling
Miao
a and
Da-Qi
Wang
b
aSchool of Chemistry and Chemical Engineering, University of Jinan, Jinan, 250022, China. E-mail: chm_boqb@ujn.edu.cn
bCollege of Chemistry and Chemical Engineering, Liaocheng University, Liaocheng, 252059, China
First published on 2nd October 2012
Abstract
Two pairs of novel Zn-based MOFs with the same structures are synthesized. The exchange mechanism between the primary MOFs and the substituted MOFs is discussed. The utilization of the single metal site substitution in pre-formed Zn-based MOFs appears suited to the design of isostructural Zn-based hetero-MOFs with different photoluminescence properties.
Introduction
Studies in functional metal–organic frameworks (MOFs) have been focused on the design and construction of novel MOFs, as well as the relationships between their structures and properties.1 During the course of our investigations on the synthesis of functional MOFs, the reaction of zinc salt with 5-tertbutyl isophthalic acid (H2tbip) led to a novel Zn-based homo-MOF, [Zn3(H2O)4(tbip)3·2(H2O)]n (ZnZn-tbip) (tbip = 5-tertbutyl isophthalate anion). Single crystal X-ray diffraction reveals that the second building unit (SBU) of ZnZn-tbip consists of two kinds of zinc ions: one in a tetrahedral environment and the other in an octahedral environment (ESI 1a†). Here, the zinc ion adopting the octahedral geometry catches our attention. It is known that Zn2+ generally favors tetrahedral coordination while alkaline earth metal ions and Cd2+ favor octahedral coordination. This kind of octahedral metal site in the SBU of ZnZn-tbip is like an “active” point, so can this kind of zinc ion be replaced by an alkaline earth metal ion through a single metal site substitution? If so, the alkaline earth metal salts may be added to the reaction solution during the synthesis of ZnZn-tbip, and the octahedral site of the primary SBU could be completely replaced by the alkaline earth metal ion, generating the substituted Zn-based hetero-MOF with the same structure as ZnZn-tbip. The isostructural Zn-based homo-MOFs have been synthesized by Bisht and Rasmus.2 However, it is important to synthesize the isostructural Zn-based hetero-MOFs through exchange of the metal center under similar synthetic conditions, because they can give a convenient method to investigate the effect of a single metal substitution site on the luminescence properties of the parent MOFs. On the other hand, although the fascinating physical properties of the Zn-based hetero-MOFs containing transition metals or main group metals have been investigated, many of them exhibit different structures.3 Occasionally, although parts of the hetero-metal doped MOFs with isostructures have been reported,4 the stoichiometry doping degree of the second metal is only obtained by ICP or atomic absorption spectroscopic analysis inaccurately. Therefore, the accurate tracking of the incorporation of alkaline earth metal or d10 ions into crystal lattices of Zn-based parent MOFs resulting in isostructural hetero-MOFs with different photoluminescent properties has been a current challenge for the design of the luminescent MOFs.Inspired by the aforementioned considerations, we added calcium nitrate into the reaction solution during the synthesis of ZnZn-tbip by means of similar synthetic conditions to verify our hypothesis. As expected, the single crystal [Zn2Ca(H2O)4(tbip)3·3(H2O)]n (ZnCa-tbip) was obtained, and single crystal X-ray diffraction reveals that both ZnCa-tbip and ZnZn-tbip are indeed isostructural, and possess similar SBUs (ESI. 1b†).
For comparison with the synthesis of ZnCa-tbip mentioned above, the reaction containing mixtures of single crystals of ZnZn-tbip, calcium nitrate and water was also performed. Unfortunately, no single crystal was found, only a colorless solution. However, when calcium nitrate was added to the remaining reaction mixtures after the synthesis of ZnZn-tbip, many single crystals of ZnCa-tbip were obtained through the hydrothermal reaction. This shows that during the synthesis of ZnCa-tbip, ZnZn-tbip is formed first and the parent reaction solution for ZnZn-tbip plays an important role in the construction of the final hetero-MOFs. Furthermore, the preparation of the pair of isostructural MOFs from Zn-based homo-MOFs, is further extended to the Zn-based hetero-MOFs. By changing the ligand from H2tbip to 5-methyl isophthalic acid (H2mip), the other Zn-based hetero-MOF [Zn2Ca(H2O)2(mip)3·2(H2O)]n (ZnCa-mip) (mip = 5-methyl isophthalate anion) containing both tetrahedral and octahedral metal sites is first synthesized (ESI. 1c†). Considering the similarities of the ionic radius and coordination nature of Ca2+ and Cd2+ ions, we speculated that the substitution of Ca2+ (dwelling at the octahedral site in ZnCa-mip) with Cd2+ should result in another isostructural hetero-MOF. The experimental results confirm that the anticipated hetero-MOF [Zn2Cd(H2O)2(mip)3·2(H2O)]n (ZnCd-mip) was obtained, when the Cd salt was added to the reaction solution, in resemblance to the synthesis of ZnCa-mip (ESI. 1d†).
The crystallographic study reveals that two pairs of the isostructural Zn-based MOFs crystallize in a 3D open framework with helical channels. Each of them can be simplified to a uninodal 6-connected net with pcu α-Po topology (Schlafli symbol is (412.63)), considering the ligands (tbip and mip) and Zn2M clusters (M = Zn,Ca and Cd) as the linkers and nodes, respectively. ZnZn-tbip and ZnCa-tbip are isostructural, and possess the same orthorhombic crystal system with space group Pnna. For ZnCa-tbip, the Zn atom is in a tetrahedral geometry, and is four-coordinated to three different carboxyl groups from three tbip ligands and one coordinated water molecule with the Zn–O distances falling into the range 1.9374(1)–2.0263(45) Å. The Ca atom dwelling at the octahedral site is coordinated to four tbip ligands and two coordinated water molecules. The Ca–O distances fall in the range of 2.2505(1)–2.3544(1) Å. Each Ca atom is corner-shared with two adjacent Zn atoms through carboxyl groups, leading to a trinuclear cluster unit Zn2Ca(COO)6, which can be regarded as a 6-connected SBU with Zn1···Zn1A and Zn1···Ca1 separations of 7.5911(3) and 4.0500(1) Å, respectively (Fig. 1a). Interestingly, the Zn2Ca clusters are interconnected by the carboxylate groups (O–C11–O and O–C12–O labeled with mauve and blue parts) to result in a 2D bilayer involving two kinds of helical chains, in which the right and left-handed helical chains are in an alternate array, as shown in Fig. 1b. Then, the adjacent helical bilayers are pillared by an infinite chain of carboxylate groups (O–C13–O) bridging Zn2Ca clusters (Fig. 1c), resulting in a novel 3D meso-helical MOF containing large parallelogram channels filled with free water molecules (Fig. 1d). The PLATON analysis5 shows that approximately 9.4% (406.6 out of the 4334.3 Å3) and 8.5% (382.9 out of the 4517.1 Å3) of the crystal volumes are occupied by free water molecules for ZnZn-tbip and ZnCd-tbip, respectively.
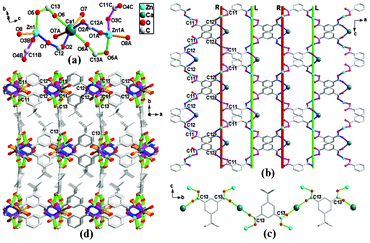 | ||
| Fig. 1 (a) Schematic representation of a 6-connected SBU Zn2Ca(COO)6 for ZnCa-tbip; (b) A 2D bilayer involving the alternated right-hand and left-hand helices along the c axis, where R and L denote the right-hand and left-hand helices, respectively; (c) An infinite chain of carboxylate group O–C13–O and the Zn2Ca clusters; (d) A 3D MOF constructed from the Zn2Ca clusters and tbip ligands for ZnCa-tbip; Symmetry codes: A (x, 0.5 − y, 1.5 − z), B (0.5 + x, y, 1 − z), C (0.5 + x, 0.5 − y, 0.5 + z). | ||
Detail structural analysis reveals that isostructural MOFs ZnCa-mip and ZnCd-mip crystallize in the orthorhombic space group Pnma. For ZnCd-mip, each Cd atom is coordinated by six carboxylate oxygen donors from six different mip ligands, and presents an octahedral coordination arrangement. The Cd–O bond lengths are in the range of 2.2282(3)–2.3585(2) Å. Each Zn atom adopts a tetrahedral geometry, and is coordinated by four oxygen atoms from three different mip ligands and one coordinated water molecule with a Zn–O bond length range of 1.9566(2)–2.0228(21) Å. Fig. 2a shows that two symmetry-related Zn atoms (Zn1 and Zn1A) and one Cd atom (Cd1) are edge-bridged by six different carboxylate groups of the mip ligands to give a 6-connected trinuclear cluster SBU Zn2Cd(COO)6, with Zn···Cd and Zn···Zn interatomic separations of 3.3872(4) and 6.7744(6) Å, respectively. Through corner sharing, the adjacent SBUs for ZnCd-mip are first connected by O–C4–O and O–C5–O carboxylate groups (labeled with mauve and green parts in Fig. 2a) into a 2D meso-helical layer featuring the alternated right-hand and left-hand helices along the b axis (Fig. 2c). At the same time, the adjacent meso-helical layers are further cross-linked by a 1D single chain resulting from the coordination of another carboxylate group (O–C10–O, labeled with blue parts in Fig. 2d) with the Zn2Cd clusters. Finally, the 3D meso-MOFs with helical channels are formed (Fig. 2b). The PLATON analysis reveals that the free volumes of the channels are 18.2% (585.6 out of the 3209.8 Å3) and 18.0% (584.6 out of the 3247.5 Å3) of the crystal volumes for ZnCd-mip and ZnCa-mip, respectively. By comparision, the solvent accessible void volumes of the isostructural MOFs are nearly same, and MOFs containing the mip ligands possess larger free volumes than those containing tbip ligands due to the more bulky tertbutyl groups (vs. methyl).
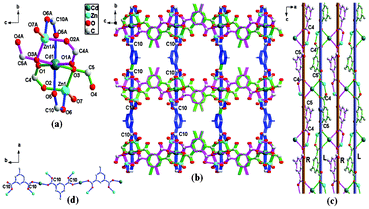 | ||
| Fig. 2 (a) Schematic representation of a 6-connected SBU Zn2Cd(COO)6 for ZnCd-mip; (b) A 3D MOF constructed from the Zn2Cd clusters and mip ligands for ZnCd-mip, where blue denotes the mip ligands used as pillars; (c) A 2D meso-helical layer featuring the alternated right-hand and left-hand helices along the b axis, where R and L denote the right-hand and left-hand helices, respectively; (d) 1D single chain resulting from the coordination of the carboxylate group O–C10–O with the Zn2Cd clusters; Symmetry codes: A (1 − x, 1 − y,1 − z). | ||
Thermal analyses show that the thermal decomposition behaviors of all the MOFs are similar. Below 400 °C, accompanied by the endothermal peaks, weight losses of 11.27% (calcd 11.19%) , 13.18% (calcd 13.16%), 9.86% (calcd 9.26%) and 8.90% (calcd 8.47%) are attributed to the release of the crystal and coordinated water molecules per formula unit for ZnZn-tbip, ZnCa-tbip, ZnCa-mip and ZnCd-mip, respectively (ESI 2–5†). Evidently, the measured weight loss values are all in good agreement with the calculated ones from the corresponding compositions. Beyond the temperature of 400 °C, the tremendous weight loss and the obvious exothermal peaks are observed, which means the burning of the organic groups and continuing collapse of the frameworks. Finally, the thermal decomposition of the MOFs leads to the formation of metal oxide as the residue. As shown in ESI 6,† the burning residuals for ZnCd-mip are attributed to mixtures of ZnO (JCPDS 005-0664) and CdO (JCPDS 001-1049). Mixtures of ZnO (JCPDS 75-0576) and CaO (JCPDS 82-1690) are found in the final residues from ZnCa-tbip (ESI 7†). The X-ray powder diffraction (XRPD) patterns of the burning residues confirm the presence of the heterometallic ions in the coresponding hetero-MOFs. On the other hand, as shown in ESI 8–11,† the XRPD patterns measured for the as-synthesized samples of the Zn-based MOFs are all in good agreement with the XRPD patterns simulated from the respective single-crystal X-ray data using the Mercury program, demonstrating the good phase purity of the MOFs.
The photoluminescent properties of two free ligands (H2tbip and H2mip) and four Zn-based MOFs are all investigated in the solid state at room temperature (ESI 12–17†). Upon excitation at 315 nm, H2mip and H2tbip display ultraviolet emissions at 357 and 345 nm, respectively, which could be attributed to the π*→π transition. When excited at 320 nm, the MOFs display emission bands centered at 546 nm for ZnZn-tbip, 436 nm for ZnCa-tbip, 524 nm for ZnCa-mip and 448 nm for ZnCd-mip. It is evident that the emission bands of the MOFs are all red-shifted compared to the corresponding free ligands, and the emission bands fall in the range of 400–600 nm. As shown in ESI 12–13,† the free ligands H2tbip and H2mip exhibit no observable fluorescent emission in the range of 400–600 nm, which eliminates ligand-centered (LC) and ligand-to-ligand charge transfer (LLCT) excited states. Therefore, taking the emission bands of the two free ligands into consideration, the fluorescent emissions in the Zn-based MOFs may be proposed to originate from the coordination of tbip (or mip) to the metal atoms and tentatively assigned to ligand-to-metal charge transfer (LMCT), which is similar to the reported literature for Zn-based MOFs.6 Furthermore, it is noticeable that the ZnZn-tbip and ZnCa-tbip exhibit different fluorescence emissions although they possess the same structures. From the view of the correlations between structure and photoluminescent property, the homo-MOF ZnZn-tbip can be viewed as having a matrix similar to the inorganic host material ZnO, and the activated Ca2+ ions can be structurally incorporated into the crystal lattice of the ZnZn-tbip, resulting in the tunable emission band positions from 546 nm (ZnZn-tbip) to 436 nm (ZnCa-tbip). Accordingly, it can be concluded that the uni-metallic and bi-metallic centers play different roles in adjusting the luminescent properties of Zn-based MOFs. For the isostructural MOFs ZnCa-mip (λem = 524 nm) and ZnCd-mip (λem = 448 nm), by exchanging the metal ions between Ca2+ and Cd2+, their photoluminescence properties can also be changed in a similar way to the isostructural MOFs ZnZn-tbip and ZnCa-tbip mentioned above. These results mean that emission band positions of the Zn-based hetero-MOFs can also be affected by substituting one heterometallic ion with the other one.
In summary, two pairs of Zn-based isostrucutural MOFs, ZnZn-tbip and ZnCa-tbip, ZnCa-mip and ZnCd-mip, were successfully synthesized through a single metal site substitution and characterized with a combination of FT-IR spectroscopy, elemental analysis, single-crystal X-ray diffraction, X-ray powder diffraction, thermogravimetric analysis and fluorescence spectroscopy. Here, the present MOFs containing the R-isophthalate (R = methyl or tertbutyl) ligands are novel, and are first synthesized under similar reaction conditions. Furthermore, the exchange mechanism from the primary Zn-based MOF to the substituted hetero-MOF is accurately tracked by single-crystal X-ray diffraction measurement (ESI 1†). The studies demonstrate that two of the tetrahedral and octahedral metal sites existing in the primary MOF are responsible for the single metal site substitution between the primary MOF and the substituted hetero-MOF, and the direct substitution position originates from the octahedral metal site of the Zn-based MOF. At the same time, the substituted hetero-MOFs also exhibit different fluorescent emissions compared with the primary MOFs. To the best of our knowledge, such a simple metal site substitution is unique in the luminescent functional MOFs, and its utilization in the pre-formed Zn-based homo-MOFs (or hetero-MOFs) containing both tetrahedral and octahedral metal sites appears suited for the design of isostructural Zn-based hetero-MOFs with different photoluminescence properties.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (grant no. 21171068) and the Shandong Provincial Natural Science Foundation of China (grant no. ZR2010BM036).References
- (a) K. K. Bisht, A. C. Kathalikkattil and E. Suresh, RSC Adv., 2012, 2, 8421 RSC; (b) J. R. Li, J. Sculley and H. C. Zhou, Chem. Rev., 2012, 112, 869 CrossRef CAS.
- (a) K. K. Bisht and E. Suresh, Inorg. Chem., Article ASAP, 2012, DOI: DOI:10.1021/ic301636z; (b) D. P. Rasmus, B. Anders, C. Mogens and B. I. Bo, Acta Crystallogr., Sect. B: Struct. Sci., 2006, 62, 245 Search PubMed.
- (a) X. L. Chen, B. Zhang, H. M. Hu, F. Fu, X. L. Wu, T. Qin, M. L. Yang, G. L Xue and J. W. Wang, Cryst. Growth Des., 2008, 8, 3706 CrossRef CAS; (b) R. Zou, A. I. Abdel-Fattah, H. Xu, A. K. Burrell, T. E. Larson, T. M. McCleskey, Q. Wei, M. T. Janicke, D. D. Hickmott, T. V. Timofeeva and Y. Zhao, Cryst. Growth Des., 2010, 10, 1301 CrossRef CAS; (c) G. X. Liu, K. Zhu, H. M. Xu, S. Nishihara, R. Y. Huang and X. M. Ren, CrystEngComm, 2009, 11, 2784 RSC.
- (a) W. C. Song, J. R. Li, P. C. Song, Y. Tao, Q. Yu, X. L. Tong and X. H. Bu, Inorg. Chem., 2009, 48, 3792 CrossRef CAS; (b) L. Carlucci, G. Ciani, S. Maggini, D. M. Proserpio and M. Visconti, Chem.–Eur. J., 2010, 16, 1232 CrossRef; (c) O. Kozachuk, K. Khaletskaya, M. Halbherr, A. Bétard, M. Meilikhov, R.W. Seidel, B. Jee, A. Pöppl and R. A. Fischer, Eur. J. Inorg. Chem., 2012, 1688 CrossRef CAS; (d) R. X. Yao, X. Xu and X. M. Zhang, Chem. Mater., 2012, 24, 303 CrossRef CAS.
- A. L. Spek,, PLATON 99, A Multipurpose Crystallographic Tool, Utrecht University, Utrecht, The Netherlands, 1999 Search PubMed.
- (a) W. G. Lu, L. Jiang, X. L. Feng and T. B. Lu, Cryst. Growth Des., 2006, 6, 564 CrossRef CAS; (b) P. X. Li, C. L. Hu, Q. P. Lin, N. Zhao and J. G. Mao, Inorg. Chem., 2009, 48, 5454 CrossRef CAS; (c) Q. B. Bo, Z. W. Zhang, J. L. Miao, D. Q. Wang and G. X. Sun, CrystEngComm, 2011, 13, 1765 RSC; (d) L. Sun, L. Ma, J. B. Cai, L. Liang and H. Deng, CrystEngComm, 2012, 14, 890 RSC; (e) C. Ji, B. Li, M. L. Ma, S. Q. Zang, H. W. Houa and T. C. W. Mak, CrystEngComm, 2012, 14, 3951 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of experimental synthesis, thermal analysis, FT-IR spectra, XRPD patterns, excitation and emission spectra. CCDC reference numbers 796514, 865281, 884315 and 884319. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra21863a |
‡ Crystal data for ZnZn-tbip: C36H48O18Zn3, M = 964.85, orthorhombic, a = 16.0781(5) Å, b = 21.0471(6) Å, c = 12.8084(4) Å, α = 90.00°, β = 90.00°, γ = 90.00°, V = 4334.3(2) Å3, T = 293(2) K, space group Pnna, Z = 4, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 179 reflections measured, 4418 independent reflections (Rint = 0.0327). The final R1 values were 0.0577 (I > 2σ(I)). The final wR(F2) values were 0.1454 (I > 2σ(I)). The final R1 values were 0.0696 (all data). The final wR(F2) values were 0.1547 (all data). Crystal data for ZnCa-tbip: C36H50CaO19Zn2, M = 957.58, orthorhombic, a = 15.7091(7) Å, b = 21.6243(9) Å, c = 13.2974(5) Å, α = 90.00°, β = 90.00°, γ = 90.00°, V = 4517.1(3) Å3, T = 293(2) K, space group Pnna, Z = 4, 14 179 reflections measured, 4418 independent reflections (Rint = 0.0327). The final R1 values were 0.0577 (I > 2σ(I)). The final wR(F2) values were 0.1454 (I > 2σ(I)). The final R1 values were 0.0696 (all data). The final wR(F2) values were 0.1547 (all data). Crystal data for ZnCa-tbip: C36H50CaO19Zn2, M = 957.58, orthorhombic, a = 15.7091(7) Å, b = 21.6243(9) Å, c = 13.2974(5) Å, α = 90.00°, β = 90.00°, γ = 90.00°, V = 4517.1(3) Å3, T = 293(2) K, space group Pnna, Z = 4, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 733 reflections measured, 4615 independent reflections (Rint = 0.0396). The final R1 values were 0.0539 (I > 2σ(I)). The final wR(F2) values were 0.1415 (I > 2σ(I)). The final R1 values were 0.0778 (all data). The final wR(F2) values were 0.1596 (all data). Crystal data for ZnCa-mip: C27H26CaO16Zn2, M = 777.30, orthorhombic, a = 7.35853(19) Å, b = 23.5965(5) Å, c = 18.7029(4) Å, α = 90.00°, β = 90.00°, γ = 90.00°, V = 3247.49(13) Å3, T = 293(2)K, space group Pnma, Z = 4, 9617 reflections measured, 2929 independent reflections (Rint = 0.0226). The final R1 values were 0.0393 (I > 2σ(I)). The final wR(F2) values were 0.1233 (I > 2σ(I)). The final R1 values were 0.0433 (all data). The final wR(F2) values were 0.1263 (all data). Crystal data for ZnCd-mip: C27H26CdO16Zn2, M = 849.62, orthorhombic, a = 7.30775(10) Å, b = 23.3444(3) Å, c = 18.8151(3) Å, α = 90.00°, β = 90.00°, γ = 90.00°, V = 3209.75(7) Å3, T = 293(2)K, space group Pnma, Z = 4, 11 733 reflections measured, 4615 independent reflections (Rint = 0.0396). The final R1 values were 0.0539 (I > 2σ(I)). The final wR(F2) values were 0.1415 (I > 2σ(I)). The final R1 values were 0.0778 (all data). The final wR(F2) values were 0.1596 (all data). Crystal data for ZnCa-mip: C27H26CaO16Zn2, M = 777.30, orthorhombic, a = 7.35853(19) Å, b = 23.5965(5) Å, c = 18.7029(4) Å, α = 90.00°, β = 90.00°, γ = 90.00°, V = 3247.49(13) Å3, T = 293(2)K, space group Pnma, Z = 4, 9617 reflections measured, 2929 independent reflections (Rint = 0.0226). The final R1 values were 0.0393 (I > 2σ(I)). The final wR(F2) values were 0.1233 (I > 2σ(I)). The final R1 values were 0.0433 (all data). The final wR(F2) values were 0.1263 (all data). Crystal data for ZnCd-mip: C27H26CdO16Zn2, M = 849.62, orthorhombic, a = 7.30775(10) Å, b = 23.3444(3) Å, c = 18.8151(3) Å, α = 90.00°, β = 90.00°, γ = 90.00°, V = 3209.75(7) Å3, T = 293(2)K, space group Pnma, Z = 4, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 167 reflections measured, 2900 independent reflections (Rint = 0.0174). The final R1 values were 0.0270 (I > 2σ(I)). The final wR(F2) values were 0.0790 (I > 2σ(I)). The final R1 values were 0.0311 (all data). The final wR(F2) values were 0.0802 (all data). 167 reflections measured, 2900 independent reflections (Rint = 0.0174). The final R1 values were 0.0270 (I > 2σ(I)). The final wR(F2) values were 0.0790 (I > 2σ(I)). The final R1 values were 0.0311 (all data). The final wR(F2) values were 0.0802 (all data). |
| This journal is © The Royal Society of Chemistry 2012 |
