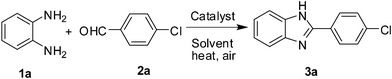
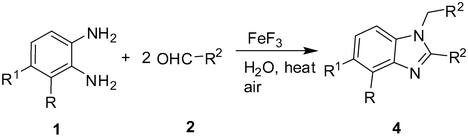
Catalysis by FeF3 in water: a green synthesis of 2-substituted 1,3-benzazoles and 1,2-disubstituted benzimidazoles†
T.
Bhaskar Kumar
ab,
Ch.
Sumanth
a,
A. V.
Dhanunjaya Rao
a,
Dipak
Kalita
a,
M.
Srinivasa Rao
a,
K. B.
Chandra Sekhar
b,
K.
Shiva Kumar
c and
Manojit
Pal
*c
aCustom Pharmaceutical Services, Dr Reddy's Laboratories Limited, Bollaram Road Miyapur, Hyderabad 500 049, India
bDepartment of Chemistry, Institute of Science and Technology, JNT University, Anantapur 515002, Andhra Pradesh, India
cInstitute of Life Sciences, University of Hyderabad Campus, Gachibowli, Hyderabad 500046, India. E-mail: manojitpal@rediffmail.com
First published on 27th September 2012
Abstract
FeF3 in water facilitated the reaction of 1,2-phenylenediamine and 2-aminothiophenol with 1 equivalent of an alkyl- or aryl-aldehyde leading to 1,3-benzazoles in open air. The process afforded 1,2-disubstituted benzimidazoles when 1,2-phenylenediamine was reacted with 2 equivalents of aryl aldehyde. The methodology is operationally simple, free from the use of hazardous organic solvents and chemoselective. The products were isolated by simple filtration and the catalyst can be recovered and recycled.
Introduction
Development of atom-efficient chemical processes using environmentally benign chemistry that minimizes or eliminates the formation of by-products has become a frontier area of research in modern organic synthesis.1 Thus a number of transition metal catalysts have been developed that enable chemical synthesis to proceed via simple addition reactions.1eAs an important class of heterocyclic compounds, 1,3-benzazoles (A, Fig. 1) i.e. benzimidazole and benzothiazole, are considered to be privileged structures in the area of medicinal chemistry.2 This is exemplified by a range of commonly used drugs such as the proton-pump inhibitor Omeprazole (B), TTX-sensitive sodium channels blocker Riluzole (C), H1 receptor antagonist Mizolastin (D), AT1 receptor antagonist Telmisartan (E) and direct thrombin inhibitor Dabigatran (Fig. 1).
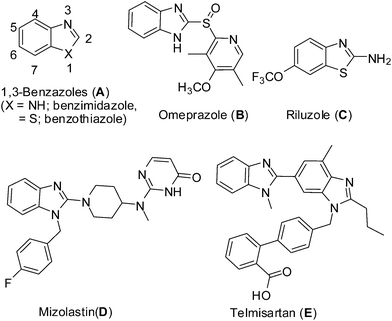 | ||
| Fig. 1 Examples of 1,3-benzazole based drugs. | ||
The most commonly used synthetic methods for accessing benzimidazole derivatives include condensation of 1,2-phenylenediamines with (i) carboxylic acids3,4 or their derivatives5 or (ii) aldehydes followed by oxidative cyclodehydrogenation.6,7 Similarly, 2-substituted benzothiazoles can be synthesized8via condensation of 2-aminothiophenol with (i) carboxylic acids9 followed by dehydration or (ii) aldehydes under oxidative conditions.10 While many of these methods are quite effective and useful most of them however suffer from the use of acidic or similar reagents or hazardous organic solvents that are not environmentally compatible and produce a large amount of waste. Moreover, the requirement for longer reaction times, higher temperatures and expensive reagents and catalysts are the other drawbacks of these methodologies. In some of these cases the formation of side products, e.g. 1,2-disubstituted benzimidazoles, along with the desired 2-substituted derivatives was also observed.
For several years iron salts have been reported as promising and alternative transition-metal catalysts that have received much attention due to their low price, sustainability, ready availability, nontoxicity, and environmentally friendly properties.11 More recently, in addition to its commercial use in the production of ceramics, FeF3 has also received attention in organic synthesis.12 This includes its use in the chemoselective addition of cyanide to aldehydes, synthesis of polyhydroquinolines, direct thiolation of an arene C–H bond, etc. Additionally, the use of water as an inexpensive and environmentally friendly solvent in commercially available and water stable FeF3 catalyzed reactions has also been explored.12a This is remarkable as the use of large volumes of volatile hazardous organic solvents in industrial processes poses a serious threat to the environment. Thus, procedures involving alternative benign solvents for reaction, isolation and purification are of high priority in industry. This prompted us to develop a green and atom-efficient one-pot synthesis of 1,3-benzazoles (benzimidazole and benzothiazole) via an FeF3 catalyzed reaction in water. Herein we report our preliminary findings on the FeF3 mediated condensation of 1,2-phenylenediamine and 2-aminothiophenol (1) with 1.0 equivalent of an alkyl- or aryl-aldehyde (2) leading to 1,3-benzazoles (3) in open air (Scheme 1). The synthesis of 1,2-disubstituted benzimidazoles is also presented.
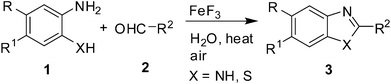 | ||
| Scheme 1 FeF3 catalyzed synthesis of 1,3-benzazoles in water. | ||
Results and discussion
Initially, we examined the reaction of 1,2-phenylenediamine (1a) with p-chloro benzaldehyde (2a) in the presence of FeCl3 in water at 60 °C for 7 h when the desired benzimidazole (3a) was isolated in 75% yield (entry 1, Table 1). While the yield of 3a was increased when FeCl3·6H2O was used in place of FeCl3 (entry 2, Table 1) the maximum yield however was achieved using FeF3 (entry 3, Table 1) indicating it is the most suitable for the present reaction. The use of other catalysts e.g. NH4F and TBAF was examined but found to be less effective (entries 4–5, Table 1). The yield of 3a was decreased when the reaction was carried out at a lower temperature (entry 6, Table 1). The use of other solvents was also examined (entries 7–8, Table 1) among which 1,4-dioxane, EtOH and MeOH were found to be effective. Nevertheless, being an inexpensive and readily available green solvent, water was chosen for further study of the present FeF3 mediated condensation reaction. To test the recyclability of the catalyst used FeF3 was recovered by simple filtration followed by evaporating the filtrate to dryness (see the experimental section) and reused in the same reaction when 3a was isolated without significant loss of yield. The yield of 3a was found to be 83, 81 and 78 after the 1st, 2nd and 3rd recovery and reuse of the catalyst. A comparison of the XRD spectrum obtained for fresh FeF3 and the reused catalyst indicated no change in its crystalline nature (Fig. 2). Notably, all these reactions were carried out in open air and therefore free from the use of an inert and anhydrous atmosphere thereby avoiding possible pressure development in a closed reaction vessel especially in scale-up synthesis. Overall, the combination of FeF3 in water was found to be optimal for the preparation of 3a.| Entry | Catalyst (mmol) | Solvent | T (°C); t (h) | Yield (%)b |
|---|---|---|---|---|
| a All the reactions were carried out using 1,2-phenylenediamine 1a (1.0 mmol), aldehyde 2a (1.0 mmol), a catalyst (0.02 mmol) in a solvent (5 mL) in open air. b Isolated yields. c Catalyst was reused for additional three runs and figures within parentheses indicate the corresponding yield for each run. | ||||
| 1 | FeCl3 (0.05) | H2O | 60; 7 | 75 |
| 2 | FeCl3·6H2O (0.05) | H2O | 60; 7 | 81 |
| 3 | FeF3 (0.02) | H2O | 60; 7 | 85 (83, 81, 78)c |
| 4 | NH4F (0.02) | H2O | 60; 7 | 50 |
| 5 | TBAF (0.02) | H2O | 60; 7 | 65 |
| 6 | FeF3 (0.02) | H2O | 25; 7 | 78 |
| 7 | FeF3 (0.02) | Toluene | 110; 12 | 73 |
| 8 | FeF3 (0.02) | 1,4-Dioxane | 100; 8 | 80 |
| 9 | FeF3 (0.02) | MeOH | 65; 2 | 83 |
| 10 | FeF3 (0.02) | EtOH | 80; 2 | 85 |
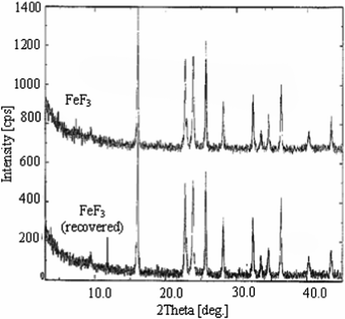 | ||
| Fig. 2 XRD spectrum of fresh FeF3 and the reused catalyst after the first cycle. | ||
Having the optimized reaction conditions in hand we then examined the generality and scope of the present FeF3 mediated reaction in water. Thus, a range of aldehydes (2) was initially reacted with 1a and the results are summarized in Table 2. Various electron donating groups, e.g. Cl, Br, tBu, Me and NMe2 (entries 1–5, Table 2), or electron withdrawing groups, e.g. CF3 and COOH (entries 6 and 7, Table 2), present on the aryl ring of the aldehyde were well tolerated. The use of heteroaromatic (entries 8 and 9, Table 2) and aliphatic aldehydes (entry 10, Table 2) was also successful and afforded the desired 2-substituted benzimidazoles in good yields. The reaction proceeded smoothly with other 1,2-phenylenediamines as well, e.g.1b–d (entries 11–14, Table 2).
| Entry | o-Phenylenediamine/o-aminobenzenethiol (1) | Aldehyde (2) | Product (3)b | Yield (%)c |
|---|---|---|---|---|
| a All the reactions were carried out using 1,2-phenylenediamine or 2-aminobenzenethiol 1 (1.0 mmol), aldehyde 2 (1.0 mmol), FeF3 (0.02 mmol) in water (5 mL) at 60 °C for 7–8 h in open air. b Identified by 1H NMR, IR and MS. c Isolated yields. d The reaction was carried out using 1.2 mmol of aldehyde. | ||||
| 1 |
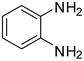 1a
1a
|
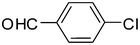 2a
2a
|
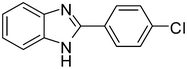 3a
3a
|
85 |
| 2 | 1a |
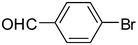 2b
2b
|
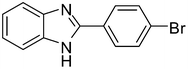 3b
3b
|
86 |
| 3 | 1a |
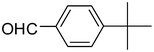 2c
2c
|
 3c
3c
|
88 |
| 4 | 1a |
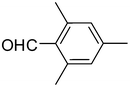 2d
2d
|
 3d
3d
|
80 |
| 5 | 1a |
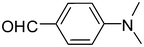 2e
2e
|
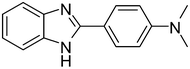 3e
3e
|
81 |
| 6 | 1a |
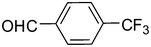 2f
2f
|
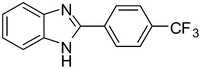 3f
3f
|
83 |
| 7 | 1a |
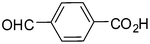 2g
2g
|
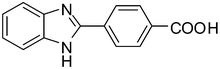 3g
3g
|
88 |
| 8 | 1a |
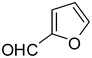 2h
2h
|
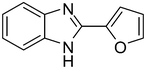 3h
3h
|
89 |
| 9 | 1a |
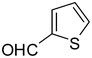 2i
2i
|
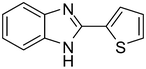 3i
3i
|
90 |
| 10 | 1a |
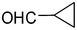 2j
2j
|
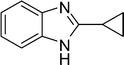 3j
3j
|
90 |
| 11 |
 1b
1b
|
2b |
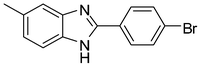 3k
3k
|
84 |
| 12 | 1b | 2g |
 3l
3l
|
78 |
| 13 |
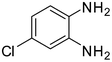 1c
1c
|
2c |
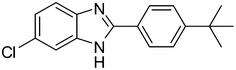 3m
3m
|
80 |
| 14 |
 1d
1d
|
2b |
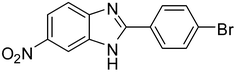 3n
3n
|
75 |
| 15 |
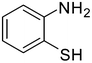 1e
1e
|
2g |
 3o
3o
|
92d |
| 16 | 1e |
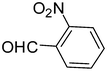 2k
2k
|
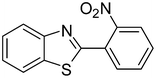 3p
3p
|
92d |
| 17 | 1e |
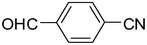 2l
2l
|
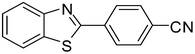 3q
3q
|
90d |
| 18 | 1a |
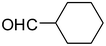 2m
2m
|
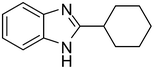 3r
3r
|
85 |
| 19 | 1a |
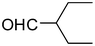 2n
2n
|
 3s
3s
|
81 |
| 20 | 1a |
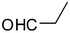 2o
2o
|
 3t
3t
|
79 |
To extend the scope of this methodology further we examined the use of 2-aminothiophenol (1e) in place of 1a and the corresponding 2-aryl substituted benzothiazoles were obtained in good yields (entries 15–17, Table 2). Furthermore, the present method afforded 2-alkyl benzimidazoles in good yields (entries 18–20, Table 2), the preparation of which was not very convenient previously as most of the reported methods were either less effective or ineffective in terms of product yields.
Prompted by the fact that 1,2-disubstituted benzimidazoles can be accessed by a direct one-step condensation of 1,2-phenylenediamines with aryl aldehydes13 we decided to explore the potential of the present FeF3 catalyzed reaction for the synthesis of these compounds in a single pot. Accordingly, 2.0 equiv of aldehyde were reacted with 1.0 equiv of 1,2-phenylenediamine under the conditions mentioned earlier (entry 3, Table 1). To our satisfaction the reaction proceeded smoothly to give the desired 1,2-disubstituted benzimidazoles (4) in good yields (Table 3). Thus, the present methodology can be used to prepare mono- or di-substituted derivatives depending on the conditions employed. The experimental procedure is simple and the products can be isolated by filtration followed by purification through crystallization.
| Entry | o-Phenylenediamine (1) | Aldehyde (2) | Product (4)b | Yield (%)c |
|---|---|---|---|---|
| a All the reactions were carried out using 1,2-phenylenediamines 1 (1.0 mmol), aldehyde 2 (2.0 mmol), FeF3 (0.02 mmol) in water (5 mL) at 60 °C for 10 h in open air. b Identified by 1H NMR, IR and MS. c Isolated yields. | ||||
| 1 |
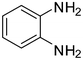 1a
1a
|
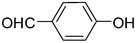 2m
2m
|
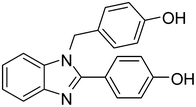 4a
4a
|
85 |
| 2 | 1a |
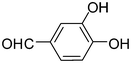 2n
2n
|
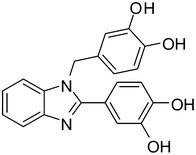 4b
4b
|
80 |
| 3 | 1b | 2c |
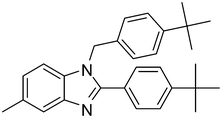 4c
4c
|
78 |
| 4 | 1f | 2c |
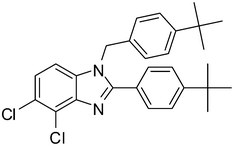 4d
4d
|
77 |
| 5 | 1a |
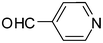 2o
2o
|
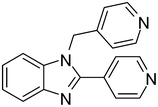 4e
4e
|
80 |
| 6 | 1a |
 2p
2p
|
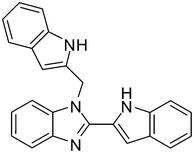 4f
4f
|
73 |
Mechanistically, the reaction seems to proceed via a sequence (Scheme 2) involving the FeF3 promoted formation of the Schiff base E-2 (via the intermediate E-1) followed by intramolecular ring closure leading to E-3. The oxidative dehydrogenation of E-3 by air affords the desired product 3. In the presence of 2.0 equivalents of the diamine (1a–b and 1f), the reaction undergoes FeF3 mediated formation of the Schiff base E-4 (i.e. N1,N2-bis(arylidene)benzene-1,2-diamine) which, after intramolecular cyclization followed by 1,3-hydride migration, affords the 1,2-disubstituted benzimidazole 4 (Scheme 3).13 While air seems to have no role in this case its presence however did not affect the reaction.
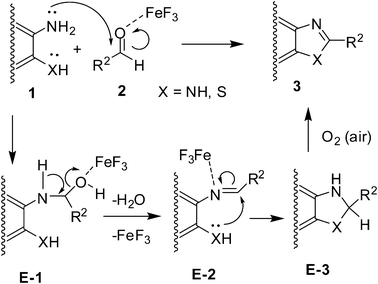 | ||
| Scheme 2 Proposed mechanism for the formation of 1,3-benzazoles (3). | ||
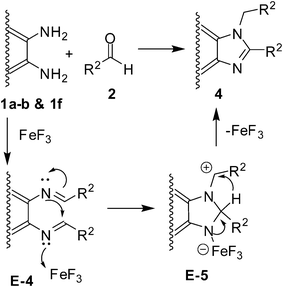 | ||
| Scheme 3 Proposed mechanism for the formation of 1,2-disubstituted benzimidazoles (4). | ||
Conclusions
In conclusion, a green, efficient and simple method has been developed for the facile and one-pot synthesis of 2-substituted benzimidazoles, 2-substituted benzothiazoles and 1,2-disubstituted benzimidazoles. The methodology involves catalysis by FeF3 in water, which facilitates the reaction of 1,2-phenylenediamine and 2-aminothiophenol with 1 equivalent of an alkyl- or aryl-aldehyde leading to 1,3-benzazoles (A, Fig. 1) in open air. The process afforded 1,2-disubstituted benzimidazoles when 1,2-phenylenediamine was reacted with 2 equivalents of aryl aldehyde. The products were isolated by simple filtration and the catalyst can be recovered and recycled. The operational simplicity, excellent yields of the products, and high chemoselectivity are the main advantages of this method, and furthermore, this procedure is inexpensive, safe and environmentally benign. The present example of the first FeF3 mediated synthesis of 1,3-benzazoles and 1,2-disubstituted benzimidazoles therefore would find wide applications.Experimental section
General methods
Unless stated otherwise, reactions were performed in open air. Reactions were monitored by thin layer chromatography (TLC) on silica gel plates (60 F254), visualizing with ultraviolet light or iodine spray. Flash chromatography was performed on silica gel (230–400 mesh) using distilled hexane, ethyl acetate or dichloromethane. 1H NMR and 13C NMR spectra were determined in DMSO-d6 solution using 400 or 100 MHz spectrometers, respectively. Proton chemical shifts (δ) are relative to tetramethylsilane (TMS, δ = 0.00) as internal standard and expressed in ppm. Spin multiplicities are given as s (singlet), d (doublet), t (triplet) and m (multiplet) as well as b (broad). Coupling constants (J) are given in hertz. Infrared spectra were recorded on a FT-IR spectrometer. Melting points were determined using melting point B-540 apparatus and are uncorrected. MS spectra were obtained on a mass spectrometer. HRMS was determined using waters LCT premier XETOF ARE-047 apparatus. The catalyst FeF3 (light green powder; purity 98%; CAS No: 7783-50-8) used is commercially available.Acknowledgements
The author (TBK) thanks Dr V. Dahanukar for his encouragement. The authors thank the analytical group of DRL for spectral data.References
- (a) B. M. Trost, Science, 1991, 254, 1471 CAS; (b) R. A. Sheldon, CHEMTECH, 1994, 38 CAS; (c) R. A. Sheldon, Pure Appl. Chem., 2000, 72, 1233 CrossRef CAS; (d) B. M. Trost, Acc. Chem. Res., 2002, 35, 695 CrossRef CAS; (e) D. B. G. Williams and M. C. Lawton, Green Chem., 2008, 10, 914 RSC.
- F. Kallashi, D. Kim, J. Kowalchick, Y. J. Park, J. A. Hunt, A. Ali, C. J. Smith, M. L. Hammond, J. V. Pivnichny, X. Tong, S. S. Xu, M. S. Anderson, Y. Chen, S. S. Eveland, Q. Guo, S. A. Hyland, D. P. Milot, A. M. Cumiskey, M. Latham, L. B. Peterson, R. Rosa, C. P. Sparrow, S. D. Wright and P. J. Sinclair, Bioorg. Med. Chem. Lett., 2011, 21, 558 CrossRef CAS.
- (a) M. R. Grimmett, in Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky and C. W. Rees, Pergamon Press, New York 1984, vol. 5, p. 457 Search PubMed; (b) J. B. Wright, Chem. Rev., 1951, 48, 397 CrossRef CAS; (c) R. W. Middleton and D. G. Wibberley, J. Heterocycl. Chem., 1980, 17, 1757 CrossRef CAS; (d) T. Hisano, M. Ichikawa, K. Tsumoto and M. Tasaki, Chem. Pharm. Bull., 1982, 30, 2996 CrossRef CAS; (e) J. D. Geratz, F. M. Stevens, K. L. Polakoski and R. F. Parrish, Arch. Biochem. Biophys., 1979, 197, 551 CrossRef CAS.
- (a) P. N. Preston, in Chemistry of Heterocyclic Compounds, Vol. 40, ed. A. Weissberger and E. C. Taylor, John Wiley and Sons, 1981 Search PubMed; (b) D. W. Hein, R. J. Alheim and J. J. Leavitt, J. Am. Chem. Soc., 1957, 79, 427 CrossRef CAS; (c) Y. -C. Chi and C.-M. Sun, Synlett, 2000, 591 CAS; (d) W. Huang and R. M. Scarborough, Tetrahedron Lett., 1999, 40, 2665 CrossRef CAS; (e) L. M. Dudd, E. Venardou, E. Garcia-Verdugo, P. Licence, A. J. Blake, C. Wilson and M. Poliakoff, Green Chem., 2003, 5, 187 RSC.
- (a) A. Czarny, W. D. Wilson and D. W. Boykin, J. Heterocycl. Chem., 1996, 33, 1393 CrossRef CAS; (b) R. R. Tidwell, J. D. Geratz, O. Dann, G. Volz, D. Zeh and H. Loewe, J. Med. Chem., 1978, 21, 613 CrossRef CAS; (c) T. A. Fairley, R. R. Tidwell, I. Donkor, N. A. Naiman, K. A. Ohemeng, R. J. Lombardy, J. A. Bentley and M. Cory, J. Med. Chem., 1993, 36, 1746 CrossRef CAS; (d) P. N. Preston, in Chemistry of Heterocyclic Compounds, vol. 40, Benzimidazoles and Congeneric Tricyclic Compounds, ed. A. Weissberger and E. C. Taylor, Wiley, New York, 1981, part 1, p. 6 Search PubMed; (e) M. R. Grimmett, in Comprehensive Heterocyclic Chemistry, vol. 5, Imidazoles and their Benzo Derivatives (ed. A. R. Katritzky and C. W. Rees), Pergamon Press, Oxford, 1984, p. 457 Search PubMed; (f) Y. Wang, K. Sarris, D. R. Sauer and S. W. Djuric, Tetrahedron Lett., 2006, 47, 4823 CrossRef CAS; (g) T. Benincori and F. Sannicolo, J. Heterocycl. Chem., 1998, 25, 1029 CrossRef; (h) K. Bourgrin, A. Loupy and M. Soufiaoui, Tetrahedron, 1998, 54, 8055 CrossRef; (i) G. V. Reddy, V. V. V. N. S. Ramarao, B. Narsaiah and P. S. Rao, Synth. Commun., 2002, 32, 2467 CrossRef CAS; (j) A. Ben-Alloum, S. Bakkas and M. Soufiaoui, Tetrahedron Lett., 1998, 39, 4481 CrossRef CAS; (k) K. Niknam and A. Fatehi-Raviz, J. Iran. Chem. Soc., 2007, 4, 438 CrossRef CAS.
- (a) J. G. Smith and I. Ho, Tetrahedron Lett., 1971, 12, 3541 CrossRef; (b) K. Nagata, T. Itoh, H. Ishikawa and A. Ohsawa, Heterocycles, 2003, 61, 93 CrossRef CAS; (c) T. Itoh, K. Nagata, H. Ishikawa and A. Ohsawa, Heterocycles, 2004, 63, 2769 CrossRef CAS; (d) S. Perumal, S. Mariappan and S. Selvaraj, Arkivoc, 2004, 8, 46 Search PubMed; (e) M. Chakrabarty, S. Karmakar, A. Mukherji, S. Arima and Y. Harigaya, Heterocycles, 2006, 68, 967 CrossRef CAS; (f) P. Sun and Z. Hu, J. Heterocycl. Chem., 2006, 43, 773 CrossRef CAS; (g) P. Salehi, M. Dabiri, M. A. Zolfigol, S. Otokesh and M. Baghbanzadeh, Tetrahedron Lett., 2006, 47, 2557 CrossRef CAS; (h) R. Varala, A. Nasreen, R. Enugala and S. R. Adapa, Tetrahedron Lett., 2007, 48, 69 CrossRef CAS; (i) B. H. Kim, R. Han, J. S. Kim, Y. M. Jun, W. Baik and B. M. Lee, Heterocycles, 2004, 63, 41 CrossRef CAS.
- (a) A. Ben Alloum, K. Bougrin and M. Soufiaoui, Tetrahedron Lett., 2003, 44, 5935 CrossRef CAS; (b) P. Gogoi and D. Konwar, Tetrahedron Lett., 2006, 47, 79 CrossRef CAS; (c) T. Itoh, K. Nagata, H. Ishikawa and A. Ohsawa, Heterocycles, 2004, 62, 197 CrossRef CAS; (d) R. Trivedi, S. K. De and R. A. Gibbs, J. Mol. Catal. A: Chem., 2006, 245, 8 CrossRef CAS.
- (a) A. Couture and P. Grandclaudon, Heterocycles, 1984, 22, 1383 CrossRef CAS; (b) R. H. Tale, Org. Lett., 2002, 4, 1641 CrossRef CAS; (c) D. Alagille, R. M. Baldwin and G. D. Tamagnan, Tetrahedron Lett., 2005, 46, 1349 CrossRef CAS; (d) T. Itoh and T. Mase, Org. Lett., 2007, 9, 3687 CrossRef CAS.
- (a) C. W. Phoon, P. Y. Ng, A. E. Ting, S. L. Yeo and M. M. Sim, Bioorg. Med. Chem. Lett., 2001, 11, 1647 CrossRef CAS; (b) E. Kashiyama, I. Hutchinson, M. S. Chua, S. F. Stinson, L. R. Phillips, G. Kaur, E. A. Sausville, T. D. Bradshaw, A. D. Westwell and M. F. G. Stevens, J. Med. Chem., 1999, 42, 4172 CrossRef CAS; (c) D. E. Boger, J. Org. Chem., 1978, 43, 2296 CrossRef CAS.
- A. Ben-Alloum, S. Bakkas and M. Soufiaoui, Tetrahedron Lett., 1997, 38, 6395 CrossRef CAS.
- (a) A. Correa, O. G. Mancheno and C. Bolm, Chem. Soc. Rev., 2008, 37, 1108 RSC; (b) B. D. Sherry and A. Furstner, Acc. Chem. Res., 2008, 41, 1500 CrossRef CAS; (c) C. Bolm, J. Legros, J. Le Paih and L. Zani, Chem. Rev., 2004, 104, 6217 CrossRef CAS; (d) A. Furstner and R. Martin, Chem. Lett., 2005, 34, 624 CrossRef; (e) D. D. Diaz, P. O. Miranda, J. I. Padron and V. S. Martın, Curr. Org. Chem., 2006, 10, 457 CrossRef.
- (a) B. P. Bandgar and V. T. Kamble, Green Chem., 2001, 3, 265 RSC; (b) T. Hatakeyama and M. Nakamura, J. Am. Chem. Soc., 2007, 32, 9844 CrossRef; (c) V. T. Kamble, B. P. Bandgar, S. B. G. Suryawanshi and S. N. Bavikar, Aust. J. Chem., 2006, 59, 837 CrossRef CAS; (d) R. Surasani, D. Kalita, A. V. D. Rao, K. Yarbagi and K. B. Chandrasekhar, J. Fluorine Chem., 2012, 135, 91 CrossRef CAS; (e) B. P. Bandgar, V. T. Kamble and A. Kulkarni, Aust. J. Chem., 2005, 58, 607 CrossRef CAS; (f) X.-L. Fang, R. -Y. Tang, X. -G. Zhang and J. -H. Li, Synthesis, 2011, 1099 CAS.
- J.-P. Wan, S.-F. Gan, J.-M. Wu and Y. Pan, Green Chem., 2009, 11, 1633 RSC.
- Y. Kim, M. R. Kumar, N. Park, Y. Heo and S. Lee, J. Org. Chem., 2011, 76, 9577 CrossRef CAS.
- M. Shen and T. G. Driver, Org. Lett., 2008, 10, 3367 CrossRef CAS.
- R. M. Acheson and M. S. Verlander, J. Chem. Soc., Perkin Trans. 1, 1973, 2348 RSC.
- M. D. Bhor and B. M. Bhanage, Synth. Commun., 2010, 40, 1743 CrossRef CAS.
- J. C. Lewis, A. M. Berman, R. G. Bergman and J. A. Eliman, J. Am. Chem. Soc., 2008, 130, 2493 CrossRef CAS.
- D. Yang, H. Fu, L. Hu, Y. Jiang and Y. Zhao, J. Org. Chem., 2008, 73, 7841 CrossRef CAS.
- S. Rostamizadeh, A. M. Amani, R. Aryan, H. R. Ghaieni and L. Norouzi, Monatsh. Chem., 2009, 140, 547 CrossRef CAS.
- L. S. Tan, R. Kannan, J. C. Spain and L. J. Nadeau, U. S. Patent No US7495106 B1, 2009 Search PubMed.
- K. Xie, Z. Yang, X. Zhou, X. Li, S. Wang, Z. Tan, X. An and C.-C. Cuo, Org. Lett., 2010, 12, 1564 CrossRef CAS.
- Y. Cheng, J. Yang, Y. Qu and P. Li, Org. Lett., 2012, 14, 98 CrossRef CAS.
- J. N. Moorthy and and I. Neogi, Tetrahedron Lett., 2011, 52, 3868 CrossRef.
- M. Rekha, A. Hamza, B. R. Venugopal and N. Nagaraju, Chin. J. Catal., 2012, 33, 439 CrossRef CAS.
- L. D. Luca and A. Porcheddu, Eur. J. Org. Chem., 2011, 29, 5791 CrossRef.
Footnote |
| † Electronic Supplementary Information (ESI) available: Copies of NMR spectra for all new compounds. For ESI see DOI: 10.1039/c2ra22302c |
| This journal is © The Royal Society of Chemistry 2012 |