Enhanced proliferation of pre-osteoblastic cells by dynamic piezoelectric stimulation
C.
Ribeiro
a,
S.
Moreira
b,
V.
Correia
ac,
V.
Sencadas
ad,
J.G.
Rocha
c,
F. M.
Gama
b,
J. L.
Gómez Ribelles
ef and
S.
Lanceros-Méndez
*ag
aCentro/Departamento de Física da Universidade do Minho, Campus de Gualtar, 4710-057 Braga, Portugal
bIBB – Institute for Biotechnology and Bioengineering, Centre of Biological Engineering, Universidade do Minho, Campus de Gualtar, 4710-057 Braga, Portugal
cCetro Algoritmi, Universidade do Minho, Campus de Azurem, 4800 Guimarães, Portugal
dInstituto Politécnico do Cávado e do Ave, Campus do IPCA, 4750-810, Barcelos, Portugal
eCenter for Biomaterials and Tissue Engineering, Universitat Politècnica de València, Camino de Vera s/n, 46022 Valencia, Spain
fNetworking Research Center on Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN), Valencia, Spain
gINL – International Iberian Nanotechnology Laboratory, 4715-330 Braga, Portugal
First published on 26th September 2012
Abstract
This work reports on the influence of the polarization of electroactive poly(vinylidene fluoride), PVDF, on the biological response of cells cultivated under static and dynamic conditions. Non-poled and “poled +” β-PVDF with and without a titanium layer were thus prepared. A thin titanium layer was deposited on PVDF films in order to obtain a more homogeneous surface charge. The MC3T3-E1 osteoblast cell culture exhibited different responses in the presence of PVDF films. The positively charged β-PVDF films promote higher osteoblast adhesion and proliferation, which is higher under dynamic conditions on poled samples, showing that the surface charge under mechanical stimulation improves the osteoblast growth. Therefore, electroactive membranes and scaffolds can provide the necessary electrical stimuli for the growth and proliferation of specific cells.
1. Introduction
Cell/biomaterial compatibility and cell response are strongly influenced by the surface properties of the biomaterial, such as surface charge, chemical composition and surface energy.1,2 In particular, surface charge and therefore electric field have been proven to influence growth and differentiation of some cells types.3,4 The quality of cell/biomaterial interactions influences cell adhesion, migration and proliferation, thus playing a decisive role in tissue engineering applications.2,5 Furthermore, different cells may behave differently on materials, according to surface morphology, hydrophobicity and roughness.1,5,6 For instance, Huag et al.5 found that osteoblasts (hFOB1.19) and fibroblasts (L929) exhibit different responses on surfaces with different morphologies. In general, it can be stated that osteoblastic cells prefer rougher surfaces, whereas fibroblasts, the most common cell type found in connective tissue, favor smoother ones.7,8 Furthermore, the surface charge influences the cell attachment and behavior.6Indeed, it has been shown that electrically charged surfaces can influence different aspects of cell behavior such as growth, adhesion or morphology of different cell types including osteoblast, nervous and cardiac cells.3,5,9 In this respect, piezoelectric materials have an interesting ability to vary surface charge when a mechanical load is applied,10 without the need for an external power source or connection wires, a property that can be taken advantage of in novel tissue engineering strategies. Verma et al.11 verified that surface charge is a critical factor for osteoblast adhesion, it was shown that positively charged surfaces promote higher adhesion.12 Schneider et al.13 observed that charged poly(hydroxyethyl methacrylic) acid (HEMA) promotes higher osteoblast attachment and spreading, and positively charged scaffolds supported higher cell attachment and spreading than neutral charges.
Many body tissues react to mechanical and electrical stimuli, thus the use of electroactive films, membranes and scaffolds shows a novel and potentially interesting approach for tissue engineering applications.14 Poly(vinylidene fluoride) (PVDF) is a semi-crystalline and biocompatible polymer with the largest piezoelectric response,15,16 good mechanical properties and excellent electroactive properties such as piezo-, pyro and ferroelectricity.10 The material can be prepared in the form of films, fibers17 or porous structures,18 allowing the production of materials with a customized microstructure for biomedical applications, among others. Depending on the processing conditions, four different crystalline structures can be obtained, known as α, β, γ and δ, with the β-phase having the greatest piezoelectric and pyroelectric properties.10,19 The semicrystalline nature of PVDF is reflected by the piezoelectric activity at the mesoscale. At the mesoscopic scale, the piezoelectric activity of β-PVDF is formed by dispersed nanoregions instead of classical regions.16 The charge distribution is therefore not totally homogeneous on the PVDF film. It has been proven that the charge surface of PVDF influences the cell viability and proliferation, being higher in poled (larger net surface charge) than in non-poled samples.14,20
Considering the suitability of PVDF, two challenges remain to enable the exploitation of electrical stimuli for cell culture purposes: the evaluation of the cellular response when a thin metal layer is deposited on top of a polymer surface, which is necessary in order to obtain a more homogeneous surface charge and to eventually use the material as an in vivo sensor and/or actuator, and to evaluate the effect under dynamic conditions. In this sense, the aim of the present work is to provide answers to the aforementioned issues by evaluating the cells cultured directly on the polymer or on the polymer coated with a conductive thin titanium layer. Further, experiments were performed under both static and dynamic conditions. MC3T3-E1 osteoblasts were selected for this work, since physiologically these cells are subjected to mechanical perturbations and can therefore be stimulated by the corresponding varying charge density on the surface of the materials, to evaluate cell adhesion, viability and proliferation in an in vitro environment.
2. Materials and methods
2.1 Preparation of PVDF samples
PVDF films were prepared as described previously.21,22 Briefly, PVDF (Solef 1010 from Solvay) was mixed with N,N-dimethyl formamide (DMF) (20 wt% PVDF), and films were obtained by spreading the solution on a glass slide that was then kept inside an oven at a controlled temperature of 120 °C for 60 min, to ensure solvent removal by evaporation and the isothermal crystallization of PVDF. Then, the sample was melted at 220 °C for 10 min, removed from the oven and cooled at room temperature. The polymer obtained by this procedure is predominantly α-PVDF, and the transformation into the β-phase was achieved by the conventional stretching procedure.15,22 Corona discharge was used to obtain the electrical poling of β-PVDF inside a home-made chamber and the piezoelectric response (d33) verified with a wide range d33-meter (model 8000, APC Int Ltd). The obtained value of the piezoelectric d33 coefficient for the poled samples was ∼−32 pC N−1.15A thin titanium layer (approximately 30 nm) was deposited on top of some of the non-poled and “poled +” β-PVDF samples by magnetron sputtering.
For the in vitro assays, circular PVDF films with 13 mm diameter were cut from the prepared films and sterilized by immersing several times in 70% ethanol for 30 min. Then, the samples were washed 5 times for 5 min in sterile phosphate-buffered saline (PBS) to eliminate any residual ethanol.
The films used in the present study were non-poled β-PVDF, “poled +” β-PVDF (cell culture on the positively charged side of the material), non-poled β-PVDF with titanium (titanium deposited on the side in which the cells were cultured) and “poled +” β-PVDF with titanium.
2.2 Substrate topography and contact angle measurements
The samples were measured using Tapping Mode with a MultiMode connected to a NanoScope III, both supplied from Veeco, USA, with non-contacting silicon (ca. 47–76 kHz, k: 1.2–6.4 N m−1) from AppNano purchased from USA. All images (10 μm wide) were fitted to a plane using the 1st degree flatten procedure included in the NanoScope software version 4.43rd8. The surface roughness was calculated as Sq (root mean square from average flat surface) and Sa (average absolute distance from average flat surface).Contact angle measurements (sessile drop in dynamic mode) were performed at room temperature in a Data Physics OCA20 set up using ultrapure water as the test liquid. Water drops (3 μL) were placed onto the surface of the PVDF samples. The contact angles were measured using the software SCA20. Each sample was measured at six different locations and the contact angle was taken as the average obtained for each sample.
2.3 Cell culture
MC3T3-E1 osteoblasts (Riken cell bank, Japan) were cultivated in Dulbecco's Modified Eagle's Medium (DMEM) containing 1 g L−1 glucose (Gibco) supplemented with 10% Fetal Bovine Serum (FBS) (Biochrom) and 1% Penicillin/Streptomycin (P/S), at 37 °C in 95% humidified air containing 5% CO2. The medium was changed every 3 days.Circular PVDF samples and glass covers used as control were placed in a 24-well tissue culture polystyrene plate (TPCS) and 0.5 mL of cell suspension (3 × 104 cells mL−1) was added to each well and incubated at 37 °C. Also, after 3 h of static culture, part of the cell-cultured membranes were transferred onto a home-made bioreactor system.
A dynamic culture was performed with MC-3T3 E1 osteoblasts on the same samples on a home-made bioreactor system with mechanical stimulation by placing the culture plate on a vertical vibration module at a frequency of 1 Hz with amplitude of ∼1 mm (Fig. 1).
 | ||
| Fig. 1 Home-made bioreactor system used for dynamic cell culture at 1 Hz: a) schematic system and b) photograph of the system. | ||
2.4 Cell viability and proliferation
The viability of MC-3T3 E1 osteoblasts on the different PVDF films under static conditions was analyzed by Live/Dead Viability/Cytotoxicity kit (Invitrogen, CA, USA), by observation with a fluorescence microscope (Olympus BX51 Microscope).The evaluation of the cell viability/proliferation was also carried out by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The MTT assay measures the mitochondrial activity of the cells, which reflects the viable cell number, and was carried out after 1, 3 and 5 days for the experiments performed under static conditions and dynamic conditions. At each time point, the cell/films were transferred to new wells and fresh medium containing MTT was added. After 3 h of incubation, the MTT crystals were dissolved and optical density was measured at 570 nm.
2.5 Statistical analysis
All quantitative results were obtained from triplicate samples. The results were expressed as mean ± standard deviation. Statistical differences were determined by ANOVA using F-test for the evaluation of different groups. P values <0.05 were considered to be statistically significant.3. Results
3.1 Surface topography
The phase content, morphology and electroactivity of PVDF films depend on the processing conditions.16 The topography of the β-PVDF obtained after stretching of α-PVDF is characterized by an oriented microfibillar microstructure. The poling process of PVDF films induces no significant differences in morphology22 and sample topography, which maintains the same mean roughness.16 The AFM analysis of the local piezoresponse data of the non-poled β-PVDF and poled samples show16 that a clear piezoresponse signal exists in both samples, the domain contrast being therefore more pronounced in the poled ones.The AFM pictures of β-PVDF samples with and without titanium are displayed in Fig. 2, maintaining the same scale and scan size for comparison. The deposition of a titanium thin layer on PVDF films increases the mean roughness (rms) of the samples, as can be observed in Fig. 2, from 20.79 nm and 24.60 nm for the non-coated samples (non-poled β-PVDF, “poled +” β-PVDF, respectively) to 29.72 nm and 26.06 nm, on average, for the coated ones (non-poled β-PVDF with titanium and “poled +” β-PVDF with titanium, respectively). Considering the titanium coated films, it can be observed that the mean roughness is lower on “poled +” β-PVDF films (26,06 nm vs. 29,72 nm, for the poled and non-poled ones, respectively). This is explained by the fact that the positive polymer charge promotes the titanium adhesion during the first deposition steps, leading to a more homogeneous and therefore less rough surface.
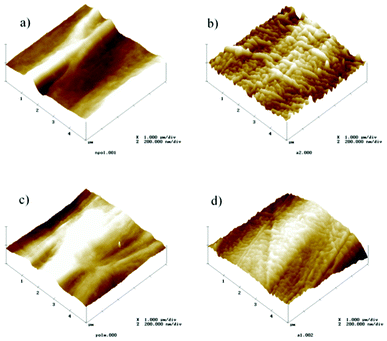 | ||
| Fig. 2 AFM pictures recorded in a 5 × 5 μm area of different PVDF samples: a) non-poled; b) non-poled with titanium; c) poled + and d) poled + with titanium. | ||
3.2 Contact angle measurements
The surface energy, which is intimately connected with wettability, is one of the key factors governing biological interaction with a given material.23–25 It is usually reported that biomaterial surfaces with moderate hydrophilicity show improved cell growth and higher biocompatibility.23The comparative wettability of the different PVDF samples was assessed by static contact angle measurements as shown in Fig. 3. It is observed that the contact angles of the different PVDF films are all below 90° and the “poled +” β-PVDF film is the most hydrophilic one, with a contact angle around 60°. When the β-PVDF films are poled by corona treatment their surface wettability increases, as shown in Fig. 3, due to the variations in the surface energy induced by the increased surface charge in the poled samples.26
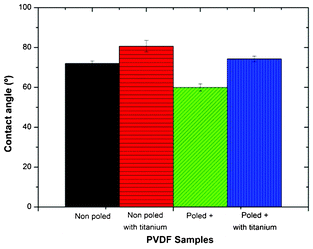 | ||
| Fig. 3 Contact angles of the different PVDF films. Values are mean ± SD. | ||
Comparing β-PVDF films with and without titanium, it is observed that surface wettability decreases with the titanium deposition. This can be ascribed to the increase in roughness with the deposition of the thin titanium layer (Fig. 2): the roughness effect overshadows the influence of interfacial interactions and the contact angle value increases with increasing surface roughness.23,27,28
3.3 Cell viability and proliferation
The viability of MC-3T3-E1 osteoblasts was investigated by the LIVE/DEAD assay, confirming the integrity of the cell membrane in all cases. Fig. 4 shows that virtually no dead MC-3T3-E1 osteoblasts were observed 3 days after cell seeding on PVDF films.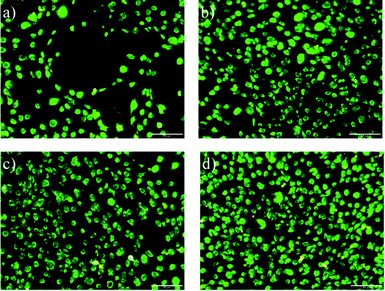 | ||
| Fig. 4 LIVE/DEAD staining of MC-3T3-E1 osteoblasts a) PVDF non-poled and b) PVDF non-poled with titanium; c) PVDF poled + and d) PVDF poled + with titanium after cell culture for 3 days. The scale bar is 50 μm for all the images. | ||
The proliferation of the attached cells on the different PVDF films and TPCS throughout 5 days of culture under static and dynamic conditions is shown in Fig. 5. The absorbance (Abs) was measured at 570 nm for all the samples at each time.
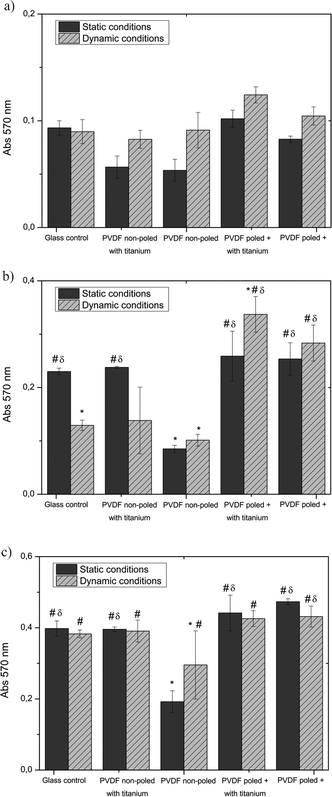 | ||
| Fig. 5 MTT results from proliferation assays of MC-3T3 osteoblasts seeded on different PVDF samples and on the control surface under static and dynamic conditions after a) 1 day, b) 3 days and c) 5 days. * P ≤ 0.05 vs. Glass control under static conditions; #P ≤ 0.05 vs. PVDF non-poled under static conditions; δP ≤ 0.05 vs. PVDF non-poled under dynamic conditions. | ||
At day 1, the cell proliferation on PVDF under static conditions was similar to the TPCS except for “poled +” β-PVDF with titanium that was higher. Comparing PVDF samples, non-poled β-PVDF shows the lowest cell proliferation and osteoblasts seem to prefer titanium surfaces. At day 3, “poled +” β-PVDF with titanium presents a higher cell proliferation.
The influence of a dynamic culture on cell proliferation in PVDF films was also studied (Fig. 5). It is observed than the dynamic culture improves the cell viability on the piezoelectric PVDF samples both with and without titanium coating.
After 5 days, both control TCPS and non-poled β-PVDF with titanium layer samples under static and dynamic culture yield the same results.
4. Discussion
The piezoelectric effect has been explored in bone tissue regeneration since this effect was first observed in bone by Fukada and Yasuda.29 Piezoelectric materials such as PVDF films were shown to induce in vivo formation of periosteal bone30 but no specific strategies to fully evaluate the potential of this material have been undertaken. Instead, materials such as hydroxyapatite (HA) have been more widely used for stimulating bone regeneration.31 It is interesting in this sense that HA films also exhibit piezo- and pyroelectricity, and studies of the effect of polarization of HA on the production of new bone32 show that charged surfaces effectively accelerates the bone formation.Previous studies on osteoblast–PVDF interactions showed that they can be used clinically for promoting tissue growth.13 The different types of β-PVDF films affect in a different way the adhesion and proliferation of osteoblasts, as the cellular response to different surfaces primarily depend on the cell type.5 Furthermore, as mentioned previously, surface topography5,33,34 and surface charge have a deep influence on cell adhesion and proliferation.4,11 In particular, it has been shown that positively charged surfaces supported higher cell attachment than neutral ones12 and induce cell adhesion and proliferation in a different way depending on the cell types.5,13 It was also observed that positively charged β-PVDF films promote higher osteoblast adhesion and proliferation. Thus, the combination of surface roughness and charge is a key point for promoting cell adhesion and proliferation on the material surface.
The goal of this work was the investigation of a dynamic mechanical stimulus of a flat surface with an electric charge distribution, and consequent effect on the response of pre-osteoblastic cells in monolayer culture. Electroactive β-PVDF has an all-trans planar “zig-zag” configuration and the unit cell has a permanent dipole moment. In non-poled polymer samples (samples non-poled β-PVDF or non-poled β-PVDF with titanium), dipoles are randomly oriented in the material and no net charge appears at the surface. In these samples, dynamic mechanical perturbations have no effect on electric charge distribution. Nevertheless, the same material presents permanent surface distribution of positive charges once electrically poled (samples “poled +” β-PVDF or “poled +” β-PVDF with titanium), since polymer dipoles during the electrical poling process rotate and align in the direction of the applied electrical field, acquiring a net orientation in the space. When the polymer chains of these samples are dynamically deformed, the net surface charge oscillates with the same frequency as that of the mechanical stimulus.
Regarding static and dynamic conditions, it was observed that cell proliferation on “poled +” β-PVDF over 3 days was higher in dynamic conditions than in static conditions, suggesting that the mechanical stimulus improves osteoblast growth. Additionally, this behaviour was not verified in the non-poled samples. As a result, the observed effect was ascribed to the variation of the charge density due to the mechanical stimulation. These results suggest that surface charge is a relevant parameter to be considered in the design of proper scaffolds and membranes for specific tissue engineering applications, and piezoelectric materials may provide the necessary electrical stimulus for cell growth, especially under a mechanically stimulated environment.
Assessment of the effect of this dynamic electric stimulation is demonstrated by the higher proliferation observed in cells cultured on “poled +” β-PVDF under dynamic conditions than in static wells. Further, this is also proven by the fact that there is no significant difference (or even the opposite effect is found) between cells cultured on non-poled β-PVDF in static and dynamic conditions (Fig. 5), where the surface charge variations should be negligible. So, the effect of dynamic stimulation is not due to mechanical action itself but to the electrical stimulation induced by the piezoelectric effect in the poled electroactive PVDF substrate. It is worthy of note that results corresponding to the first days of culture must be considered since proliferation rate of these cells is high and cultures reach confluence in a short culture time (in just five days in poled PVDF substrates), thus, proliferation tends to be similar in all membranes and in all conditions after 5 days of growth. An interesting exception is non-coated and non-poled β-PVDF substrates, in which proliferation is clearly slower than that observed for the control samples, as seen in fluorescence images such as those shown in Fig. 4 and in MTT measurements (Fig. 5). This feature was already demonstrated in our previous investigation,14 showing a significant difference between fibronectin adsorption on poled and non-poled substrates and significantly smaller cell numbers in non-poled β-PVDF with respect to both negatively or positively charged PVDF surfaces.
Titanium coated samples allow the same conclusion to be reached. The titanium layer has a positive effect in poled samples, which is significant in the first day although diminishes over longer culture periods. In the case of non-poled samples, proliferation of the titanium coated samples, non-poled β-PVDF with titanium samples, is like that of control TCPS wells, thus clearly improving proliferation with respect to non-coated samples. In the case of poled samples the titanium layer increases roughness but also increases hydrophobicity, two factors that are expected to affect cell proliferation in opposite ways. However, certainly the main effect of titanium coating in these samples is the improvement of charge surface distribution.
5. Conclusions
Piezoelectric poly(vinylidene fluoride) has been studied as a suitable material for tissue engineering applications due to its piezoelectric effect. In order to isolate the piezoelectric effect on cell response, poled and non-poled material, as well as material coated with a thin titanium layer to obtain a more homogeneous charge distribution, has been tested in osteoblasts under static and dynamic conditions. The polarization and titanium layer deposition modifies mean roughness of the PVDF film surface and therefore cell adhesion and proliferation on the samples. Osteoblast adhesion and proliferation is different depending on the samples, adhesion being more influenced by the piezoelectric material. The positive charge of β-PVDF promotes higher adhesion and proliferation on osteoblasts. Dynamic culture with MC3T3-E1 cells showed higher cell proliferation on “poled +” β-PVDF. In this way, these results demonstrated that varying surface electrical charge when a mechanical perturbation is applied influences cell response and confirms the potential of electroactive polymers for cell culture and tissue engineering by providing the necessary electrical stimuli for the growth and proliferation of specific cells.Acknowledgements
This work is funded by FEDER funds through the “Programa Operacional Factores de Competitividade—COMPETE” and by national funds by FCT- Fundação para a Ciência e a Tecnologia, project reference NANO/NMed-SD/0156/2007. C. R. thanks the INL for a PhD grant. V.S. thanks the FCT for the SFRH/BPD/63148/2009 grants. Authors thank Armando Ferreira and Filipe Vaz for the help with titanium deposition on PVDF films.JLGR acknowledge the support of the Spanish Ministry of Education through project No. MAT2010-21611-C03-01 (including the FEDER financial support) and project EUI2008-00126.
References
- K. Anselme, Biomaterials, 2000, 21, 667–681 CrossRef CAS.
- Y. Chen, M. R. Cho, A. F. T. Mak, J. S. Li, M. Wang and S. Sun, J. Mater. Sci.: Mater. Med., 2008, 19, 2563–2567 CrossRef CAS.
- Y.-S. Lee and T. L. Arinzeh, Tissue Eng. Part A, 2012, 19–20, 2063–2072 CrossRef.
- S. Tofail, Biological Interactions with Surface Charge in Biomaterials, Royal Society of Chemistry, Cambridge, UK, 2011 Search PubMed.
- H.-S. Huag, S.-H. Chou, T.-M. Don, W.-C. Lai and L.-P. Cheng, Polym. Adv. Technol., 2009, 20, 1082–1090 CrossRef CAS.
- H.-I. Chang and Y. Wang, in Regenerative Medicine and Tissue Engineering – Cells and Biomaterials, ed. D. Eberli, InTech, 2011, ch. 27 Search PubMed.
- T. P. Kunzler, T. Drobek, M. Schuler and N. D. Spencer, Biomaterials, 2007, 28, 2175–2182 CrossRef CAS.
- R. J. Kroeze, M. N. Helder, L. E. Govaert and T. H. Smit, Materials, 2009, 2, 833–856 CrossRef CAS.
- N. Weber, Y. S. Lee, S. Shanmugasundaram, M. Jaffe and T. L. Arinzeh, Acta Biomater., 2010, 6, 3550–3556 CrossRef CAS.
- A. J. Lovinger, Developments in Crystalline Polymers, Elsevier Applied Science, London, 1982 Search PubMed.
- D. Verma, K. S. Katti and D. R. Katti, Philos. Trans. R. Soc. London, Ser. A, 2010, 368, 2083–2097 CrossRef CAS.
- I. F. Amaral, A. L. Cordeiro, P. Sampaio and M. A. Barbosa, J. Biomater. Sci., Polym. Ed., 2007, 18, 469–485 CrossRef CAS.
- G. B. Schneider, A. English, M. Abraham, R. Zaharias, C. Stanford and J. Keller, Biomaterials, 2004, 25, 3023–3028 CrossRef CAS.
- C. Ribeiro, J. A. Panadero, V. Sencadas, S. Lanceros-Méndez, M. N. Tamaño, D. Moratal, M. Salmerón-Sánchez and J. L. G. Ribelles, Biomed. Mater., 2012, 7, 035004 CrossRef CAS.
- J. Gomes, Smart Mater. Struct., 2010, 19, 065010 CrossRef.
- J. S. Nunes, A. Wu, J. Gomes, V. Sencadas, P. M. Vilarinho and S. Lanceros-Mendez, Appl. Phys. A: Mater. Sci. Process., 2009, 95, 875–880 CrossRef.
- C. Ribeiro, V. Sencadas, J. L. G. Ribelles and S. Lanceros-Mendez, Soft Mater., 2010, 8, 274–287 CrossRef CAS.
- A. California, V. F. Cardoso, C. M. Costa, V. Sencadas, G. Botelho, J. L. Gomez-Ribelles and S. Lanceros-Mendez, Eur. Polym. J., 2011, 47, 2442–2450 CrossRef CAS.
- H. S. Nalwa, Ferroelectric Polymers: Chemistry, Physics and Applications, Marcel Dekker, Inc., New York, 1995 Search PubMed.
- M. T. Rodrigues, M. E. Gomes, J. F. Mano and R. L. Reis, in Advanced Materials Forum Iv, ed. A. T. Marques, A. F. Silva, A. P. M. Baptista, C. Sa, F. Alves, L. F. Malheiros and M. Vieira, Trans Tech Publications Ltd, Stafa-Zurich, 2008, vol. 587–588, pp. 72–76 Search PubMed.
- M. C. Branciforti, V. Sencadas, S. Lanceros-Mendez and R. Gregorio, J. Polym. Sci., Part B: Polym. Phys., 2007, 45, 2793–2801 CrossRef CAS.
- V. Sencadas, R. Gregorio and S. Lanceros-Mendez, J. Macromol. Sci., Part B: Phys., 2009, 48, 514–525 CrossRef CAS.
- J. I. Rosales-Leal, M. A. Rodriguez-Valverde, G. Mazzaglia, P. J. Ramon-Torregrosa, L. Diaz-Rodriguez, O. Garcia-Martinez, M. Vallecillo-Capilla, C. Ruiz and M. A. Cabrerizo-Vilchez, Colloids Surf., A, 2010, 365, 222–229 CrossRef CAS.
- G. B. Sigal, M. Mrksich and G. M. Whitesides, J. Am. Chem. Soc., 1998, 120, 3464–3473 CrossRef CAS.
- P. B. Vanwachem, T. Beugeling, J. Feijen, A. Bantjes, J. P. Detmers and W. G. Vanaken, Biomaterials, 1985, 6, 403–408 CrossRef CAS.
- S. Bodhak, S. Bose and A. Bandyopadhyay, Acta Biomater., 2009, 5, 2178–2188 CrossRef CAS.
- R. N. Wenzel, Ind. Eng. Chem., 1936, 28, 988–994 CrossRef CAS.
- S. J. Marshall, S. C. Bayne, R. Baier, A. P. Tomsia and G. W. Marshall, Dent. Mater., 2010, 26, E11–E16 CrossRef.
- E. Fukada and I. Yasuda, J. Phys. Soc. Jpn., 1957, 12, 1158–1162 CrossRef.
- J. J. Ficat, M. Thiechart, P. Ficat, C. Lacabanne, F. Micheron and I. Bab, Ferroelectrics, 1984, 60, 313–316 CrossRef CAS.
- S. B. Lang, S. A. M. Tofail, A. A. Gandhi, M. Gregor, C. Wolf-Brandstetter, J. Kost, S. Bauer and M. Krause, Appl. Phys. Lett., 2011, 98 Search PubMed.
- N. C. Teng, S. Nakamura, Y. Takagi, Y. Yamashita, M. Ohgaki and K. Yamashita, J. Dent. Res., 2001, 80, 1925–1929 CrossRef CAS.
- Y. Q. Wan, Y. Wang, Z. M. Liu, X. Qu, B. X. Han, J. Z. Bei and S. G. Wang, Biomaterials, 2005, 26, 4453–4459 CrossRef CAS.
- M. S. Lord, M. Foss and F. Besenbacher, Nano Today, 2010, 5, 66–78 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
