Mechanism of supported bilayer formation of zwitterionic lipids on SiO2 nanoparticles and structure of the stable colloids
Hairong
Wang
a,
Jelena
Drazenovic
a,
Zhenyu
Luo
a,
Jiangyue
Zhang
b,
Hongwen
Zhou
a and
Stephanie L.
Wunder
*a
aDepartment of Chemistry, Temple University, Philadelphia, PA 19122. E-mail: slwunder@temple.edu
bDepartment of Chemistry, Immaculata University, Immaculata, PA 19345
First published on 17th October 2012
Abstract
Bare, chemically surface modified and supported lipid bilayer (SLB) nanoparticles are finding expanding uses in pharmaceutical, biomedical and materials applications, where in vivo, in vitro or in the environment they come into contact with lipids in the form of cells or cellular debris, and where clustering of the nanoparticles can affect dosages and cellular uptake. Here, the mechanism of formation, nano-structure, state of aggregation and colloidal stability of SLBs for a model system consisting of ∼100 nm silica (SiO2) nanoparticles and zwitterionic lipids in the form of ∼100 nm small unilamellar vesicles (SUVs) is investigated at low and high ionic strengths, as a function of the surface area (SA) ratios of the SUVs (SASUV) and SiO2 (SASiO2). Formation of single-SLBs is suggested to be a bi-molecular collision event, both above and below the lipid phase transition temperature (Tm), between negatively charged SiO2 and neutral SUVs, with rupture of SUVs occurring at the vesicle sides where there is a small radius of curvature, followed by continued wrapping of the SiO2. At low ionic strength (5 mM NaCl), colloidal metastability occurs at SASUV/SASiO2 ≥ 1/1 due to residual electrostatic repulsion, and the nanosystem exists as independent, non-interacting particles. At high ionic strength (PBS buffer), precipitation occurs at SASUV/SASiO2 = 1/1 due to charge shielding, but at SASUV/SASiO2 ≥ 2/1, excess SUVs adsorb onto the SLBs at defects sites (bare SiO2) in the first SLB, restoring undulatory/protrusion forces, and thus colloidal metastability, by portions of the SUVs not in contact with the SLBs; for SASUV/SASiO2 = 2/1 there is an approximate 1/1 pairing of SUVs and SLBs above Tm, while below Tm, aggregation of larger but meta-stable structures occurs. When the SASUV/SASiO2 = 2/1 nanosystems in PBS buffer are reheated to a temperature above Tm, separate, non-interacting particles are formed, suggesting that SUVs trapped on SiO2 defect sites are pinched off to form a SLB in the defect area, expelling a smaller SUV.
Introduction
Nanoparticles such as SiO2 may be taken in vivo as part of drug/gene delivery systems or can be found in the environment after use, where they may pose dangers and can enter the food chain.1–3 In both cases, the nanoparticles are exposed to lipids in the form of cells or cellular debris, although there will be competition from organic matter. As a result of their high specific surface area, the surface properties of nanoparticles determine their interactions with lipid bilayers and cells,4 which in turn can affect their in vitro cytotoxicity5,6 and in vivo distribution, excretion and toxicity.7–9 Nanoparticle uptake in cells can occur by a process of endocytosis, in which the nanoparticles are enclosed by a surrounding membrane. This process is therefore similar to supported lipid bilayer (SLB) formation around nanoparticles, if mediation via proteins or cell-penetrating peptides is excluded. Nanoparticle uptake in cells has been observed to be size-dependent10,11 and to scale with the surface area,12 while nanoparticle settling13 or aggregation may affect the interpretation of results of in vitro or in vivo studies.3,4 In applications that depend on nanoparticle dosage, the calculated number density of nanoparticles may not reflect true dosages if there is aggregation. Since proteins can absorb to nanoparticles and affect their biological response,14 neutral ligands such as polyethylene glycol (PEG),15 hydroxyl groups,16 and zwitterionic ligands17 are used to inhibit non-specific protein adsorption and cell binding/uptake, and this effect can also occur when zwitterionic lipids surround nanoparticles.18 It has been observed that after formation of a zwitterionic phosphatidyl choline single-supported lipid bilayer around SiO2 nanoparticles, further engulfment by another vesicle did not occur.19The mechanism of formation of supported lipid bilayers from zwitterionic lipids on planar substrates has been extensively investigated and reviewed20,21 and has been shown to depend on the type of substrate,22–24 surface charge density,25 average phospholipid curvature,26 the ionic strength of the medium,27–30 the charge of the lipids31 and the buffer.25,32 While similar considerations are expected for SLB formation on nanoparticles, the mechanism is likely to be different. On planar substrates, SLB formation can occur via adsorption and deformation of the small unilamellar vesicles (SUVs) followed by fusion to form a SLB, or a critical density of adsorbed SUVs, independent of vesicle size,22 can be required before SLB formation.20,33 When the SUV is larger or comparable in size to the substrate dimensions, as is the case for nanoparticles, the latter mechanism is unlikely, and a mechanism more similar to that of passive endocytosis should occur. Further, nanoparticle aggregation before and/or after SLB formation, and the colloidal stability of the nano-systems are important considerations in many applications. Quantification of the amount of adsorbed lipid, often using difference spectroscopy methods, does not provide information on the amount of supported lipid bilayer formation, aggregation, or adsorption of excess vesicles.
We previously investigated supported lipid bilayer formation on SiO2 nanoparticles using zwitterionic phosphatidylcholine lipids, and found that in cases where the amount of lipid was just sufficient to form a single-SLB, i.e. where the surface area of the lipid (SASUV) matched the surface area of the SiO2 (SASiO2) so that SASUV/SASiO2 = 1/1, the SLBs were colloidally stable (at least on the order of hours) at low ionic strengths, but precipitated at high ionic strengths.34 Further, addition of excess lipid (SASUV/SASiO2 = 2/1) was found to stabilize the suspensions.35 In the case of equimolar mixtures of the zwitterionic lipid 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and the cationic lipid 1,2-dimyristoyl-3-trimethylammonium-propane (chloride salt) (DMTAP), single-SLB formation occurred with concurrent precipitation at high ionic strength. In the presence of excess lipid (SASUV/SASiO2 = 2/1), the nanosystem was colloidally stable above the gel-to-liquid phase transition temperature, Tm, of the mixed lipids, but precipitated immediately below Tm. The precipitates consisted of SLB aggregates surrounded by a contiguous close-fitting bilayer sheath.36
Here we investigate supported lipid bilayers formed from 100 nm SiO2 nanoparticles with ∼100 nm SUVs prepared with the zwitterionic lipid 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), when the SASUV/SASiO2 is increased from 1/1 to 4/1, both at low (5 mM NaCl) and high (0.08 M PBS buffer, 109 mM NaCl, 2 mM KCl) ionic strengths. The mechanism of formation, nano-structure, state of aggregation and colloidal stability of SLBs is investigated using dynamic light scattering, nano-differential scanning calorimetry and transmission electron microscopy (TEM) in order to understand the morphology of the nano-systems, the mechanism of colloidal stabilization and to determine conditions where separation of SUVs and SLBs is possible.
Experimental
Materials
1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 PC) was obtained from Avanti Polar Lipids (Alabaster, AL) and used without further purification. Snowtex™ colloidal silica (SiO2) nanoparticle suspensions, with densities of 2.2–2.6 g cm−3 (reported by manufacturer) and prepared by a water glass process, were a gift from Nissan Chemical America (Houston, TX). The nominal diameter of the SiO2 was 100 nm (MP-1040, 40.7 wt% SiO2, lot 200
0 PC) was obtained from Avanti Polar Lipids (Alabaster, AL) and used without further purification. Snowtex™ colloidal silica (SiO2) nanoparticle suspensions, with densities of 2.2–2.6 g cm−3 (reported by manufacturer) and prepared by a water glass process, were a gift from Nissan Chemical America (Houston, TX). The nominal diameter of the SiO2 was 100 nm (MP-1040, 40.7 wt% SiO2, lot 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 109, pH 9.3, specific gravity 1.300). All solutions/suspensions were prepared with HPLC grade water, chloroform, NaCl (5 mM) or 0.08 M phosphate buffer saline (PBS with 109 mM NaCl and 2 mM KCl) purchased from Fisher Chemicals (Fairlawn, NJ). An Avanti Mini-Extruder from Avanti Polar Lipids was employed for extrusion of the lipids, using 100 nm polycarbonate filters.
109, pH 9.3, specific gravity 1.300). All solutions/suspensions were prepared with HPLC grade water, chloroform, NaCl (5 mM) or 0.08 M phosphate buffer saline (PBS with 109 mM NaCl and 2 mM KCl) purchased from Fisher Chemicals (Fairlawn, NJ). An Avanti Mini-Extruder from Avanti Polar Lipids was employed for extrusion of the lipids, using 100 nm polycarbonate filters.
Preparation of suspensions
Dry lipid films were formed after evaporation from chloroform solutions under a stream of nitrogen and then in a vacuum oven overnight to remove any residual solvent. The lipid films were redispersed in 5 mM NaCl solution or PBS buffer at a concentration of 10 mg ml−1 and incubated at a temperature of 45 °C, above the Tm (23 °C) of DMPC for ∼2 h with periodic shaking to form hydrated multilamellar vesicles (MLVs). Small unilamellar vesicles (SUVs) were obtained from MLVs by subjecting them to 5 freeze/thaw cycles followed by extrusion using polycarbonate filters with 100 nm pores. Approximately 1 ml of a 10 mg ml−1 lipid solution was passed back and forth for up to 20 times, until a clear suspension was formed. LC/MS was used to confirm that there was no loss of lipid during this process (99.6% SUV lipids were collected from MLVs lipids after extrusion). Additional 5 mM salt solution or PBS buffer was added to the extrusion product to yield vesicle suspensions of ∼2 mg ml−1 lipid.The vesicles and nanobeads were mixed (in equal volumes) and incubated at 45 °C or 10 °C for 1 h. The amount of lipid required to achieve single bilayer coverage was calculated using the surface area occupied by the lipid headgroup (0.59 nm2 for DMPC) and the total surface area of the nanoparticles, with the assumption that the latter was a planar surface and using a value for the density of 2.4 g cm−3. The amount of lipid required for single bilayer coverage of the nanoparticles is achieved when the surface area of the SUVs (SASUV) was equal to the surface area of the SiO2 (SASiO2), SASUV/SASiO2 = 1; other coverages will be referred to as multiples of this amount. The suspensions were investigated as prepared, as a function of time, or the supernates/precipitates investigated after centrifugation at different speeds (7000 rpm or 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 rpm) and times (4 or 8 min). The supernate was removed from the pelleted material, and the pellets rinsed, so that the compositions of the supernate and precipitate could be separately analyzed. For the high ionic strength precipitates (also called pellets), after the original supernate was removed, additional buffer was added without disturbing the pellet and also removed, or the pellet was agitated/stirred with successive removal of the supernate.
200 rpm) and times (4 or 8 min). The supernate was removed from the pelleted material, and the pellets rinsed, so that the compositions of the supernate and precipitate could be separately analyzed. For the high ionic strength precipitates (also called pellets), after the original supernate was removed, additional buffer was added without disturbing the pellet and also removed, or the pellet was agitated/stirred with successive removal of the supernate.
Analysis
Results
Low ionic strength conditions (5 mM NaCl)
At low (5 mM NaCl) ionic strength, colloidal stability was maintained during the time scale (∼ days) of the experiments both above and below Tm at SASUV/SASiO2 ≥ 1/1 as previously observed.34,35 The zeta potential (ζ) was ζ ≈ 0 for the SUVs, ζ ≈ −40 mV for the 100 nm SiO2 and ζ ≈ −22 mV for the single-SLBs (SASUV/SASiO2 = 1).34 The negative zeta potential for the single-SLBs (SASUV/SASiO2 = 1/1) was sufficient to maintain colloidal stability.Nano-DSC data for the DMPC SUVs and nano-systems formed with DMPC SUVs and SiO2 at SASUV/SASiO2 = 1/1 and 2/1 at low ionic strength after incubation at 45 °C for 1 h is shown in Fig. 1 (left). Tm of the SUV peak at 24.2 °C is easily distinguished from that of lipids forming a supported lipid bilayer, which occurs at 21.9 °C, as has been previously reported.37,38 When SASUV/SASiO2 = 1/1, only the SLB transition is seen, while at SASUV/SASiO2 = 2/1, both transitions are observed. As will be discussed further below, SLB formation also occurred for SASUV/SASiO2 = 1/1 below Tm, as shown in Fig. 1 (right).
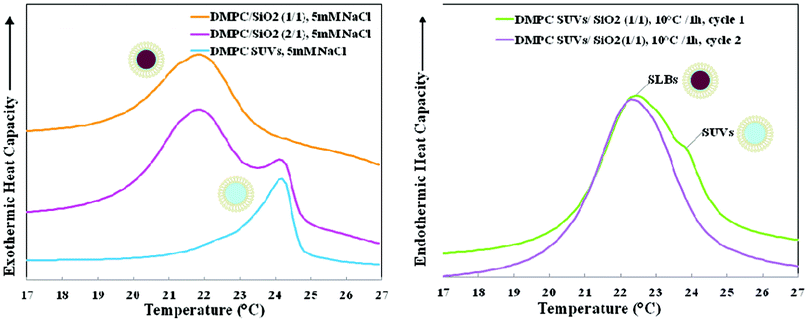 | ||
| Fig. 1 (left). Nano-DSC data for 5 mM NaCl suspensions of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC): small unilamellar vesicles (SUVs), nano-systems formed at SASUV/SASiO2 = 2/1, and supported lipid bilayers (SLBs) formed at SASUV/SASiO2 = 1/1 after incubation at 45 °C/1 h; (right). Nano-DSC scans of DMPC SUVs and SiO2 in 5 mM NaCl, cooled separately to 10 °C, mixed at a ratio of SASUV/SASiO2 = 1/1 at 10 °C, incubated in the nano-DSC at 10 °C for 1 h and scanned twice. | ||
DLS data (volume averages) of the DMPC SUVs, nominal 100 nm SiO2 and DMPC SUVs incubated with nominal 100 nm SiO2 at SASUV/SASiO2 = 1/1 and 2/1 at 45 °C/1 h is shown at 30 °C at low ionic strength in Fig. 2a. Nominal 100 nm SiO2 nanoparticles (diameter of ∼119 nm) increase in size after fusion with SUVs (∼93 nm) to form SLBs (∼136 nm). The size increase is slightly larger than expected based on the addition of a bilayer to the nanoparticle radius (bilayer thickness is ∼4–5 nm). This may arise since DLS measures the hydrodynamic radius, which includes the hydration sphere (surrounding water and salt). For SASUV/SASiO2 = 2/1, the size (∼124 nm) is intermediate between that of the SLBs (∼136 nm) and SUVs (93 nm). This strongly suggests that there is a mixed population of approximately equal numbers of SUVs (93 nm) and SLBs (136 nm).
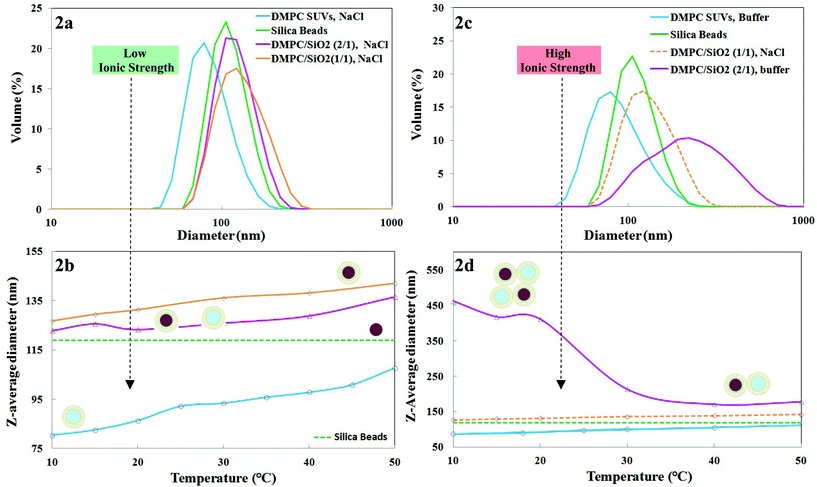 | ||
| Fig. 2 (a). Dynamic light scattering (DLS) data at 30 °C at low ionic strength (5 mM NaCl) for 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC): small unilamellar vesicles (SUVs), nominal 100 nm SiO2, supported lipid bilayers (SLBs) formed at SASUV/SASiO2 = 1/1, nano-systems formed at SASUV/SASiO2 = 2/1; and (b) their temperature dependent sizes. Dashed line indicates DLS data for nominal 100 nm SiO2 obtained at room temperature in 5 mM NaCl. Schematic of proposed structures shown alongside the plots; (c). DLS data at 30 °C at high ionic strength (PBS buffer) for DMPC: small unilamellar vesicles (SUVs), nominal 100 nm SiO2, SLBs formed at SASUV/SASiO2 = 1/1 (taken from 2a, since precipitation occurs in PBS buffer), nano-systems formed at SASUV/SASiO2 = 2/1; and (d) their temperature dependent sizes. Dashed line indicates DLS data for nominal 100 nm SiO2 obtained at room temperature in PBS buffer. Schematic of proposed structures shown alongside the plots. | ||
The temperature dependence of the z-average diameters of the SUVs and nano-systems with SASUV/SASiO2 = 1/1 and 2/1 is shown in Fig. 2b for low ionic strength conditions. The size of the SiO2 is not expected to be temperature dependent and is shown as a dashed line. The SUVs and SLBs formed at SASUV/SASiO2 = 1/1 increase in size with increase in temperature with a change in slope at Tm, which is more apparent for the SUVs. The bilayer itself has been shown to thin and widen with increasing temperature: the bilayer thickness decreases from the gel (Lgel = 43 Å2) to the liquid crystalline (Lliqxtal = 35 Å2) phase, while the area/molecule increases from the gel (Agel = 47 Å2) to the liquid crystalline phase (Aliqxtal = 47 Å2).39 As was observed for the nano-system at 30 °C (Fig. 2a), the temperature dependent increase in size for the SASUV/SASiO2 = 2/1 nano-system is intermediate between the constituent SUVs and SLBs at all temperatures, both above and below Tm, indicating a mixed population of SUVs and SLBs. At low ionic strength, the populations of SUVs and SLBs were separately stable and remained so when mixed. Further, at low ionic strength there does not appear to be hysteresis in the nanosystem on short time scales: raising the temperature from 10 °C to 45 °C results in the same diameters previously observed for the SUVs and SLBs at SASUV/SASiO2 = 1/1 and 2/1.
TEM images for nanosysems prepared at low ionic strength above Tm, show supported lipid bilayer formation at high and low resolution for SASUV/SASiO2 = 1/1 (Fig. 3A1). TEM images prepared above Tm of the lipids at SASUV/SASiO2 = 2/1 (Fig. 3A2) confirm that the SUVs and SLBs were non-interacting, and show regions on the same grid where there are only SLBs, and other regions where there are only fused vesicles and lipid fragments, supporting the independence of the SUVs and SLBs, i.e. the lack of SUV adsorption to the SLBs. TEM images of lipids without SiO2 are the same as shown in Fig. 3A2 right; the SiO2 support acts to prevent lipid collapse.
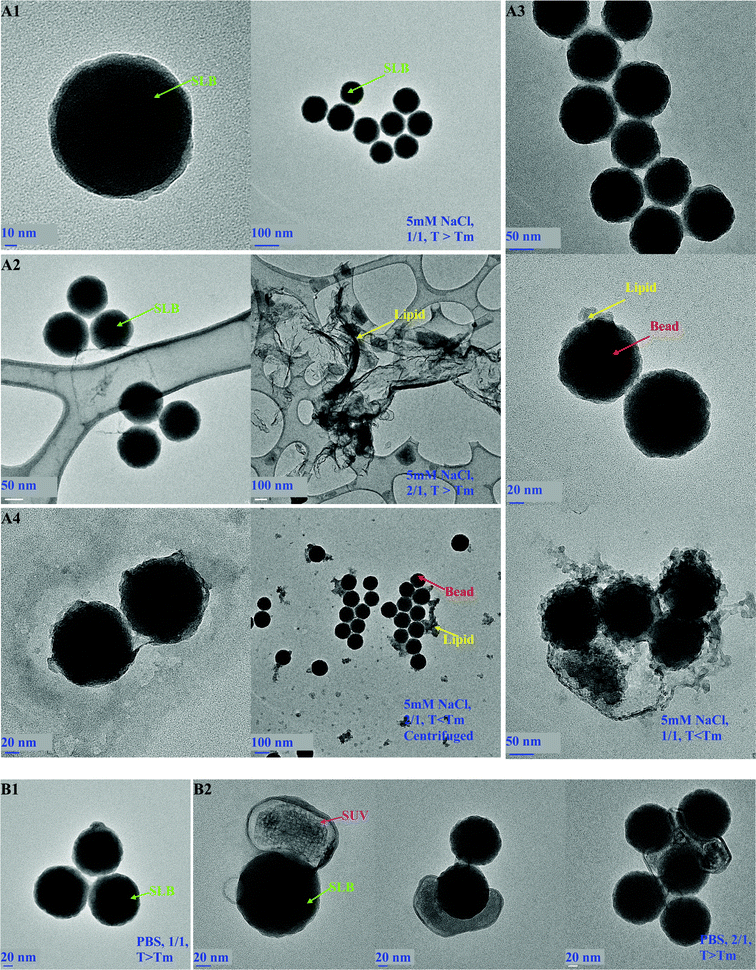 | ||
| Fig. 3 TEM images of DMPC nano-systems prepared at: (A) low ionic strength (5 mM NaCl): (A1) single-SLBs, SASUV/SASiO2 = 1/1, prepared above Tm at high and low resolution; (A2) SASUV/SASiO2 = 2/1, prepared above Tm, showing separate regions of SLBs and fused SUVs on same TEM grid; (A3) SASUV/SASiO2 = 1/1 below Tm (10 °C) showing SLBs with desorbed lipid; (A4) SASUV/SASiO2 = 2/1 centrifuged below Tm (10 °C) showing SLBs with desorbed lipid; (B) high ionic strength above Tm: (B1) SASUV/SASiO2 = 1/1 showing aggregates of SLBs; (B2) SASUV/SASiO2 = 2/1 showing adsorbed SUVs. | ||
However, at low ionic strength, the SLBs are not stable below Tm when stored. In contrast with TEM images of SLBs formed at SASUV/SASiO2 = 1/1 above Tm (Fig. 3A1 and 3A2), which exhibit intact SLBs, TEM images of SASUV/SASiO2 = 1/1 samples kept below Tm, at 10 °C for ∼1 h exhibit debonding of the lipid layer from the SiO2 (Fig. 3 A3). Similarly, for low ionic strength SASUV/SASiO2 = 2/1 suspensions centrifuged below Tm, the centrifuged pellet with the supernate removed also shows lipid debonding from the SiO2 (Fig. 3 A4). This may occur due to the decrease in lipid volume (and area) when the SLB is first cooled below Tm. Upon reheating, as occurs when the lipids are drying on the TEM grids, local expansion of the lipid area on the SLB may result in “buckling” and removal of the lipid from the grid. Instabilities resulting from temperature changes have also been observed for DMPC on planar SiO2 surfaces.40 Desorption of lipid from planar substrates, particularly in unsaturated solutions and water, has also been observed.41
Nano-DSC data for low ionic strength nanosystems at SASUV/SASiO2 = 1/1 and SASUV/SASiO2 = 2/1 was consistent with the TEM data, i.e. only a SLB transition for the former and both SLB and SUV transitions for the latter (Fig. 1 left). However, for the SASUV/SASiO2 = 1/1 low ionic strength suspensions held below Tm (at 10 °C) in the nanoDSC, we expected, but did not observe, both a SUV and a SLB transition upon heating (since we observed lipid desorption in the TEM images). We speculated that lipid fusion was occurring below Tm during the heating scan, at odds with conventional wisdom that fusion occurs preferentially above Tm. In order to confirm that SUV fusion occurred below Tm, SiO2 and SUVs were cooled separately to 10 °C, mixed and held at 10 °C for 1 h in the nano-DSC. The subsequent nano-DSC scan (Fig. 1 right) confirmed that fusion in fact occurred below Tm, as shown by the large SLB and small SUV peak observed initially; the single SLB peak observed after the second DSC cycle results from heating the sample above Tm.
High ionic strength conditions (PBS buffer)
In PBS buffer, the nano-system with SASUV/SASiO2 = 1/1 precipitates rapidly (< seconds) above and below (slightly longer, but still seconds) Tm. With the addition of “extra” lipid e.g. 1.1–1.3/1 in PBS buffer, the samples still precipitate. Nano-DSC data confirms supported lipid bilayer formation, and TEM images (Fig. 3B1) show individual SLBs, not clusters of SiO2 nanoparticles surrounded by lipid. This suggests that the SLB formation occurs as a bimolecular collision between a SiO2 nanoparticle and an SUV, and confirms the low ionic strength results that SLB formation below Tm does occur.In contrast, in PBS buffer the nano-system with SASUV/SASiO2 = 2/1 remains colloidally metastable both above and below Tm. Suspensions kept at 45 °C were stable for 3 days, those kept at 10 °C were stable for ∼4 h, and those cooled to 10 °C/1 h and then kept at 45 °C were stable for ∼12 h, after which time “settling” occurred for each. However, uniform suspensions could be reformed after shaking.
DLS data for the SUVs, SiO2 and freshly prepared, uniform suspensions formed from SASUV/SASiO2 = 2/1 is shown in Fig. 2c and temperature dependent sizes are presented in Fig. 2d. The SUVs themselves exhibit the same increase in size with increase in temperature observed for the SUVs at low ionic strength (Fig. 2a). The SASUV/SASiO2 = 1/1 nano-system precipitates and thus cannot be measured by DLS, but is expected to be similar to the low ionic strength nano-system; the SASUV/SASiO2 = 1/1 low ionic strength data is included for comparison in Fig. 2c and d. However, the SASUV/SASiO2 = 2/1 high ionic strength nano-systems (Fig. 2d) exhibit quite different behavior compared with the low ionic strength nano-systems (Fig. 2a). Above Tm the z-average diameter of the nanoparticles is ∼200 nm, almost the size of a single SLB and an SUV. Unlike the case for the 5 mM NaCl suspensions, the size measured is greater than that of the SUVs, SiO2 or SLBs (where a size similar to 5 mM NaCl expected). This strongly suggests that the size increase above Tm is the result of SUV adsorption onto the SLBs. TEM images (Fig. 3 B2) show SUVs flattened and partially surrounding the SLBs and aggregates with SUVs between the SLBs. While cryo-TEM data would more clearly demonstrate this effect we note here that these types of images were never observed for the SASUV/SASiO2 = 2/1 low ionic strength system. The contours of the SUVs around the SLBs suggest that the adsorption has occurred before drying of the nano-system (but does not eliminate the possibility that the SUVs collapsed onto the SLB during the drying process).
Below Tm for the high ionic strength SASUV/SASiO2 = 2/1 nano-system (Fig. 2d), the diameter increases even more steeply with decrease in temperature, strongly indicating that further aggregation is occurring, although the nano-system remains stable (for ∼4 h). Reheating the high ionic strength SASUV/SASiO2 = 2/1 nano-system kept at 10 °C/1 h to above Tm (25 °C or 45 °C), results in “break-up” of the larger aggregates to a size similar to a system of approximately equal size SLBs and SUVs, the same as observed for low ionic strength SASUV/SASiO2 = 2/1 nanosystems (Fig. 2a).
It is interesting to compare sizes measured above and below Tm using different size averages, e.g. z-, intensity-, volume- and number- averages. The z-average represents the size the system would have if the particles consisted of single diameter nanoparticles. When the system contains nanoparticles of different sizes, the greater scattering intensity of larger nanoparticles is taken into account in the cumulants analysis program. Fig. 4 left presents intensity, volume and number averages from the DLS data for SASUV/SASiO2 = 2/1 nanosystems at low and high ionic strengths at 50 °C, and the same data at 10 °C (Fig. 4 right). For low ionic strength nanosystems, the high and low temperature data analyzed using any of the measures show a single, narrow peak, where size (intensity) > size (volume) > size (number). This is expected since in the distribution any larger nanoparticles will scatter more light than the smaller nanoparticles, although there can be a greater number of the smaller nanoparticles. For the high ionic strength SASUV/SASiO2 = 2/1 nanosystems at 50 °C (Fig. 4 left), a single size distribution is also observed, which is broader by any comparable measure (intensity, volume, number) than for the low ionic strength SASUV/SASiO2 = 2/1 nanosystem. By intensity and volume, the size is ∼2× that for low ionic strength, suggesting that the aggregates at high ionic strength consist of a SLB with an adsorbed SUV. The number average at high ionic strength has a high molecular weight tail and a population of nanoparticles of the same size as the low ionic strength nanosystem. In this case, for example, 200 nm aggregates would contain ∼4 primary (125 nm) particles, and the 150 nm aggregates would contain ∼1.7 primary (125 nm) particles, as observed in the TEM images (Fig. 3B2). This highlights why the volume averages most closely represents the actual distribution in the nanosystem. At 10 °C and high ionic strength SASUV/SASiO2 = 2/1 nanosystems, two populations are observed by intensity, volume and number, representing both the original SUV-SLB “dimer” and larger aggregates.
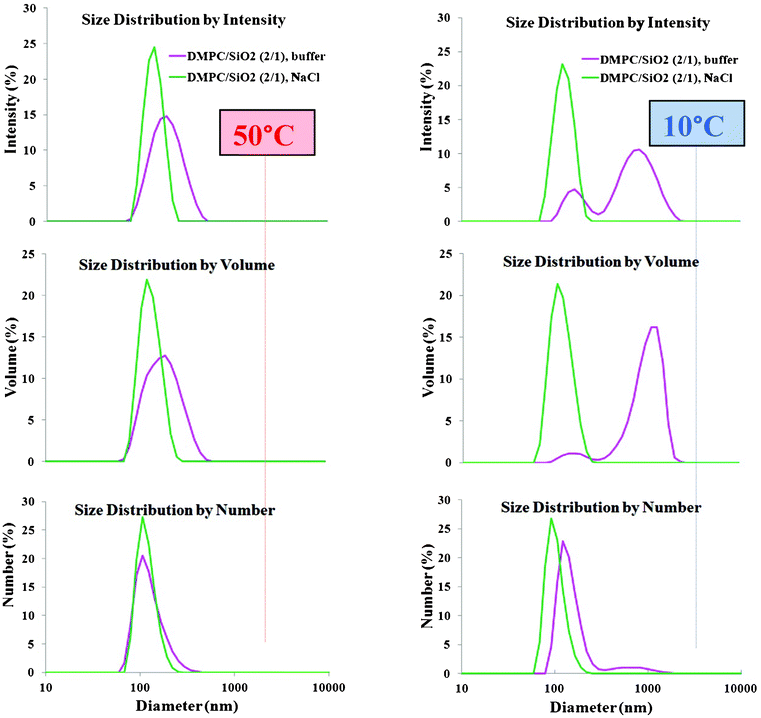 | ||
| Fig. 4 DLS analyzed by intensity, volume and number average for low (5 mM NaCl) and high (PBS buffer) ionic strength suspension with SASUV/SASiO2 = 2/1 at 50 °C and 10 °C. | ||
Separation of SUVs from SLBs
In order to determine whether the SUVs and SLBs could be separated, SASUV/SASiO2 = 2/1 or 4/1 nanosystems were centrifuged at speeds between 7000–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 rpm for different times either above or below Tm at high and low ionic strengths. The supernate/pellet was evaluated by nanoDSC and TEM images for evidence of SLBs in the supernate and SUVs in the pellet. In PBS buffer, it was always possible to find conditions for which no SLBs existed in the supernate, as monitored both by TEM images and nano-DSC data for the supernate (not shown). The pellet that formed at high ionic strength was always “sticky”, consistent with the association of the SUVs and SLBs. The nano-DSC scans (cooling cycle) of the pellets formed from SASUV/SASiO2 = 2/1 nanosystems at high ionic strength obtained after centrifugation above (T = 45 °C) and below (T = 10 °C) Tm, and after rinsing the pellet at 45 °C are compared with that of the original mixture (Fig. 5). The traces show that more SUVs are pelleted at low compared with high temperatures, and that rinsing the pellet at high temperature with agitation/stirring succeeded in removal of the SUVs. TEM images for high ionic strength SASUV/SASiO2 = 4/1 nanosystems, a more stringent test of SUV removal, confirm that SUVs originally adsorbed onto the SLBs are pelleted with them, but can be removed with successive rinsing/agitation (Fig. 6).
200 rpm for different times either above or below Tm at high and low ionic strengths. The supernate/pellet was evaluated by nanoDSC and TEM images for evidence of SLBs in the supernate and SUVs in the pellet. In PBS buffer, it was always possible to find conditions for which no SLBs existed in the supernate, as monitored both by TEM images and nano-DSC data for the supernate (not shown). The pellet that formed at high ionic strength was always “sticky”, consistent with the association of the SUVs and SLBs. The nano-DSC scans (cooling cycle) of the pellets formed from SASUV/SASiO2 = 2/1 nanosystems at high ionic strength obtained after centrifugation above (T = 45 °C) and below (T = 10 °C) Tm, and after rinsing the pellet at 45 °C are compared with that of the original mixture (Fig. 5). The traces show that more SUVs are pelleted at low compared with high temperatures, and that rinsing the pellet at high temperature with agitation/stirring succeeded in removal of the SUVs. TEM images for high ionic strength SASUV/SASiO2 = 4/1 nanosystems, a more stringent test of SUV removal, confirm that SUVs originally adsorbed onto the SLBs are pelleted with them, but can be removed with successive rinsing/agitation (Fig. 6).
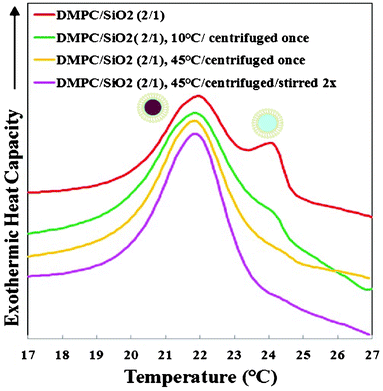 | ||
| Fig. 5 Nano-DSC data for nanosystems formed from DMPC SUVs and 100 nm SiO2 at SASUV/SASiO2 = 2/1: as prepared (showing both SLBs and SUVs); the pellet obtained after centrifugation at 10 °C with supernate removed; the pellet obtained after centrifugation at 45 °C with supernate removed; and the pellet obtained after centrifugation at 45 °C after agitation/rinsing/centrifugation twice with buffer | ||
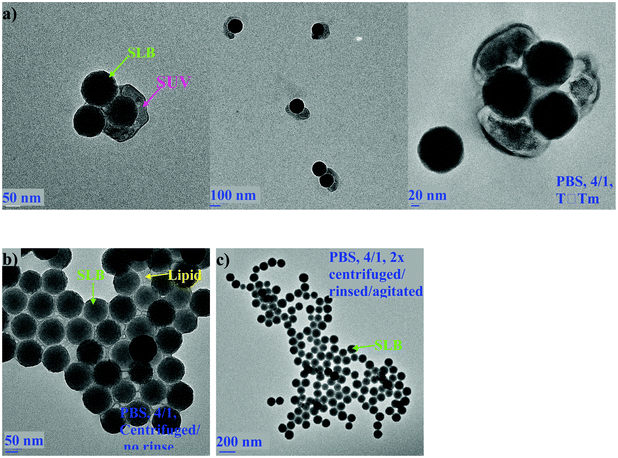 | ||
| Fig. 6 TEM images of SASUV/SASiO2 = 4/1 nanosystems at high ionic strength formed at 45 °C: (a) as prepared (no centrifugation); (b) centrifuged without rinse; (c) centrifuged/rinsed/agitated two times. | ||
At low ionic strength above Tm for SASUV/SASiO2 = 2/1, while it was possible to find conditions where there were no SLBs in the supernate and no SUVs in the pellet, it should be noted that the separation was made more difficult due the higher colloidal stability of these mixtures: the SLBs and SUVs were each separately stable and thus it was more difficult to find conditions where there was pelleting of only one species. The consistency of the centrifuged pellet for the SASUV/SASiO2 = 2/1 nanosystem obtained at 45 °C was much “looser” than for the pellet obtained at high ionic strength, consistent with the lack of adsorption of the SUVs and SLBs at low ionic strength. At low ionic strengths, since the lipid desorbed from the SiO2 below Tm, the pellet was not investigated. Lastly, another effect that has not been explored here in detail is the possible removal or partial removal of the lipid bilayer from the SiO2 under the effects of centrifugation, which appears to occur at low but not at high ionic strengths.
Discussion
Formation of single-SLBs
As has been observed in the case of planar and large spherical substrates such as SiO2 and zwitterionic lipids, salt promotes supported lipid bilayer formation,42,43 while in the absence of salt, little/no adsorption or fusion of vesicles onto SiO2 occurs.32,40,44,45 For planar substrates studied by quartz crystal microbalance with dissipative monitoring (QCM-D), SLB formation from zwitterionic vesicles can occur as a one- or two-step process, in which the time to SLB formation includes the diffusion of the vesicles to the substrate as well as the rupture event itself, depending on the vesicle concentration,40,46,47 and typically48 (but not always49,50) occurs above the Tm of the lipid.20 At low salt concentrations, SLB occurs as a one-step process, where vesicles rupture on contact and no critical coverage is required, but SLB formation occurs with slower kinetics than at high salt.28,45 At high salt concentrations (>90 mM NaCl, pH 7.4), SLB formation occurs via a two-step process (with occasional 1 step events), requiring a critical vesicle coverage before vesicle rupture, and where SLB edges enhance vesicle adhesion and rupture.20,51However, in the case of the SiO2 nanoparticles investigated here, SLB formation occurs as a single-step event at high ionic strength with similar rapidity above and below Tm, as determined by visual observation of immediate precipitation, and subsequent confirmation of SLB formation by nano-DSC scans and TEM images. The DLS data for single-SLBs at low ionic strength (where the nanosystem is stable) also clearly shows that the suspended lipids form supported lipid bilayers below Tm.
A possible reason for the difference between vesicle rupture on planar19,40,28,45 compared with nanoparticle supports comes from recent molecular dynamic simulations, which show that while larger vesicles exhibit larger deformations compared with smaller vesicles, they have no greater tendency to spontaneously rupture,52 as originally thought.53,54 Instead, the radius of curvature on the most strained portions of the vesicle, namely the sides where there is the smallest radius of curvature, determines when the vesicles rupture and is independent of vesicle size.52 As shown in Fig. 7 (bottom), this radius is smaller at the same contact area (other variables e.g. adsorption energies, bending and stretching moduli, ionic strengths, etc., the same) for nanoparticles compared with a planar surface. Stated differently, the radius needed for rupture at the vesicle sides will occur at a smaller vesicle adsorption area for curved compared with planar substrates.
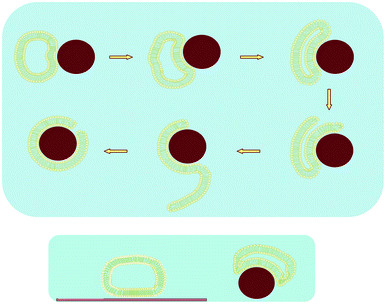 | ||
| Fig. 7 Schematics of: (top) Supported lipid bilayer (SLB) formation of zwitterionic small unilamellar vesicles (SUVs) around SiO2 nanoparticles of comparable sizes, ∼100 nm, shown to occur as a single bimolecular collision; incomplete coverage results in a defect site; (bottom) Same size SUVs adsorbed at the same surface area coverage to planar compared with curved SiO2 surfaces, showing smaller radii of curvature for SUVs at edges near the SiO2 support; not to scale. | ||
The size dependence of SLB formation,55,56 which is also dependent on the relative size of the nanoparticle, RSiO2, and the size of the SUV, RSUV, has been explained as a balance between the favorable adhesive forces (between the nanoparticle and SUV, with w the adhesion energy per unit area57) and the unfavorable elastic force necessary to wrap (bend the membrane or vesicle around) the nanoparticle, which has both bending (k2 is the mean bending modulus) and lateral stress components.58–60 Previous results indicate that formation of SLBs occurs on nanoparticles above Tm with diameters greater than ∼10–25 nm.34,55,56
Although the SUV does not “engulf” the nanoparticle here, the initial stages of nanoparticle envelopment are similar to this wrapping process. For tensionless membranes, the wrapping criterion for the particle radius is RC = (2 k2/w)1/2, i.e. smaller nanoparticles can be wrapped by membranes with smaller bending moduli. Since k2 has been reported to vary by an order of magnitude (above and below Tm),61 the formation of SLBs both above and below Tm in the current investigation also suggests that rupture occurs at the edges of the SUVs, and precedes by the mechanism shown in Fig. 7 (top). For RSUV ≈ RSiO2 (the case considered here), vesicles adsorb and fuse on contact with the surface during a single bimolecular collisional event between a SiO2 nanoparticle and an SUV (there is no critical coverage regime); it would not be possible for 100 nm SUVs to deform and completely surround the 100 nm SiO2 nanoparticles, since at best, even completely flattened, <½ of the SiO2 nanoparticle could be covered before rupture. Since the SiO2 nanoparticles are isolated from each other, once the SUV ruptures, the lipid bilayer continues to wrap around the SiO2 nanoparticle, consistent with the TEM and cryo-TEM images of the independent, single-SLBs.
Cryo-TEM results using tip sonicated SUVs (RSUV < RSiO2) and 110 nm SiO2 showed that after 1–2 min incubation (the smallest observable time), bilayer patches, corresponding to ruptures of 30–50 nm SUVs were (but adsorbed SUVs were rarely) observed,19 and were also consistent with the one-step mechanism of SLB formation. For RSUV > RSiO2 (30–10 nm SiO2 NPs in 100 to 300 nm SUVs) a process of invagination has been reported, where a radius of curvature of ∼6 nm ± 1 nm for the edges was measured before neck formation (not observed) and membrane fission completed the internalization process.56
Mechanism of colloidal stabilization/precipitation
Theoretical models of interaction between surfaces consider long-range attractive van der Waal (VW(l)) interactions (determined by the Hamaker constant),62,63 electrostatic interactions (VE(l)),62–64 short-range hydration repulsion (VH(l))65 and thermally excited “shape fluctuations” (thermally excited bending modes) that give rise to an effective repulsion (Vs(l)),58 where l is the separation distance between the two interacting surfaces. The cohesion necessary for the formation of stable adhesion is provided by long range attractive van der Waals forces,62 or electrostatic interactions if they are attractive. At short separation distances, there are strong repulsions as a result of hydration forces62 and short range steric repulsions, Vs(l)58 due to undulation and protrusion forces.66 The total interaction energy per unit area (VTOT), for two parallel segments (either membrane/membrane or membrane/SiO2) separated by a distance l is given by:| VTOT (l) = VW (l) + VE (l) + VH (l) + Vs (l) | (1) |
For SASUV/SASiO2 = 1/1, i.e. when all of the lipid is fused onto the SiO2, the Vs(l) term decreases dramatically for both low and high ionic strengths, since all of the lipid is attached to a rigid substrate, where undulations/protrusions are suppressed,68 so that only the VW (l), VE (l) and VH (l) interactions remain. When excess lipid (i.e. SASUV/SASiO2 ≥ 2/1) is used, the nanosystem now consists of approximately equal numbers of SUVs and SLBs (with the caveat that more lipid than calculated may actually be required to form a single-SLB). The SUVs are zwitterionic and the SLBs now have less negative charge than the native SiO2. More importantly, while the lipids on the SLBs have dramatically reduced undulatory/protrusion forces,68 the SUVs still maintain these motions.
Recently, a useful model has been proposed that accounts for further addition of vesicles onto an existing SLB in the presence of monovalent salt.23 For a negatively charged surface such as SiO2 and phosphatidyl choline lipids in NaCl solution, the Na+ counterions exist in the diffuse layer; Na+41 or Cl−69 ions are not expected to bind to the SiO2 or to the zwitterionic head groups.41 In this model, attractive van der Waals and electrostatic interactions are considered, and a restructuring of the diffuse double layer is proposed, the thickness of which is determined by the Debye screening length, (1/κ) found from:
| κ2 = (e2/ε0εkT) Σinizi2 |
At low ionic strength, the initial electrical double layer (κ−1 = 4.3 nm) is greater than the interstitial water layer, so in these cases the counterions are displaced by the lipid and removed to the region beyond the lipid (which is impermeable to ions) so that both the lipid and counterions become part of a new diffuse double layer.70 For SLBs composed of 100 nm SiO2 nanoparticles and DMPC lipids at 5 mM NaCl, a zeta potential of ∼ −22 mV has been reported,34 consistent with this model. If the van der Waals attraction is determined from the original surface (since the intervening lipid is considered part of the diffuse layer in this model) the van der Waals attraction for another SUV or SLB will decrease, while the extended electrical double layer maintains the electrostatic repulsion.70 Both factors keep the single-SLBs suspended at low ionic strength. For SASUV/SASiO2 ≥ 2/1 at low ionic strength there is little charge shielding and the repulsive VE(l) and Vs(l) interactions (the latter from the SUVs) are greater than the attractive VVW(l) interactions, so that SUVs do not adsorb onto the SLBs and a system of separate SUVs and SLBs exists, as observed by DLS. “Floating” bilayers (planar bilayers next to a SLB) have also been observed to have weak interactions with the SLB next to the solid support, particularly in the fluid phase.71
At high ionic strength, the electrical double layer (κ−1 < 1 nm) is less than the amount of water (1–2 nm) between the substrate and the first supported lipid bilayer. In this case, no counterions will be displaced and the electrostatic interaction will not be extended.70 This model thus accounts for the lack or reduction of the electrostatic repulsion between the single-SLBs in PBS buffer, which, along with the negligible steric repulsion (Vs (l)) results in the observed precipitation. Van der Waals attractions in high salt have been reported as the main factor responsible for promoting adsorption and interaction of zwitterionic lipids with SiO2 nanoparticles.72 A small addition of monovalent ions (NaCl) has been shown to induce contact between zwitterionic lipids on micron-size SiO2 with a SiO2 planar substrate also having a zwitterionic SLB.73
At high ionic strength, in the presence of excess vesicles, SASUV/SASiO2 = 2/1, the adsorption of SUVs onto the SLBs may be the result of attachment of the SUVs at “defect” sites (Fig. 7) on the SLBs, where the negative surface charge of the SiO2 is exposed, since this region will have the highest negative potential and therefore ability attract the neutral lipid. The defects occur for two reasons: (i) Surface areas of the SiO2 nanoparticles and the SUVs are not exactly matched; on average the sizes of the SUVs are smaller than the SiO2 nanoparticles and there is a distribution of sizes for both (Fig. 2). In collision events where the SUV size is smaller than that of the SiO2, there may not be enough lipid for complete coverage of the SiO2, leaving a pore (i.e. a defect) with exposed SiO2 as well as energetically unfavorable bilayer edges.74,75 (ii) The defect areas may be too small to form SLBs even when excess lipid is present. The inability to form a complete bilayer without defects above Tm is confirmed by the observed precipitation of the nanosystem at high ionic strength even upon addition of a slight excess of SUVs (SASUV/SASiO2 = 1.1 − 1.3/1), presumably due to the inability of the SUVs to form SLBs on small exposed SiO2 patches. The difficulty in forming a complete SLB in the presence of defects, as might exist on planar substrates at the later stages in the fusion process, has previously been observed,76 and SUVs have been shown to “span” pores on inorganic substrates.77
What is interesting however, is that after reheating the SASUV/SASiO2 = 2/1 nanosystem in PBS buffer from 10 °C to above Tm, the “adsorbed” SUVs are no longer attached to the SLBs. Instead, there is a nanosystem consisting of noninteracting SUVs and SLBs, as occurred for the SASUV/SASiO2 = 2/1 nanosystem in 5 mM NaCl. One possibility is that upon cooling, the lipid on the SLB “shrinks”, as the result of the smaller area/volume occupied by lipids below Tm,49,78 allowing more of the lipid from the SUVs at defect sites to adsorb to the bare SiO2 surface. The opposite effect occurs upon reheating: the lipid on the “nano-complex” SLB expands, possibly “pinching off” the portion of the SUV attached to the SiO2, releasing the remaining (smaller) SUV, as shown schematically in Fig. 8.
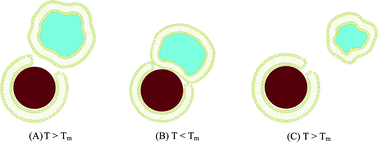 | ||
| Fig. 8 Schematic of SUVs and SLBs at high ionic strength: (A) adsorption of SUVs at defect sites on the SLB above Tm; (B) Contraction of the lipid below Tm permitting adsorption of the SUV onto the SiO2; (C) Expansion of the lipid on the SLB above Tm, “pinching” off part of the adsorbed SUV and expelling a smaller SUV | ||
However, the reduced electrostatic repulsion keeps the high ionic strength nanosystem stable for ∼12 h rather than the ∼days observed for the nanosystem at low ionic strength. Similarly, the nano-“complex” between the SUVs attached to the SLBs formed at 45 °C increases colloidal stability to ∼3 days. In this case, the nano-particle complex now has the undulatory/protrusion motions absent in the pure SLBs, and the steric repulsions (Vs (l)) promote colloidal metastability.
What is also of interest is that for the SASUV/SASiO2 = 2/1 nanosystem in PBS buffer below Tm, further (reversible) aggregation occurs, as shown by DLS data and TEM images. Here, if we assume that VE(l) is the same above and below Tm, then there can be changes in Vs(l), VVW(l) or VH(l). Insight for this problem comes from the analysis of the individual contributions and the behavior of the SUVs themselves.
(i) Vs(l): The form of Vs [Vs (l) ∼ (kBT)2/k2l2; k2 is the rigidity constant of membrane] indicates that steric repulsion should decrease with a decrease in temperature. Thus lower temperature favors aggregation. Since the steric repulsive interaction decreases as the membrane becomes stiffer,41,61 there should be more aggregation in the gel compared with the liquid crystalline phase and more for a lipid attached to a solid substrate, since the decrease in repulsion permits a greater contribution to the potential for the attractive van der Waals interaction.
(ii) VVW(l): The attractive van der Waals interaction will be greater the greater the area of interaction. The ∼200 nm nano-constructs composed of SLBs with adsorbed SUVS observed above Tm may further aggregate upon decreases in temperature, and the larger nanostructures formed will continue this process.
(iii) VH(l): There is no clear experimental evidence to indicate whether the hydration repulsion is greater in the gel or fluid phases of zwitterionic lipid bilayers. Hydration repulsion was believed to be a short range force,79,80 dominant between lipid bilayers at separation distances of ∼1.0 to 3.0 nm,65 but more recent evidence suggests that the hydration force extends to 4–5 water layers.81 However, there is more water associated with the zwitterionic headgroups in the liquid crystal phase compared with the gel phase. The maximum water content, water molecules/lipid (in swelling experiments of MLVs) is greater for the liquid crystalline phases of phospholipids,82–84 and swelling (by water) occurs at the main phase transition temperature for floating bilayers.61 Further, molecular dynamic simulations indicated that in the liquid crystalline phase the headgroups are separated by 1 (at 6.8 Å) or 2 water layers (at 9.6 Å), while in the gel phase they were separated only be a single (at 6.8 Å) water layer. The lipids in the SLBs and SUVs can therefore come into closer contact in the low temperature phase, promoting increased van der Waals attraction.
(iv) When more excess vesicles are present, several SUVs can be adsorbed to and/or be trapped between the SLBs. In both cases, the nano-systems remain colloidally stable due to the adsorbed SUVs.
Thus, the above (i–iii) factors all favor increased aggregation, but persistent colloidal stability (iv), below Tm.
Separation of SLBs and SUVs
In applications where adsorption of lipids with and without cargo on nanoparticles are investigated, the loading on the nanoparticles can be measured using a difference method, in which the solution concentration before and after centrifugation or precipitation of the nanoparticles is measured. Excess vesicles, both for planar and nanoparticle surfaces are typically employed. Experiments with planar surfaces use continual rinsing to remove residual SUVs.However, the stabilization imparted to the nanoparticles by SUV adsorption in the buffer—the condition often used in biological applications—means that it is necessary to investigate conditions where SUV removal is optimized. For example, it is not sufficient to simply confirm that the speeds required for centrifugation of the nanoparticles or supported lipid bilayers, do not in separate experiments, also result in pelleting of the SUVs, since it is the SUV adsorption to the SLBs that causes the SUVs to pellet along with the SLBs.
Some insight into the optimal conditions can be derived from the DLS results on the suspensions. In low salt, there was no SUV adsorption to the SLBs above or below Tm, but the lipids partially desorbed from the SiO2 below Tm. However, under these conditions, both the SUVs and SLBs were separately stable and thus the centrifuge speeds and times needed to be adjusted to ensure complete separation, with only SUVs in the supernate and only SLBs in the pellet. In high salt, adsorption of SUVs to the SLBs occurred above Tm, and became worse below Tm. However, separation of the SUVs and SLBs occurred when these nano-systems were reheated to above Tm of the lipids.
Previous investigations of SLB formation on silica beads that attempt to determine how much lipid has fused have yielded mixed results, with fractional or noninteger numbers of bilayers,27,85 or excess lipid18 observed. The current investigation suggests that this may be a separation problem, since different results can be obtained depending on the ionic strength and whether separation occurred above or below Tm of the lipid.
Conclusions
The morphology, mechanism of supported lipid bilayer (SLB) formation and stabilization of SLBs formed from zwitterionic small unilamellar vesicles (SUVs) around SiO2 nanoparticles of approximately equal (∼100 nm diameter) size was investigated at low (5 mM NaCl) and high (0.08 M PBS buffer, 100 mM NaCl) ionic strengths. SLBs formed instantaneously above and below the main gel-to-liquid crystal phase transition temperature, Tm as the result of bi-molecular collisions between the SiO2 and SUVs, faster than similar events on planar SiO2 substrates. The SLB kinetics are more rapid since the critical (small) radius required for rupture at the vesicle sides occurs at a smaller vesicle adsorption area for curved compared with planar substrates. When the surface area ratios are equal, SASUV/SASiO2 = 1, the entropic undulation/protrusion forces68 are absent for the first layer adsorbed to the SiO2 nanoparticle. In the case of the single-SLBs in low salt, the residual negative charge (zeta potential ∼ −22 mV) is sufficient to keep the single-SLBs suspended above and below Tm, but at high salt, screening effects eliminate this electrostatic repulsion, and the single-SLBs precipitate.When excess lipid is added, SASUV/SASiO2 ≥ 2/1, the SLBs are metastable above and below Tm in both high and low salt. Dynamic light scattering, nano-differential scanning calorimetry and transmission electron microscopy results show that the mechanism of stabilization is different at high and low salt. In low salt (5 mM NaCl), stabilization above and below Tm is achieved by electrostatic repulsion of the non-interacting SLBs and SUVs, the latter retaining also the repulsive undulatory/protrusion forces. In high salt (PBS buffer), the SUVs adsorb to the SLBs, possible at defect sites on the SLB. When there are about equal populations and sizes of SUVs and SLBs, as investigated here, this appears as an approximately equal pairing of the two above Tm, with the SUVs providing repulsive undulatory/protrusion forces from regions of the SUV not in contact with the SLB. At temperatures below Tm, more extensive, reversible aggregation occurs, with the SUVs still adsorbed but not fused to the SLB aggregates. When the “nano-complex” is heated from 10 °C to a temperature above Tm, there are separate populations of SUVs and SLBs, as observed for the low salt system. We suggest that this occurs from an expansion of the lipid on the SLB as it is heated above Tm, which “pinches off” the portion of the SUV adsorbed to the defect site (SiO2) on the SLB.
In order to quantify the amount of supported lipid bilayer (SLB) formation on nanoparticles such as SiO2, it is necessary to separate the SLBs from the excess SUVs used to form them. However, nanoparticles themselves, or the SLBs, can exhibit extensive aggregation as the result of their high surface area. The aggregation sometimes results in flocculation/precipitation, but stable suspensions can also form. Previous data for positively charged DMTAP/DMPC (50/50) showed that when excess lipid was present, sheaths of lipid surrounded clusters of SLBs and complete/immediate precipitation of the nano-system occurred even without centrifugation.36 Here, for zwitterionic lipids, double bilayers or sheaths surrounding many SLBs were never observed. The results indicate that information obtained by difference analysis of supernates to infer details about supported lipid bilayer formation on nanoparticles and their morphology should be viewed with some caution, since SUVs can absorb to the SLBs, or sheaths can surround the SLBs.
Abbreviations
| SLB | supported lipid bilayer |
| MLV | multilamellar vesicle |
| SUV | small unilamellar vesicle |
| MVV | multivesicular vesicle |
| DMPC | 1,2-dimyristoyl-sn-glycero-3-phosphocholine |
| PC | phosphatidylcholine |
| DLS | dynamic light scattering |
| Nano-DSC | nano-differential scanning calorimetry |
| T m | gel-to-liquid crystalline phase transition temperature |
| SASUV | surface area of SUV |
| SASiO2 | surface area of SiO2 nanoparticles |
Acknowledgements
We acknowledge NSF grant # CHE-0923077 for acquisition of the TEM.References
- C. R. Thomas, S. George, A. M. Horst, Z. X. Ji, R. J. Miller, J. R. Peralta-Videa, T. A. Xia, S. Pokhrel, L. Madler, J. L. Gardea-Torresdey, P. A. Holden, A. A. Keller, H. S. Lenihan, A. E. Nel and J. I. Zink, Nanomaterials in the Environment: From Materials to High-Throughput Screening to Organisms, ACS Nano, 2011, 5(1), 13–20 CrossRef CAS.
- J. R. Peralta-Videa, L. J. Zhao, M. L. Lopez-Moreno, G. de la Rosa, J. Hong and J. L. Gardea-Torresdey, Nanomaterials and the environment: A review for the biennium 2008–2010, Journal of Hazardous Materials, 2011, 186(1), 1–15 CrossRef CAS.
- A. Nel, T. Xia, L. Madler and N. Li, Toxic potential of materials at the nanolevel, Science, 2006, 311(5761), 622–627 CrossRef CAS.
- A. Verma and F. Stellacci, Effect of Surface Properties on Nanoparticle-Cell Interactions, Small, 2010, 6(1), 12–21 CrossRef CAS.
- T. J. Brunner, P. Wick, P. Manser, P. Spohn, R. N. Grass, L. K. Limbach, A. Bruinink and W. J. Stark, In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility, Environmental Science & Technology, 2006, 40(14), 4374–4381 CAS.
- A. E. Nel, L. Madler, D. Velegol, T. Xia, E. M. V. Hoek, P. Somasundaran, F. Klaessig, V. Castranova and M. Thompson, Understanding biophysicochemical interactions at the nano-bio interface, Nature Materials, 2009, 8(7), 543–557 CrossRef CAS.
- Z. J. Zhu, R. Carboni, M. J. Quercio, B. Yan, O. R. Miranda, D. L. Anderton, K. F. Arcaro, V. M. Rotello and R. W. Vachet, Surface Properties Dictate Uptake, Distribution, Excretion, and Toxicity of Nanoparticles in Fish, Small, 2010, 6(20), 2261–2265 CrossRef CAS.
- X. X. He, H. L. Nie, K. M. Wang, W. H. Tan, X. Wu and P. F. Zhang, In Vivo Study of Biodistribution and Urinary Excretion of Surface-Modified Silica Nanoparticles, Analytical Chemistry, 2008, 80(24), 9597–9603 CrossRef CAS.
- D. F. Moyano and V. M. Rotello, Nano Meets Biology: Structure and Function at the Nanoparticle Interface, Langmuir, 2011, 27(17), 10376–10385 CrossRef CAS.
- B. D. Chithrani, A. A. Ghazani and W. C. W. Chan, Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells, Nano Letters, 2006, 6(4), 662–668 CrossRef CAS.
- B. D. Chithrani and W. C. W. Chan, Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes, Nano Letters, 2007, 7(6), 1542–1550 CrossRef CAS.
- K. M. Waters, L. M. Masiello, R. C. Zangar, N. J. Karin, R. D. Quesenberry, S. Bandyopadhyay, J. G. Teeguarden, J. G. Pounds and B. D. Thrall, Macrophage Responses to Silica Nanoparticles are Highly Conserved Across Particle Sizes, Toxicological Sciences, 2009, 107(2), 553–569 CrossRef CAS.
- J. G. Teeguarden, P. M. Hinderliter, G. Orr, B. D. Thrall and J. G. Pounds, Particokinetics in vitro: Dosimetry considerations for in vitro nanoparticle toxicity assessments, Toxicological Sciences, 2007, 95(2), 300–312 CrossRef CAS.
- D. Dutta, S. K. Sundaram, J. G. Teeguarden, B. J. Riley, L. S. Fifield, J. M. Jacobs, S. R. Addleman, G. A. Kaysen, B. M. Moudgil and T. J. Weber, Adsorbed proteins influence the biological activity and molecular targeting of nanomaterials, Toxicological Sciences, 2007, 100(1), 303–315 CrossRef CAS.
- J. Xie, C. Xu, N. Kohler, Y. Hou and S. Sun, Controlled PEGylation of monodisperse Fe3O4 nanoparticles for reduced non-specific uptake by macrophage cells, Advanced Materials, 2007, 19(20), 3163 CrossRef CAS.
- B. A. Kairdolf, M. C. Mancini, A. M. Smith and S. M. Nie, Minimizing nonspecific cellular binding of quantum dots with hydroxyl-derivatizied surface coatings, Analytical Chemistry, 2008, 80(8), 3029–3034 CrossRef CAS.
- C. K. Kim, P. Ghosh, C. Pagliuca, Z. J. Zhu, S. Menichetti and V. M. Rotello, Entrapment of Hydrophobic Drugs in Nanoparticle Monolayers with Efficient Release into Cancer Cells, Journal of the American Chemical Society, 2009, 131(4), 1360 CrossRef CAS.
- E. E. Ross, S. W. Mok and S. R. Bugni, Assembly of Lipid Bilayers on Silica and Modified Silica Colloids by Reconstitution of Dried Lipid Films, Langmuir, 2011, 27(14), 8634–8644 CrossRef CAS.
- S. Mornet, O. Lambert, E. Duguet and A. Brisson, The Formation of Supported Lipid Bilayers on Silica Nanoparticles Revealed by Cryoelectron Microscopy, Nano Letters, 2005, 5(2), 281–285 CrossRef CAS.
- R. P. Richter, R. Berat and A. R. Brisson, Formation of Solid-Supported Lipid Bilayers: An Integrated View, Langmuir, 2006, 22(8), 3497–3505 CrossRef CAS.
- I. Czolkos, A. Jesorka and O. Orwar, Molecular phospholipid films on solid supports, Soft Matter, 2011, 7(10), 4562–4576 RSC.
- E. Reimhult, F. Hook and B. Kasemo, Vesicle adsorption on SiO2 and TiO2: Dependence on vesicle size, Journal of Chemical Physics, 2002, 117(16), 7401–7404 CrossRef CAS.
- T. A. Oleson and N. Sahai, Oxide-dependent adsorption of a model membrane phospholipid, dipalmitoylphosphatidylcholine: Bulk adsorption isotherms, Langmuir, 2008, 24(9), 4865–4873 CrossRef CAS.
- J. Xu, M. J. Stevens, T. A. Oleson, J. A. Last and N. Sahai, Role of Oxide Surface Chemistry and Phospholipid Phase on Adsorption and Self-Assembly: Isotherms and Atomic Force Microscopy, Journal of Physical Chemistry C, 2009, 113(6), 2187–2196 CrossRef CAS.
- T. Cha, A. Guo and X. Y. Zhu, Formation of supported phospholipid bilayers on molecular surfaces: Role of surface charge density and electrostatic interaction, Biophysical Journal, 2006, 90(4), 1270–1274 CrossRef CAS.
- C. Hamai, T. L. Yang, S. Kataoka, P. S. Cremer and S. M. Musser, Effect of average phospholipid curvature on supported bilayer formation on glass by vesicle fusion, Biophysical Journal, 2006, 90(4), 1241–1248 CrossRef CAS.
- P. Nollert, H. Kiefer and F. Jaehnig, Lipid vesicle adsorption versus formation of planar bilayers on solid surfaces, Biophysical Journal, 1995, 69(4), 1447–55 CrossRef CAS.
- B. Seantier and B. Kasemo, Influence of Mono- And Divalent Ions on the Formation of Supported Phospholipid Bilayers via Vesicle Adsorption, Langmuir, 2009, 25(10), 5767–5772 CrossRef CAS.
- J. Radler, H. Strey and E. Sackmann, Phenomenology and Kinetics of Lipid Bilayer Spreading on Hydrophilic Surfaces, Langmuir, 1995, 11(11), 4539–4548 CrossRef.
- P. S. Cremer and S. G. Boxer, Formation and spreading of lipid bilayers on planar glass supports, Journal of Physical Chemistry B, 1999, 103(13), 2554–2559 CrossRef CAS.
- K. Dimitrievski and B. Kasemo, Influence of Lipid Vesicle Composition and Surface Charge Density on Vesicle Adsorption Events: A Kinetic Phase Diagram, Langmuir, 2009, 25(16), 8865–8869 CrossRef CAS.
- R. Rapuano and A. M. Carmona-Ribeiro, Supported bilayers on silica, Journal of Colloid and Interface Science, 2000, 226(2), 299–307 CrossRef CAS.
- R. Richter, A. Mukhopadhyay and A. Brisson, Pathways of lipid vesicle deposition on solid surfaces: A combined QCM-D and AFM study, Biophysical Journal, 2003, 85(5), 3035–3047 CrossRef CAS.
- S. Savarala, S. Ahmed, M. A. Ilies and S. L. Wunder, Formation and Colloidal Stability of DMPC Supported Lipid Bilayers on SiO2 Nanobeads, Langmuir, 2010, 26(14), 12081–12088 CrossRef CAS.
- S. Savarala, F. Monson, M. A. Ilies and S. L. Wunder, Supported Lipid Bilayer NanoSystems: Stabilization by Undulatory-Protrusion Forces and Destabilization by Lipid Bridging, Langmuir, 2011, 27(10), 5850–5861 CrossRef CAS.
- S. Ahmed, S. Savarala, Y. Chen, G. Bothun and S. L. Wunder, Formation of Lipid Sheaths around Supported Lipid Bilayer Nanoparticles, Small, 2012 Search PubMed , in press.
- C. Naumann, T. Brumm and T. M. Bayerl, Phase transition behavior of single phosphatidylcholine bilayers on a solid spherical support studied by DSC, NMR and FT-IR, Biophysical Journal, 1992, 63(5), 1314–19 CrossRef CAS.
- S. Ahmed and S. L. Wunder, Effect of High Surface Curvature on the Main Phase Transition of Supported Phospholipid Bilayers on SiO2 Nanoparticles, Langmuir, 2009, 25(6), 3682–3691 CrossRef CAS.
- A. V. Hughes, S. J. Roser, M. Gerstenberg, A. Goldar, B. Stidder, R. Feidenhans'l and J. Bradshaw, Phase behavior of DMPC free supported bilayers studied by neutron reflectivity, Langmuir, 2002, 18(21), 8161–8171 CrossRef CAS.
- T. H. Anderson, Y. J. Min, K. L. Weirich, H. B. Zeng, D. Fygenson and J. N. Israelachvili, Formation of Supported Bilayers on Silica Substrates, Langmuir, 2009, 25(12), 6997–7005 CrossRef CAS.
- J. Marra and J. Israelachvili, Direct measurements of forces between phosphatidylcholine and phosphatidylethanolamine bilayers in aqueous electrolyte solutions, Biochemistry, 1985, 24(17), 4608–18 CrossRef CAS.
- R. Rapuano and A. M. Carmona-Ribeiro, Physical adsorption of bilayer membranes on silica, Journal of Colloid and Interface Science, 1997, 193(1), 104–111 CrossRef CAS.
- T. M. Bayerl and M. Bloom, Physical properties of single phospholipid bilayers adsorbed to micro glass beads. A new vesicular model system studied by deuterium nuclear magnetic resonance, Biophysical Journal, 1990, 58(2), 357–62 CrossRef CAS.
- S. Savarala, S. Ahmed, M. A. Ilies and S. L. Wunder, Stabilization of Soft Lipid Colloids: Competing Effects of Nanoparticle Decoration and Supported Lipid Bilayer Formation, ACS Nano, 2011, 5(4), 2619–2628 CrossRef CAS.
- S. Boudard, B. Seantier, C. Breffa, G. Decher and O. Felix, Controlling the pathway of formation of supported lipid bilayers of DMPC by varying the sodium chloride concentration, Thin Solid Films, 2006, 495(1–2), 246–251 CrossRef CAS.
- C. A. Keller, K. Glasmastar, V. P. Zhdanov and B. Kasemo, Formation of supported membranes from vesicles, Physical Review Letters, 2000, 84(23), 5443–5446 CrossRef CAS.
- E. Reimhult, F. Hoeoek and B. Kasemo, Intact Vesicle Adsorption and Supported Biomembrane Formation from Vesicles in Solution: Influence of Surface Chemistry, Vesicle Size, Temperature, and Osmotic Pressure, Langmuir, 2003, 19(5), 1681–1691 CrossRef CAS.
- B. Seantier, C. Breffa, O. Felix and G. Decher, Dissipation-enhanced quartz crystal microbalance studies on the experimental parameters controlling the formation of supported lipid bilayers, Journal of Physical Chemistry B, 2005, 109(46), 21755–21765 CrossRef CAS.
- M. Beckmann, P. Nollert and H. A. Kolb, Manipulation and molecular resolution of a phosphatidylcholine-supported planar bilayer by atomic force microscopy, Journal of Membrane Biology, 1998, 161(3), 227–233 CrossRef CAS.
- B. Seantier, C. Breffa, O. Felix and G. Decher, In situ investigations of the formation of mixed supported lipid bilayers close to the phase transition temperature, Nano Letters, 2004, 4(1), 5–10 CrossRef CAS.
- K. L. Weirich, J. N. Israelachvili and D. K. Fygenson, Bilayer Edges Catalyze Supported Lipid Bilayer Formation, Biophysical Journal, 2010, 98(1), 85–92 CrossRef CAS.
- K. Dimitrievski, Deformation of Adsorbed Lipid Vesicles as a Function of Vesicle Size, Langmuir, 2010, 26(5), 3008–3011 CrossRef CAS.
- U. Seifert and R. Lipowsky, Adhesion of Vesicles, Physical Review A, 1990, 42(8), 4768–4771 CrossRef CAS.
- U. Seifert, Configurations of fluid membranes and vesicles, Advances in Physics, 1997, 46(1), 13–137 CrossRef CAS.
- Y. Roiter, M. Ornatska, A. R. Rammohan, J. Balakrishnan, D. R. Heine and S. Minko, Interaction of nanoparticles with lipid membrane, Nano Letters, 2008, 8(3), 941–944 CrossRef CAS.
- O. Le Bihan, P. Bonnafous, L. Marak, T. Bickel, S. Trepout, S. Mornet, F. De Haas, H. Talbot, J. C. Taveau and O. Lambert, Cryo-electron tomography of nanoparticle transmigration into liposome, Journal of Structural Biology, 2009, 168(3), 419–425 CrossRef CAS.
- R. Lipowsky and H. G. Dobereiner, Vesicles in contact with nanoparticles and colloids, Europhysics Letters, 1998, 43(2), 219–225 CrossRef CAS.
- W. Helfrich, Steric Interaction of Fluid Membranes in Multilayer Systems, Zeitschrift Fur Naturforschung Section a-a Journal of Physical Sciences, 1978, 33(3), 305–315 Search PubMed.
- M. Deserno and W. M. Gelbart, Adhesion and wrapping in colloid-vesicle complexes, Journal of Physical Chemistry B, 2002, 106(21), 5543–5552 CrossRef CAS.
- M. Deserno and T. Bickel, Wrapping of a spherical colloid by a fluid membrane, Europhysics Letters, 2003, 62(5), 767–773 CrossRef CAS.
- J. Daillant, E. Bellet-Amalric, A. Braslau, T. Charitat, G. Fragneto, F. Graner, S. Mora, F. Rieutord and B. Stidder, Structure and fluctuations of a single floating lipid bilayer, Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(33), 11639–11644 CrossRef CAS.
- R. P. Rand, Interacting Phospholipid Bilayers- Measured Forces and Induced Structural Changes, Annual Review of Biophysics and Bioengineering, 1981, 10, 277–314 CrossRef CAS.
- L. J. Lis, M. McAlister, N. Fuller, R. P. Rand and V. A. Parsegian, Interactions between Neutral Phospholipid-Bilayer Membranes, Biophysical Journal, 1982, 37(3), 657–665 CAS.
- J. Israelachvili, Intermolecular and Surface Forces, Academic Press: London, 2007 Search PubMed.
- S. Leikin, V. A. Parsegian, D. C. Rau and R. P. Rand, Hydration Forces, Annual Review of Physical Chemistry, 1993, 44, 369–395 CrossRef CAS.
- W. Helfrich and R. M. Servuss, Undulations, Steric Interactions and Cohesion of Fluid Membranes, Nuovo Cimento Della Societa Italiana Di Fisica D-Condensed Matter Atomic Molecular and Chemical Physics Fluids Plasmas Biophysics, 1984, 3(1), 137–151 Search PubMed.
- R. Lipowsky and S. Leibler, Unbinding Transitions of Interacting Membranes, Physical Review Letters, 1986, 56(23), 2541–2544 CrossRef CAS.
- J. N. Israelachvili and H. Wennerstrom, Entropic Forces Between Amphiphilic Surfaces in Liquids, Journal of Physical Chemistry, 1992, 96(2), 520–531 CrossRef CAS.
- H. I. Petrache, T. Zemb, L. Belloni and V. A. Parsegian, Salt screening and specific ion adsorption determine neutral-lipid membrane interactions, Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(21), 7982–7987 CrossRef CAS.
- T. A. Oleson and N. Sahai, Interaction energies between oxide surfaces and multiple phosphatidylcholine bilayers from extended-DLVO theory, Journal of Colloid and Interface Science, 2010, 352(2), 316–326 CrossRef CAS.
- B. Stidder, G. Fragneto and S. J. Roser, Structure and stability of DPPE planar bilayers, Soft Matter, 2007, 3(2), 214–222 RSC.
- A. Vakurov, R. Brydson and A. Nelsont, Electrochemical Modeling of the Silica Nanoparticle-Biomembrane Interaction, Langmuir, 2012, 28(2), 1246–1255 CrossRef CAS.
- E. L. Kendall, E. Mills, J. W. Liu, X. M. Jiang, C. J. Brinker and A. N. Parikh, Salt-induced lipid transfer between colloidal supported lipid bilayers, Soft Matter, 2010, 6(12), 2628–2632 RSC.
- F. Y. Jiang, Y. Bouret and J. T. Kindt, Molecular dynamics simulations of the lipid bilayer edge, Biophysical Journal, 2004, 87(1), 182–192 CrossRef CAS.
- P. M. Kasson and V. S. Pande, Molecular dynamics simulation of lipid reorientation at bilayer edges, Biophysical Journal, 2004, 86(6), 3744–3749 CrossRef CAS.
- E. Reimhult, M. Zach, F. Hook and B. Kasemo, A multitechnique study of liposome adsorption on Au and lipid bilayer formation on SiO2, Langmuir, 2006, 22(7), 3313–3319 CrossRef CAS.
- K. Kumar, L. Isa, A. Egner, R. Schmidt, M. Textor and E. Reimhult, Formation of Nanopore-Spanning Lipid Bilayers through Liposome Fusion, Langmuir, 2011, 27(17), 10920–10928 CrossRef CAS.
- L. K. Tamm and H. M. McConnell, Supported phospholipid bilayers, Biophysical Journal, 1985, 47(1), 105–13 CrossRef CAS.
- D. M. Leneveu, R. P. Rand and V. A. Parsegian, Measurement of Forces Between Lecithin Bilayers, Nature, 1976, 259(5544), 601–603 CrossRef CAS.
- V. A. Parsegian and T. Zemb, Hydration forces: Observations, explanations, expectations, questions, Current Opinion in Colloid & Interface Science, 2011, 16(6), 618–624 CAS.
- M. Hishida and K. Tanaka, Long-Range Hydration Effect of Lipid Membrane Studied by Terahertz Time-Domain Spectroscopy, Physical Review Letters, 2011, 106(15), 158102-1–158102-4 CrossRef.
- M. J. Ruocco and G. G. Shipley, Characterization of the Sub-transition of Hydrated Dipalmitpylphosphotidylcholine Bilayers-Kinetic, Hydration and Structural Study, Biochimica Et Biophysica Acta, 1982, 691(2), 309–320 CrossRef CAS.
- K. Gawrisch, D. Ruston, J. Zimmerberg, V. A. Parsegian, R. P. Rand and N. Fuller, Membrane Dipole Potentials, Hydration Forces and the Ordering of Water at Membrane Surfaces, Biophysical Journal, 1992, 61(5), 1213–1223 CrossRef CAS.
- A. S. Ulrich, M. Sami and A. Watts, Hydration of DOPC Bilayers by Differential Scanning Calorimetry, Biochimica Et Biophysica Acta-Biomembranes, 1994, 1191(1), 225–230 CrossRef CAS.
- K. Esumi and T. Yamada, Characterization of a Phospholipid Adsorbed Layer on Silica From Small Unilamellar Vesicles, Langmuir, 1993, 9(2), 622–624 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
