A facile route to functionalised, protic and chiral ionic liquids based on the triaminocyclopropenium cation†
Owen J.
Curnow
*,
Michael T.
Holmes
,
Leonardus C.
Ratten
,
Kelvin J.
Walst
and
Ruhamah
Yunis
Department of Chemistry, University of Canterbury, Private Bag 4800, Christchurch, New Zealand. E-mail: owen.curnow@canterbury.ac.nz; Fax: +64 3 364 2110; Tel: +64 3 364 2819
First published on 26th September 2012
Abstract
Readily prepared alkoxydiaminocyclopropenium salts provide an easy access to triaminocyclopropenium (tac) ionic liquids containing functionalised, protic and chiral cations.
Ionic liquid technology has undergone rapid development in recent years and many books and reviews have now been published in the area.1 There are many factors that make ionic liquids attractive materials, including their extremely low volatility, ability to conduct electricity, and general tendency to dissolve a wide range of solutes. An additional advantage of ionic liquids over conventional solvents is the ability to tune the properties of the liquid, particularly the viscosity and conductivity, but also solvent properties such as hydrogen bond donor and acceptor ability, effective polarity and polarisability, and also chirality.
In 2011, we introduced the triaminocyclopropenium (tac) cation as a new class of cation for ionic liquids.2 However, one of the significant limitations that hinders the further development of tac salts as ionic liquid materials is the lack of structural variety and ease of functionalisation. Currently, only the D3h- and C3h-symmetric systems of the [C3(NR2)3]+ and [C3(NRR′)3]+ kinds, respectively, are readily accessible via addition of the corresponding dialkylamine to either C3Cl5H or C3Cl4. A limited number of cations of the kind [C3(NR2)2(NR′R′′)]+, in which R is isopropyl or cyclohexyl, are accessible via reaction of the readily isolated chlorodiaminocyclopropenium cations [C3(N-i-Pr2)2Cl]+ and [C3(NCy2)2Cl]+, respectively, with the corresponding amine.3–5 The tris(diisopropylamino)cyclopropenium cation is only formed with difficulty due to the steric strain imposed by the diisopropylamino groups.3,6 This steric strain similarly limits the additional amino group to less sterically-demanding R′ and R′′ moieties. Notably, the ethyl analogue, [C3(NEt2)2Cl]ClO4, can be obtained in only about 15% yield by careful reaction of C3Cl4 with diethylamine, as most of the product is found to be [C3(NEt2)3]ClO4.3 More generally then, this class of compounds is currently limited in a practical sense to bulky R groups and less bulky R′ and R′′ groups. It should be recognized that there are alternative routes to reduced symmetry cations. For example, reaction of a diaminoacetylene with an isocyanide gives a cyclopropenonimine which, after protonation or alkylation, leads to a tac salt, however, this route is hindered by the availability of these reagents.7 Similarly, Weiss has reported the synthesis of [C3(NMe2)2Cl]SbCl6via [C3Cl3]SbCl6 and two equivalents of Me3SiNMe2.8 This is an elegant route, but, unfortunately, it is not very convenient or versatile. Cyclopropenones can be converted to the corresponding dichlorocyclopropene by treatment with SOCl2,3 however, we find that this gives a product that is not easily purified to a satisfactory degree for subsequent steps. In 1965, Breslow reported the alkylation of diphenylcyclopropenone with [Et3O]BF4 followed by treatment with a secondary amine to give a monoaminodiphenylcyclopropenium salt.9 This reaction type was later used by Krebs to make the only tac salt that we are aware of with three different amino groups.7 Here we make use of this alkylation process to illustrate a route to a variety of easily-accessible ionic liquids containing cations of the kind [C3(NR2)2(NR′R′′)]+ in which the nature of the R, R′ and R′′ groups is much less restricted.
Conversion of tac salts (1) to the corresponding cyclopropenone (2) with the loss of one equivalent of amine is readily achieved by hydrolysis in a 10% aqueous NaOH solution at 70 °C for ca. 24 h (Scheme 1). We also find it beneficial to carry this out in an open vessel to allow the escape of the volatile amine by-product. The cyclopropenone can then be alkylated with a variety of alkylating agents to give alkoxydiaminocyclopropenium salts (3X). Trialkyloxonium tetrafluoroborate will produce tetrafluoroborate salts 3BF4 in good yields (clearly, the hexafluorophosphate analogue could be used to generate PF6− salts). However, BF4− is not always easily exchanged for other anions and we find that dimethylsulfate, as well as being inexpensive, gives high yields of 3MeSO4 with a methylsufate anion that is readily exchanged. For some applications, ethyliodide can be useful for directly generating iodide salts 3I, though we generally find that the yields are lower. Similarly, methyltriflate will give the triflate salts 3OTf directly. It should be noted that, if the dimethylsulfate is not fresh, significant amounts of the sulfate dianion will be present. Fortunately, sulfate is readily exchanged along with the methylsulfate, so its presence does not affect the final product, unless it is the methylsulfate salt that is required.
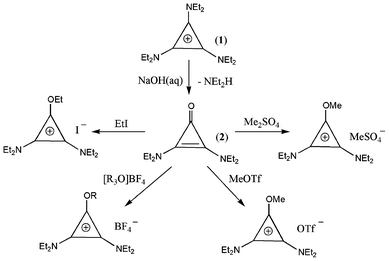 | ||
| Scheme 1 Formation of alkoxydiaminocyclopropenium salts. | ||
Treatment of 3X with the desired amine quickly gives a new tac salt 4X via loss of an alcohol (Scheme 2). Importantly, this reaction can be carried out with primary amines and is also tolerant of functional groups. Here we show the formation of ionic liquids with protic, alkenyl, hydroxy and carboxylic acid functional groups. It would be reasonable to expect, due to the mild conditions and strength of the cyclopropenium-amine bond that is formed, that this reaction would be tolerant of a very wide range of groups.
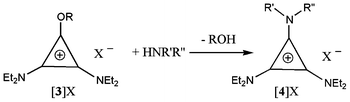 | ||
| Scheme 2 Formation of triaminocyclopropenium salts (R = Me or Et; R′, R′′ and X− are detailed in Table 1 and 2). | ||
Table 1 provides DSC and selected viscosity data for the NTf2− salts we prepared and Table 2 provides data for salts with other anions. Reaction of 3X with a secondary dialkyl amine produces peralkyl tac salts whereas a primary amine RNH2 produces the protic tac ILs [C3(NEt2)2(NHR)]X.
| NRR′ | T g/°Ca | T m/°Ca | T d/°Cb | Viscosity at 20 °C/cP | Viscosity at 60 °C/cP |
|---|---|---|---|---|---|
| a Determined by DSC at 10 °C min−1. b Determined by TGA at 10 °C min−1. c T s-s at −30 and 20 °C. d 4% zwitterion. e 15% zwitterion. f 19% zwitterion. g 8% zwitterion. h At 75 °C. i n.o. = not observed | |||||
| NMe2 | n.o.i | 17 | 379 | 83.6 | 17.9 |
| NEt2 | n.o. | 23c | 393 | 95.0 | 20.0 |
| NBu2 | −86 | −4 | 403 | 126 | 23.1 |
| NHex2 | −81 | n.o. | 396 | 182 | 30.2 |
N(CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)2 CH2)2 |
−87 | −26 | 358 | 111 | 20.9 |
| N(CH2CH2OH)2 | −62 | n.o. | 266 | — | 89.0 |
| NBuMe | −88 | n.o. | 384 | 106 | 21.1 |
| NHexMe | −84 | n.o. | 396 | 82.1 | 16.7 |
| NHBu | –80 | n.o. | 301 | 176 | 25.5 |
| NHHex | −82 | n.o. | 371 | 171 | 27.9 |
| NH2 | −59 | 94 | 334 | — | — |
| Alaninod | −30 | n.o. | 253 | — | 329h |
| Prolinoe | −22 | n.o. | 275 | — | 230h |
| Valinof | −41 | n.o. | 247 | — | 574h |
| Threoninog | −22 | n.o. | 176 | — | 884h |
| [C3(N(allyl)2)3)]NTf2 | −82 | 9 | 306 | 126 | 21.1 |
| NRR′/X | T g/°Ca | T m/°Ca | T d/°Cb | Viscosity at 20 °C/cP | Viscosity at 60 °C/cP |
|---|---|---|---|---|---|
| a Determined by DSC at 10 °C min−1. b Determined by TGA at 10 °C min−1. c With an exothermic event. d n.o. = not observed | |||||
| NMe2/DCA | −85 | 32 | 322 | 58.4 | 13.2 |
| NEt2/DCA | n.o.d | 9 | 330 | 64.2 | 14.6 |
| NBu2/DCA | −82 | 30 | 334 | 105 | 19.4 |
| NHex2/DCA | −80 | n.o. | 348 | 131 | 22.2 |
| NBuMe/DCA | −83 | 8 | 332 | 73.7 | 15.3 |
| NHexMe/DCA | −82 | n.o. | 348 | 69.0 | 14.3 |
| NHBu/DCA | −70 | n.o. | 233 | 251 | 31.6 |
| N(CH2CH2OH)2/DCA | −50 | 80 | 216c | — | — |
| NHex2/I | −46 | n.o. | 292 | — | 123 |
| NHex2/OTf | −69 | n.o. | 314 | 444 | 54.7 |
| NHBu/BF4 | −63 | n.o. | 228 | 131 | 14.7 |
| [C3(N(allyl)2)3)]DCA | −68 | 24 | 270c | 211 | 27.2 |
As would be hoped, all of the melting points for the peralkyl tac salts are near or below room temperature. Unfortunately, in many cases the melting point was not observed, and so it is difficult to discern any clear trends from the available data. On the other hand, the viscosity data for the peralkyl cations is seen to increase with molecular weight (Fig. 1) and the DCA salts are found to exhibit lower viscosities than the NTf2− salts, as is generally seen with other cations. An interesting exception from these trends are the [C3(NEt2)2(NHexMe)]+ salts which show lower viscosities. This is possibly a shape effect arising from the single long hexyl chain. Thermal decomposition onset temperatures follow the expected trends with the peralkyl NTf2− salts generally around 400 °C, although the dimethyl and diallyl examples are a little lower (379 and 358 °C, respectively) but not as low as their permethyl and perallyl analogues (339 and 306 °C, respectively).2 The DCA salts are correspondingly lower and lie in the range 322–348 °C.
![Viscosity at 20 °C vs. molecular weight of the cation for NTf2 ( and ) and DCA ( and ) salts. The outliers from the exponentially-fitted lines are the protic salts (high) and [C3(NEt2)2(NHexMe)]+ salts (low).](/image/article/2012/RA/c2ra22078d/c2ra22078d-f1.gif) | ||
| Fig. 1 Viscosity at 20 °C vs. molecular weight of the cation for NTf2 ( and ) and DCA ( and ) salts. The outliers from the exponentially-fitted lines are the protic salts (high) and [C3(NEt2)2(NHexMe)]+ salts (low). | ||
For comparison, the perethyl ([C3(NEt2)3]+) and perallyl (C3(N(CH2CHCH2)3)]+) NTf2− and DCA tac salts are also reported here. Interestingly, the perallyl DCA salt has higher Tg, Tm and viscosity than the NTf2− salt whereas the perethyl salts fit the usual trends. It is also interesting to note that both the perallyl DCA salt and C3(NEt2)2(N(CH2CH2OH)2)]DCA exhibited an exothermic event upon thermal decomposition.
The protic ILs show higher viscosities than the peralkyl ILs of similar molecular weight due to increased hydrogen bonding between the cation and anion. It is thus noteworthy that the DCA protic salts have higher viscosities than the NTf2− protic salts, presumably due to the increased basicity of DCA. Similarly, the NH proton chemical shift is also indicative of the strength of the hydrogen bond to the anion10 with δ NH decreasing as the basicity of the anion decreases: For the cation [C3(NEt2)2(NHBu)]+, δ NH in CDCl3 decreases MeSO4− (8.14 ppm), DCA (7.92 ppm), BF4− (6.81 ppm) and NTf2− (6.22 ppm).11 The Tg for [C3(NEt2)2(NHBu)]DCA (−70 °C) is also higher than the peralkyl DCA salts and the NTf2− salts (−90 to −80 °C) and this reflects the increased viscosity of this salt. As would also be expected, the Td values are lower than for the peralkyl salts, with the DCA salt [C3(NEt2)2(NHBu)]DCA (233 °C) being the lowest for the protic salts. Nonetheless, Td values for the peralkyl DCA salts are still over 300 °C.
The protic salts are easily deprotonated (e.g. with BuLi or NaOH(aq)) to the corresponding diaminocyclopropenimine which can then be alkylated to provide another route to tac salts (Scheme 3). Here we have illustrated the use of BuLi and Me2SO4, however, it can reasonably be expected that a variety of alkylating agents could be used. Bandar and Lambert have recently reported the use of chiral diaminocyclopropenimines to carry out enantioselective Brønsted base catalysis which involves a protic tac intermediate.5
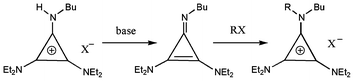 | ||
| Scheme 3 | ||
Reaction of 3X with ammonia gas gives the diprotic IL [C3(NEt2)2(NH2)]X. Unfortunately, these materials are very water sensitive with their conversion back to the cyclopropenone being driven by the loss of gaseous NH3.
Diethanolamine also gives a protic IL. In this case we also used iodoethane as the alkylating agent to give the iodide which was then converted to the NTf2− salt. As expected, these materials have higher Tg, Tm and viscosities than the peralkyl and NH protic salts due to their additional cation–cation hydrogen bonding. Td is quite low at 266 °C.
3MeSO4 was treated with the amino acids alanine, proline, valine and threonine to give the corresponding chiral ILs. When the salts were isolated by extraction of an aqueous solution with CH2Cl2, they were found to have significant amounts of the zwitterion (ca. 25%, 38%, 50% and 55%, respectively). The amount of zwitterion present did not vary significantly with the pH of the aqueous solution. When the anion was exchanged to NTf2−, the ILs were isolated with significantly less zwitterion (4%, 15%, 19% and 8%, respectively).
We carried out a series of catalysed aldol reactions (benzaldehyde with acetone) in the presence of our chiral ILs to investigate their potential effectiveness for enantioselective syntheses (Table 3). We found that whereas the proline- and valine-derived tac salts gave slight enhancements of ee (from 61% to 64%) for the L-proline-catalysed reaction, the alanine-derived tac salt gave a significant decrease in enantioselectivity (to 47%) indicating that the two chiral catalysts are uncooperative. We were surprised that changing to D-proline gave a similar decrease in ee (to 42%). Without added proline, the tac salts we tested gave only mild enhancements in ee.
| Tac salt | Added proline | ee (%) | Yield (%) |
|---|---|---|---|
| None | L-proline | 61 | 41 |
| [C3(NEt2)2(alanino)]MeSO4 | L-proline | 47 | 30 |
| [C3(NEt2)2(alanino)]MeSO4 | D-proline | 42 | 13 |
| [C3(NEt2)2(prolino)]MeSO4 | L-proline | 64 | 20 |
| [C3(NEt2)2(valino)]MeSO4 | L-proline | 64 | 34 |
| [C3(NEt2)2(alanino)]MeSO4 | none | 12 | 8 |
| [C3(NEt2)2(prolino)]MeSO4 | none | 9 | 2 |
| [C3(NEt2)2(valino)]MeSO4 | none | 33 | 2 |
In summary, we have described a facile synthesis to functionalised and reduced-symmetry tac ILs of the general formula [C3(NR2)2(NR′R′′]X. For the enantioselective reaction we investigated, only low enhancements in ee were found. We expect that this synthetic route will pave the way for a large variety of new tac ILs.
References
- For example: (a) N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123 RSC; (b) Ionic Liquids in Synthesis, ed. P. Wasserscheid and T. Welton, Wiley-VCH, 2003 Search PubMed; (c) Ionic Liquids as Green Solvents: Progress and Prospects, ed. R. D. Rogers and K. R. Seddon, ACS Symp. Ser., vol. 856, American Chemical Society, Washington D.C., 2003 Search PubMed; (d) Ionic Liquids IIIB: Fundamentals, Progress, Challenges, and Opportunities – Transformations and Processes, ed. R. D. Rogers and K. R. Seddon, ACS Symp. Ser., vol. 902, American Chemical Society, Washington D.C., 2005 Search PubMed; (e) Electrochemical Aspects of Ionic Liquids, ed. H. Ohno, Wiley-Interscience, Hoboken, 2005 Search PubMed.
- O. J. Curnow, D. R. MacFarlane and K. J. Walst, Chem. Commun., 2011, 47, 10248 RSC.
- R. Gompper and K. Schönafinger, Chem. Ber., 1979, 112, 1514 CrossRef CAS.
- (a) H. Bruns, M. Patil, J. Carreras, A. Vazquez, W. Thiel, R. Goddard and M. Alcarazo, Angew. Chem., Int. Ed., 2010, 49, 3680 CrossRef CAS; (b) R. Weiss, K. G. Wagner, C. Priesner and J. Macheleid, J. Am. Chem. Soc., 1985, 107, 4491 CrossRef CAS; (c) R. Weiss and R. H. Lowack, Bull. Soc. Chim. Belg., 1991, 100, 483 CrossRef CAS; (d) R. Gompper and K. Schönafinger, Chem. Ber., 1979, 112, 1535 CrossRef CAS.
- J. S. Bandar and T. H. Lambert, J. Am. Chem. Soc., 2012, 134, 5552 CrossRef CAS.
- (a) J. R. Butchard, O. J. Curnow, R. J. Pipal, W. T. Robinson and R. Shang, J. Phys. Org. Chem., 2008, 21, 127 CrossRef CAS; (b) J. R. Butchard, O. J. Curnow, D. J. Garrett and R. G. A. R. Maclagan, Angew. Chem., Int. Ed., 2006, 45, 7550 CrossRef CAS.
- A. Krebs, A. Güntner, S. Versteylen and S. Schulz, Tetrahedron Lett., 1984, 25, 2333 CrossRef CAS.
- R. Weiss, Tetrahedron Lett., 1979, 3295 Search PubMed.
- R. Breslow, T. Eicher, A. Krebs, R. A. Peterson and J. Posner, J. Am. Chem. Soc., 1965, 87, 1320 CrossRef CAS.
- M. S. Miran, H. Kinoshita, T. Yasuda, Md. A. B. H. Susan and M. Watanabe, Chem. Commun., 2011, 47, 12676 RSC.
- It should be recognised that there is also a concentration dependence to these chemical shifts.
Footnote |
| † Electronic Supplementary Information (ESI) available: experimental details of the syntheses and characterisations, as well as microanalytical and viscosity data. See DOI: 10.1039/c2ra22078d |
| This journal is © The Royal Society of Chemistry 2012 |




