Heterogeneously catalyzed Suzuki–Miyaura conversion of broad scope
Rosaria
Ciriminna
a,
Valerica
Pandarus
b,
Genevieve
Gingras
b,
François
Béland
*b,
Piera
Demma Carà
a and
Mario
Pagliaro
*a
aSiliCycle Inc., 2500 Parc-Technologique Blvd, Quebec City, Quebec G1P 4S6, Canada. E-mail: mario.pagliaro@cnr.it
bIstituto per lo Studio dei Materiali Nanostrutturati, CNR, via U. La Malfa 153, 90146 Palermo, Italy. E-mail: FrancoisBeland@silicycle.com
First published on 7th September 2012
The palladium catalyzed Suzuki–Miyaura coupling reaction is amongst the most important reactions in organic chemistry allowing the single step synthesis of symmetrical and unsymmetrical biaryls that are widely present in the structures of many types of polymers, natural products, pharmaceuticals and fine chemicals.1 In the reaction, mediated by a palladium catalyst, organoboron compounds such as readily available arylboronic acids are cross-coupled with aryl halides (Scheme 1), even if the scope of the reaction partners is not restricted to aryls, but includes vinyls, alkyls, alkenyls and alkynyls. | ||
| Scheme 1 The Suzuki–Miyaura coupling of arylboronic acids and haloarenes. | ||
The reaction tolerates a broad range of functional groups in the coupling partners and is usually performed in solution under homogeneous conditions at T ≥ 60 °C using 2–3 mol% catalytic amounts. The catalyst is often a Pd(0) complex with triarylphosphane ligands.2
The catalytic cycle (Scheme 2) begins with the oxidative addition of an aryl halide to a Pd(0) species formed in situ to form an arylpalladium(II) halide intermediate.3
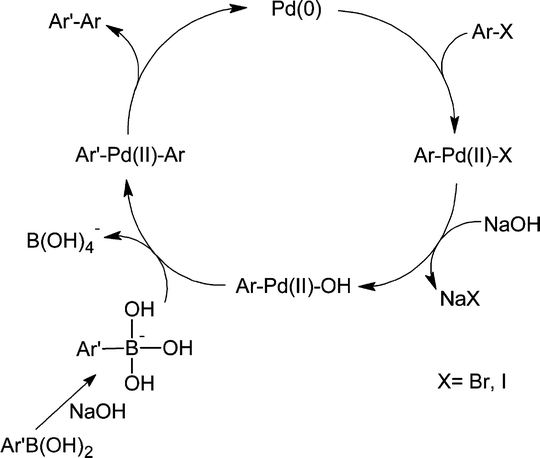 | ||
| Scheme 2 A general Suzuki–Miyaura catalytic cycle. Oxidative addition is followed by transmetallation and reductive elimination. Boronic acid is activated with base. | ||
Chloroarenes, especially nonactivated aryl chlorides, are notoriously less reactive due to the stability of the C–Cl bond (the relative reactivity of Ar–X is correlated to the respective bond dissociation energy: Ph–Cl: 96 kcal mol−1, Ph–Br: 81 kcal mol−1, Ph–I: 65 kcal mol−1). This, from a practical viewpoint adds cost to the products of traditional Suzuki–Miyaura reactions, because aryl chlorides are considerably less costly than iodo and bromoarenes.
Another significant problem preventing widespread utilization of palladium homogeneous catalysis lies in the Pd impurities left in the reaction product since the upper limit for residual Pd levels in active pharmaceutical ingredients is typically very low (less than 5 ppb).4 Removing residual palladium in a pharmaceutical substance to reduce its content to the maximum acceptable concentration limit requires rigorous product–catalyst separation processing (a purification process that often makes use of silica-based Pd scavengers).
Intense research activities have therefore being devoted in the last decade to finding heterogeneous Pd catalysts of broad scope, capable of affording the recovery and reuse of the valued palladium while avoiding time-consuming catalyst separation and product purification steps which impact cost and worsen the environmental footprint of the reaction.5
In this context, we have recently reported that SiliaCat Pd(0) heterogeneously mediates the cross-coupling of iodo and bromoarenes under reflux selectively affording high yields of the coupled products.6 This reusable catalyst is made of ultrasmall Pd(0) nanoparticles (2–5 nm, depending on the sol–gel synthesis parameters)7 highly dispersed in the inner porosity of an organosilica matrix that, in its turn, ensures the high chemical and physical stabilization of the entrapped nanoparticles.
We show herein how to broaden the scope of the method to convert also readily available aryl chlorides and access extremely high reaction rates for aryl chlorides and bromides using microwave irradiation. In each case, no inert atmosphere is required and the reaction is carried out in environmentally benign aqueous alcohol.
In general, reactions were carried out in aqueous MeOH as protic solvents are generally required for optimal reaction over the SiliaCat, while K2CO3 is the base normally employed. The synthesis of a typical SiliaCat Pd(0) catalyst has been described elsewhere.6 Catalyst samples used throughout this work are those now commercially available following optimization in light of manufacturing requirements (Table 1).
| Name | Pd Loading (mmol g−1) | Surface m2 g−1 | Pore Size Range Å (average) |
|---|---|---|---|
| Si-Pd-1 | 0.05 | 754 | 40 |
| Si-Pd-2 | 0.11 | 774 | 45 |
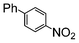
The catalysts in Table 1 were thus first tested both under reflux and at room temperature in the conversion of aryl iodides under the optimised conditions of Table 2. The results show that, the Suzuki–Miyaura conversion mediated by 0.5 mol% SiliaCat Pd(0) can also be carried out at room temperature, with complete conversion of the substrate after 6.5 h (entry 3 in Table 2). The reaction time can be lowered to 2 h upon doubling the catalyst amount to 1 mol% (entry 4).
| Run | Substrate | Catalyst mol (%) | PhB(OH)2 (eq) | Base (eq) | Solventb (M) | Temp/Time | Coupled product | Convc % | TON | TOF (h−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| a Experimental conditions: Reactions performed in HPLC grade methanol at reflux. Molar concentration is with respect to the substrate. Substrate (0.8 mmol, 1 eq), catalyst, 0.004 or 0.008 mmol Pd, MeOH (10 mL), phenylboronic acid (0.88 mmol, 1.2 eq), K2CO3 (1.6 mmol, 2 eq). b Molar concentration with respect to the substrate. c Conversion in the coupled product by GC-MS analysis. | ||||||||||
| 1 |
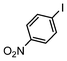
|
Si-Pd-1 | 1.1 | K2CO3 | MeOH | Reflux |
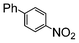
|
100 | 200 | 2500 |
| 0.5 | 2 | 0.08 M | 5 min | |||||||
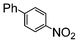
| 2 |
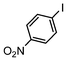
|
Si-Pd-2 | 1.1 | K2CO3 | MeOH | Reflux |
|
100 | 200 | 2500 |
| 0.5 | 2 | 0.08 M | 5 min | |||||||
| 3 |
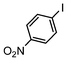
|
Si-Pd-2 | 1.1 | K2CO3 | MeOH/H2O | RT |
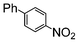
|
100 | 200 | 31 |
| 0.5 | 2 | 0.02 M | 6.5 h | |||||||
| 4 |
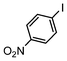
|
Si-Pd-2 | 1.1 | K2CO3 | MeOH/H2O | RT |
|
100 | 100 | 50 |
| 1 | 2 | 0.02 M | 2 h | |||||||
The coupling of aryl bromides generally requires reflux conditions. A wide variety of substrates bearing both electron withdrawing and electron donating groups are generally coupled with phenylboronic acid over 0.5 to 1 mol% catalyst affording good to excellent yields of coupled products in aqueous methanol or aqueous ethanol (Table 3); whereas aqueous propanol (entry 7) requires longer reaction times and higher amounts of catalyst. In general, reaction times of 1 to 6 h are common, depending on the substrate.
| Run | Substrate | Catalyst mol (%) | PhB(OH)2 (eq) | Base (eq) | Solventa (M) | Temp/Time | Coupled product | Conv.b (%) | TON | TOF |
|---|---|---|---|---|---|---|---|---|---|---|
| a Molar concentration with respect to the substrate. b Conversion in the coupled product determined by GC-MS analysis. | ||||||||||
| 1 |
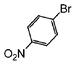
|
Si-Pd-2 | 1.1 | K2CO3 | MeOH | Reflux |
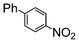
|
100 | 200 | 133 |
| 0.5 | 2 | 0.13 M | 1.5 h | |||||||
| 2 |
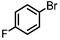
|
Si-Pd-2 | 1.1 | K2CO3 | EtOH/H2O | Reflux |
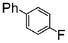
|
78 | 200 | 100 |
| 30 min | ||||||||||
| 0.5 | 2 | (0.1 M) | 2 h | 100 | ||||||
| 3 |
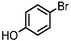
|
1.1 | K2CO3 | EtOH/H2O | Reflux |
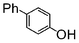
|
— | — | ||
| Si-Pd-2 | 2 h | 53 | ||||||||
| 0.5 | 2 | (0.1 M) | 6 h | 54 | ||||||
| 4 |
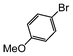
|
1.1 | K2CO3 | EtOH/H2O | Reflux |
|
— | — | ||
| Si-Pd-2 | 1 h | 75 | ||||||||
| 0.5 | 2 | (0.1 M) | 3 h | 63 | ||||||
| 5 |
|
1.1 | Cs2CO3 | EtOH/H2O | Reflux |
|
— | — | ||
| 3 h | 71 | |||||||||
| Si-Pd-2 | 6 h | 72 | ||||||||
| 2 | 2 | (0.12 M) | 24 h | 72 | ||||||
| 6 |
|
1.1 | K2CO3 | EtOH/H2O | Reflux |
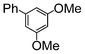
|
— | — | ||
| Si-Pd-2 | 2 h | 50 | ||||||||
| 0.5 | 2 | (0.1 M) | 4 h | 52 | ||||||
| 7 |
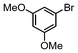
|
Si-Pd-2 | 1.25 | K2CO3 | PrOH/H2O | Reflux |
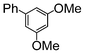
|
59 | — | — |
| 1 | 2 | (0.1 M) | 5 h | |||||||
| 8 |
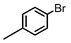
|
1.1 | K2CO3 | EtOH/H2O | Reflux |
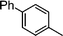
|
— | — | ||
| Si-Pd-2 | 1 h | 78 | ||||||||
| 0.5 | 2 | (0.1 M) | 3 h | 45 | ||||||
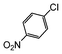
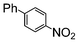
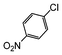
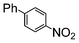
Under reflux, chloroarenes are generally not converted. For example, Table 4 shows that 1-chloro-4-nitrobenzene (entry 3) does not react with phenylboronic acid over the SiliaCat Pd(0). A modest 35% conversion into the coupled product is observed only in the presence of a phosphine ligand under an Ar atmosphere (entry 4).
| Run | Substrate | Catalyst (mol%) | PhB(OH)2 (eq) | Base (eq) | Solventa (M) | Temp/Time | Coupled product | Conv.b (%) | TON | TOF |
|---|---|---|---|---|---|---|---|---|---|---|
| a Molar concentration with respect to the substrate. b Conversion of the substrates in the coupled product determined by GC-MS analysis. | ||||||||||
| 1 |

|
Si-Pd-2 | 1.1 | K2CO3 | MeOH | Reflux |
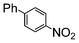
|
100 | 200 | 2500 |
| 0.5 | 2 | 0.08 M | 5 min | |||||||
| 2 |
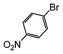
|
Si-Pd-2 | 1.1 | K2CO3 | MeOH | Reflux |
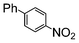
|
100 | 200 | 133 |
| 0.5 | 2 | 0.3 M | 1 h 30 | |||||||
| 3 |
|
Si-Pd-2 | 1.13 | K2CO3 | EtOH/H2O | Reflux |
|
0 | — | — |
| 0.1 | 2 | 0.125 M | 23 h | |||||||
| 4 |
|
Si-Pd-2 | 1.1 | K2CO3 | PrOH/H2O | Reflux under Ar |
|
35 | — | — |
| 1 | ||||||||||
| P(t-Bu)3 (0.03 eq) | 2 | 0.125 M | 22 h | |||||||
Leadbeater has shown that microwaves are an effective alternative means to heat the reaction mixture and obtain the cross-coupling of various substrates, including unreactive chloroarenes.8 We thus conducted the conversion of aryl chlorides over SiliaCat Pd(0) under microwave irradiation (Table 5).
| Run | Substrate | Catalyst mol (%) | Solventa (M) | Microwave Conditions | Coupled product | Conv. b (%) | TON | TOF | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Power (W) | PSI | T/°C | t (min) | ||||||||
| a Molar concentration of the mixture with respect to the substrate. b Conversion of the substrates in the coupled product determined by GC-MS analysis. | |||||||||||
| 1 |
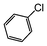
|
Si-Pd-1 | MeOH | 200 | 200 | 100 | 5 |
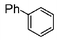
|
100 | 200 | 2500 |
| 0.5 | 0.2 M | ||||||||||
| 2 |
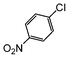
|
Si-Pd-1 | MeOH | 200 | 200 | 100 | 5 |
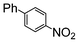
|
100 | 200 | 2500 |
| 0.5 | 0.2 M | ||||||||||
| 3 |
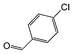
|
Si-Pd-1 | MeOH | 200 | 200 | 100 | 10 |
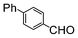
|
100 | 200 | 1250 |
| 0.5 | 0.2 M | ||||||||||
| 4 |
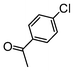
|
Si-Pd-1 | EtOH/H2O | 200 | 200 | 120 | 15 |
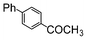
|
53 | — | — |
| 1 | 0.2 M | ||||||||||
| 5 |
|
Si-Pd-1 | MeOH | 200 | 200 | 100 | 10 |
|
64 | — | — |
| 0.5 | 0.2 M | ||||||||||
| 6 |
|
Si-Pd-1 | EtOH/H2O | 200 | 200 | 100 | 15 |
|
38 | — | — |
| 0.5 | 0.2 M | ||||||||||
| 7 |
|
Si-Pd-1 | MeOH | 200 | 200 | 100 | 15 |

|
0 | — | — |
| 0.5 | 0.2 M | ||||||||||
| 8 |
|
Si-Pd-1 | MeOH | 200 | 200 | 100 | 15 |
|
0 | — | — |
| 0.5 | 0.2 M | ||||||||||
| 9 |
|
Si-Pd-1 | MeOH | 200 | 200 | 100 | 15 |
|
0 | — | — |
| 0.5 | 0.2 M | ||||||||||
| 10 |
|
Si-Pd-1 | MeOH | 200 | 200 | 100 | 15 |
|
53 | — | — |
| 0.5 | 0.2 M | ||||||||||
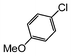
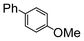
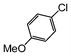
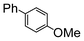
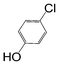
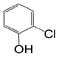
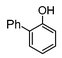
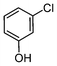
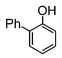
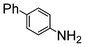
Here, most of the substrates tested were cross-coupled to phenylboronic acid in short reaction times ranging from 5 through to 15 min. Only the isomers of chlorophenol (entries 7–9) did not react, while p-chloroanisole (entry 6) afforded the coupled product in 38% yield.
The cross-coupling of aryl bromides under microwave irradiation proceeds 20-to-100 times faster than under reflux. A substrate such as p-bromophenol under reflux afforded a maximum 54% yield after 6 h (entry 3 in Table 3), however, it is entirely converted after only 5 min here (entry 3 in Table 6).
| Run | Substrate | Catalyst (mol%) | Solventa (M) | Microwave Conditions | Coupled product | Conv.b (%) | TON | TOF | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| W | PSI | T/°C | t (min) | ||||||||
| a Molar concentration of the mixture with respect to the substrate. b Conversion of the substrates in the coupled product determined by GC-MS analysis. | |||||||||||
| 1 |
|
Si-Pd-1 | MeOH | 200 | 200 | 100 | 5 |
|
100 | 1000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| 0.1 | 0.1 M | ||||||||||
| 2 |
|
Si-Pd-1 | MeOH | 150 | 150 | 75 | 5 |
|
100 | 1000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| 0.1 | 0.1 M | ||||||||||
| 3 |
|
Si-Pd-1 | MeOH | 150 | 150 | 75 | 5 |
|
100 | 1000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| 0.1 | 0.1 M | ||||||||||
| 4 |
|
Si-Pd-1 | MeOH | 200 | 200 | 100 | 5 |
|
100 | 1000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| 0.1 | 0.2 M | ||||||||||
| 5 |
|
Si-Pd-1 | MeOH | 150 | 150 | 75 | 5 |
|
100 | 1000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| 0.1 | 0.1 M | ||||||||||
| 6 |
|
Si-Pd-1 | MeOH | 150 | 150 | 75 | 5 |
|
100 | 1000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| 0.1 | 0.1 M | ||||||||||
| 7 |
|
Si-Pd-1 | MeOH | 150 | 150 | 75 | 5 |
|
100 | 1000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| 0.1 | 0.1 M | ||||||||||
| 8 |
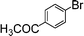
|
Si-Pd-1 | MeOH | 200 | 200 | 75 | 5 |
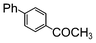
|
100 | 1000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| 0.1 | 0.2 M | ||||||||||
| 9 |
|
Si-Pd-1 | EtOH/H2O | 200 | 200 | 125 | 20 |
|
88 | — | — |
| 0.1 | 0.234 M | ||||||||||
| 10 |
|
Si-Pd-1 | EtOH/H2O | 200 | 200 | 125 | 15 |
|
73 | — | — |
| 0.1 | 0.234 M | ||||||||||
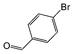
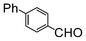
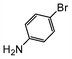
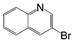
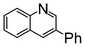
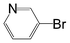
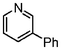
A comparison of the results in Tables 3 and 6 indeed shows that the TOF values are 10 to 100 times higher than that under reflux conditions. Showing the versatility of the method, heterocycles such as 3-bromoquinoline (entry 9) and 3-bromopyridine (entry 10) are also smoothly converted into the coupled product.
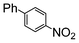
The reaction under microwave irradiation catalysis remains heterogeneous. Indeed, the hot filtration test of the reaction mixtures under reflux with 1-iodo-4-nitrobenzene in different aqueous alcohol solvents over SiliaCat Pd(0) showed that no further reaction takes place in the filtrate. This shows that the small amount of leached Pd(0) nanoparticle species are catalytically inactive. Indeed, the limited leaching of Pd was experimentally observed with the levels of Pd leached in the crude product being generally <8 ppm (Table 7).
| Run | Conditionsa | Coupled product (100% conversion) | Leachingb (ppm) | |
|---|---|---|---|---|
| Pd | Si | |||
| a 1-Iodo-4-nitrobenzene (1 eq), phenylboronic acid (1 eq), SiliaCat Pd(0) 0.5 mol%, reflux. b The levels of Pd and Si were determined by inductively coupled plasma (ICP) analysis in the crude product after work-up. | ||||
| 1 | Si-Pd-2 0.5 mol (%) |
|
7.8 | 0.2 |
| K2CO3 1.5 eq | ||||
| MeOH 0.055 M | ||||
| 2 | Si-Pd-2 0.5 mol (%) |
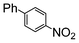
|
5.8 | 2.3 |
| K2CO3 1.5 eq | ||||
| EtOH 0.055 M | ||||
| 3 | Si-Pd-2 0.5 mol (%) |
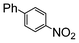
|
7.8 | 16.6 |
| K2CO3 1.5 eq | ||||
| PrOH 0.055 M | ||||
| 4 | Si-Pd-2 0.5 mol (%) |
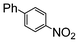
|
4.5 | 37.6 |
| K2CO3 1.5 eq | ||||
| BnOH 0.08 M | ||||
It is relevant here to note that for analogous Suzuki reactions mediated by Pd/C in aqueous solvent, Chuen et al. have shown that Pd-leaching (mainly due to the oxidative addition of aryl-bromides to Pd) is independent of the reaction solvent and temperature and that only the Pd(0) nanoparticles, and not the Pd2+ ions, once leached in solution are catalytically active.9 It is also relevant that Suzuki–Miyaura coupling reactions of aryl bromides and iodides under microwave irradiation can be catalyzed at completion even by 50 ppb of Pd contaminating the commercially available sodium carbonate.10 Recycling of the catalyst was possible, with full retention of the activity of the catalyst in all 5 consecutive reaction runs in which the catalyst was reused using 1-iodo-4-nitrobenzene as the representative substrate.
In conclusion, the important Suzuki–Miyaura reaction can be smoothly carried out over a catalytic amount (0.5–1 mol%) of nanostructured palladium (0) organosilica catalyst. The conversion of aryl iodides can be carried out even at room temperature while aryl bromides generally require conversion under reflux. Low levels of leached Pd are observed and catalysis is truly heterogeneous. The conversion of readily available chloroarenes requires microwave heating. Under 200 W power irradiation, aryl chlorides are coupled to arylboronic acid affording good yields of coupled products, while aryl bromides are converted in extremely short reaction times. In each case, no inert atmosphere is required to carry out the reaction, thereby opening the route to widespread utilization of this simple, green methodology for the clean and sustainable synthesis of organic compounds through the Suzuki–Miyaura reaction.
Experimental section
For reactions conducted under reflux, a two-necked round bottom flask was equipped with a condenser and a rubber stopper. The substrate, phenylboronic acid and base were mixed in HPLC solvent. The mixture was heated under reflux for 10 min (or until complete homogenization), after which the catalyst was added.For reactions under microwave irradiation, the substrate, phenylboronic acid and base were mixed in HPLC solvent in a microwave tube. The tube was placed in a sonicator for 5 min to ensure complete homogenization, after which the catalyst was added. The tube was inserted into the sample holder of a Discover system (CEM, Matthews, USA). Following the setting of the reaction parameters (power, pressure and temperature) the reaction was started. GC-MS analysis was used to assess conversion. Yields were also measured by isolating the reaction products showing full agreement with the yield values obtained by GC-MS analysis. In a typical procedure, the reaction mixture was washed with EtOAc and water three times. The organic layer was separated each time via gravity and stirred with sodium sulfate to remove water. The mixture was then filtered to remove Na2SO4. After extraction with EtOAc filtration, the solvent was removed by a rotovapor to obtain a solid that was weighed and analysed by GC-MS analysis to identify the reaction products. Leaching was assessed by ICP analysis of the isolated crude product.
Acknowledgements
This article is dedicated to Dr Cesare Spciale, for a memorable stay of one of us (M.P.) in New York City back in 1996. We thank Mr. Pierre-Gilles Vaillancourt and Mr Simon Bédard from the Quality Control Department of SiliCycle Inc. for their valuable contribution.References
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- C. Barnard, Platinum Met. Rev., 2008, 52, 38 CrossRef CAS.
- A. Herath and N. D. P. Cosford, Org. Lett., 2010, 12, 5182–5185 CrossRef CAS.
- C. E. Garrett and K. Prasad, Adv. Synth. Catal., 2004, 346, 889 CrossRef CAS.
- A. Molnár, Chem. Rev., 2011, 111, 2251–2320 CrossRef.
- M. Pagliaro, V. Pandarus, F. Béland, R. Ciriminna, G. Palmisano and P. Demma Carà, Catal. Sci. Technol., 2011, 1, 736–739 Search PubMed.
- V. Pandarus, F. Béland, R. Ciriminna, P. Demma Carà and M. Pagliaro, Catal. Lett., 2012, 142, 213–217 CrossRef CAS.
- N. E. Leadbeater, Chem. Commun., 2005, 2881–2902 RSC.
- J.-S. Chen, A. N. Vasiliev, A. P. Panarello and J. G. Khinast, Appl. Catal., A, 2007, 325, 76–86 CrossRef CAS.
- R. K. Arvela, N. E. Leadbeater, M. S. Sangi, V. A. Williams, P. Granados and R. D. Singer, J. Org. Chem., 2005, 70, 161–168 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
