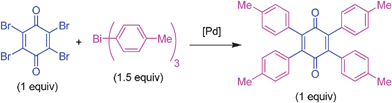
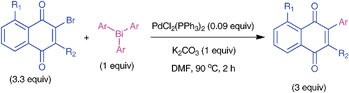
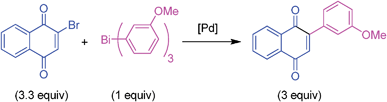Pd-catalyzed threefold arylations of mono, di and tetra-bromoquinones using triarylbismuth reagents†
Maddali L. N.
Rao
* and
Somnath
Giri
Department of Chemistry, Indian Institute of Technology Kanpur, Kanpur 208 016, India. E-mail: maddali@iitk.ac.in; Fax: +91 5122597532
First published on 11th October 2012
Abstract
The palladium-catalyzed efficient cross-couplings of mono-, di- and tetra- bromo-1,4-quinones with triarylbismuth reagents were developed, involving threefold arylation. This first time study which is demonstrated with a wide variety of substrates possesses high synthetic utility and furnished a plethora of arylated 1,4-quinones in a one-pot operation.
Introduction
Substituted 1,4-quinones are important structural motifs which are known to exhibit various pharmacological properties.1–3 These compounds are also widely used in organic synthesis.4–10 In recent years, metal catalyzed couplings are in high demand as the most dependable work tools in synthetic organic chemistry.11 These coupling processes are also useful to expand the core molecular complexity of quinone structures.12–14 For example, Liebeskind et al. reported the palladium-mediated oxidative dimerization of stannyl quinones to give symmetrical 2,2′-bisquinones.15 Arylquinone core structures are routinely used for medicinal studies.16 2-Bromo-1,4-quinones or its derivatives which are easily accessible were employed, for example, (i) in dimerization reactions,17 (ii) in cycloaddition reactions,18 (iii) in the synthesis of benz[f]indole-4,9-diones, indolequinones and other derivatives,19,20 (iv) in the synthesis of 1,6,7,8-tetrahydro-naphtho[2,3-d]-azepino[4,5-b]indol-9,14-diones useful in pro-inflammatory cytokines inhibitory effect studies,21 (v) in palladium-catalyzed intramolecular cyclizations,22etc. Additionally, these substrates also react with organometallic diphenyl diselenide to give 2-(phenylseleno)naphthoquinone.23Metal catalyzed coupling reactions of quinone boronic esters with aryl halides are known to furnish arylquiones.24 However, synthesis of quinone boronic esters is not a straight forward process. Alternately, cross-coupling reactions of bromoquinones provides an easy access to arylated quinones with organometallic reagents.25,26 However, the cross-coupling chemistry of bromoquinones with organometallic reagents with multi-coupling capabilities was not reported so far. In that context, the present study with triarylbismuths is important for the following reasons: (i) the increase in applications of cross-coupling reactions in bulk scale preparations demands new capabilities,11 (ii) arylboronic acids, organotin reagents usually provide only one C–C coupling, (iii) triarylbismuths which are non-toxic and air stable are equipped with unique threefold arylating capabilities under metal-catalyzed conditions,27 (iv) couplings with triarylbismuths would deliver a protocol which is operationally simple, will not generate toxic inorganic waste unlike organotin reagents and would react under simple and mild conditions with threefold arylation in short reaction times etc. Hence, it was envisaged that couplings of bromoquinones with triarylbismuth reagents would furnish synthetically useful cross-coupling methodology with threefold arylations. Herein, we report these findings under palladium-catalyzed conditions.
Results and discussion
Initially, the catalytic protocol was optimized for the cross-coupling reaction of 2-bromo-1,4-naphthoquinone with tris(3-methoxyphenyl)bismuth as given in Table 1. In the study with different palladium catalysts (Table 1, entries 1-3), PdCl2(PPh3)2 was found to be more efficient and the desired cross-coupling product, 2-phenyl-1,4-naphthoquinone, was obtained in 88% yield (Table 1, entry 3). Scrutiny with different solvents using N,N-dimethylacetamide (DMA), acetonitrile, N-methyl-2-pyrrolidinone (NMP) and 1,4-dioxane provided yields of 68–80% (Table 1, entries 4–7). Different bases such as Cs2CO3, K3PO4, KOAc and Na2CO3 were tested for their efficacy. These bases delivered 74–80% yields (Table 1, entries 8–11). This investigation with different solvents and bases revealed that the palladium catalytic system is more effective with K2CO3 base in DMF solvent to furnish a high product yield (Table 1, entry 3). A few control experiments with an excess amount of base did not improve the yield considerably (Table 1, entries 12 and 13). The reactions carried out at 60 °C and 40 °C yielded 71% and 56% yield respectively (Table 1, entries 14 and 15). Couplings with 1 h reaction time or without base furnished lower yields (Table 1, entries 16 and 17). Expectedly, an additional control reaction without catalyst did not furnish the cross-coupled product (Table 1, entry 18). From this optimization study, we were pleased to note that the protocol involving PdCl2(PPh3)2 K2CO3 in DMF at 90 °C for 2 h is effective to give a high cross-coupling yield (Table 1, entry 3).| Entry | Catalyst/ligand | Base | Solvent | Temperature (°C) | Yield (%)b |
|---|---|---|---|---|---|
| a Reaction conditions: 2-Bromo-1,4-naphthoquinone (0.825 mmol, 3.3 equiv.), tris(3-methoxyphenyl)bismuth (0.25 mmol, 1.0 equiv.), Pd catalyst (0.0225 mmol, 0.09 equiv.), base (0.25 mmol, 1.0 equiv.), solvent (3 mL), 2 h. b Isolated yields with reference to three aryl couplings from tris(3-methoxyphenyl)bismuthine. Homo-coupled 3,3-dimethoxy biphenyl from bismuth reagent was formed in minor amounts. c With K2CO3 (2 equiv.). d With K2CO3 (4 equiv.). e Reaction time 1 h. f Control reaction without base. g Control reaction without catalyst. | |||||
| 1. | Pd(OAc)2/4 PPh3 | K2CO3 | DMF | 90 | 66 |
| 2. | Pd(PPh3)4 | K2CO3 | DMF | 90 | 68 |
| 3. | PdCl2(PPh3)2 | K2CO3 | DMF | 90 | 88 |
| 4. | PdCl2(PPh3)2 | K2CO3 | DMA | 90 | 80 |
| 5. | PdCl2(PPh3)2 | K2CO3 | MeCN | 90 | 66 |
| 6. | PdCl2(PPh3)2 | K2CO3 | NMP | 90 | 78 |
| 7. | PdCl2(PPh3)2 | K2CO3 | 1,4-dioxane | 90 | 68 |
| 8. | PdCl2(PPh3)2 | Cs2CO3 | DMF | 90 | 76 |
| 9. | PdCl2(PPh3)2 | K3PO4 | DMF | 90 | 78 |
| 10. | PdCl2(PPh3)2 | KOAc | DMF | 90 | 80 |
| 11. | PdCl2(PPh3)2 | Na2CO3 | NMP | 90 | 74 |
| 12. | PdCl2(PPh3)2 | K2CO3 | DMF | 90 | 89c |
| 13. | PdCl2(PPh3)2 | K2CO3 | DMF | 90 | 75d |
| 14. | PdCl2(PPh3)2 | K2CO3 | DMF | 60 | 71 |
| 15. | PdCl2(PPh3)2 | K2CO3 | DMF | 40 | 56 |
| 16. | PdCl2(PPh3)2 | K2CO3 | DMF | 90 | 73e |
| 17. | PdCl2(PPh3)2 | none | DMF | 90 | 51f |
| 18. | none | K2CO3 | DMF | 90 | —g |
This optimized condition is synthetically useful from the viewpoint that the threefold arylations using triarylbismuths were effected in two hours to deliver 2-aryl-1,4-naphthoquinones in high yield. In comparison, the literature known methods involving using boron25 and tin26 reagents need longer reaction times, even for single C–C couplings.
To elaborate this study, the above optimized conditions were employed in the couplings of 2-bromo-1,4-naphthoquinone with different triarylbismuth reagents (Table 2). Coupling reactions of 2-bromo-1,4-naphthoquinone were very effective to furnish the corresponding 2-aryl-1,4-naphthoquninones in high yields. It was satisfying to see that the couplings of various triarylbismuths with electronically different aryls proved to be equally efficient with an excellent cross-coupling reactivity giving 81–93% yields.
| a Reaction conditions: 2-Bromo-1,4-naphthoquinone (0.825 mmol, 3.3 equiv.), BiAr3 (0.25 mmol, 1 equiv.), K2CO3 (0.25 mmol, 1 equiv.), PdCl2(PPh3)2 (0.0225 mmol, 0.09 equiv.), DMF (3 mL), 90 °C, 2 h. Isolated yields based on three aryl couplings from BiAr3. Homo-coupled biaryl products formed in minor amounts. All the products were identified by 1H, 13C-NMR, EI/ESI HRMS and IR spectroscopic analyses. | ||
|---|---|---|
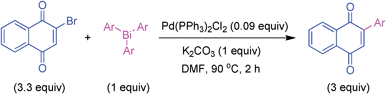
|
||
|
|
|
|
|
|
|
|
|
|
|
|
To expand the synthetic scope of this protocol further, differently substituted 2-bromo-1,4-naphthoquinones were tested (Table 3). This study demonstrated high coupling reactivity of both 2-bromo-1,4-naphthoquinones and bismuth reagents furnishing high yields in the majority of cases. For example, the coupling reaction of 2-bromo-8-methoxy-1,4-naphthoquinone readily furnished high product yields with different bismuth reagents. Similarly, 3-benzyl substituted 2-bromo-1,4-naphthoquinone also fared well despite the presence of imposing sterics due to the benzyl group. These reactions also gave coupled products in moderate to high yields. Notably, multi-functional Baylis–Hillman derivative of 2-bromo-1,4-naphthoquinone also furnished high product yields with double bond intact and without any of the side reactions that may be expected under palladium catalysis. Overall, it is of note that this coupling study provided a wide variety of mono- and di-substituted 2-aryl-1,4-naphthoquinones embedded with multiple functionalities in a short reaction time.
| a Reaction conditions: Substituted 2-bromo-1,4-naphthoquinone (0.825 mmol, 3.3 equiv.), BiAr3 (0.25 mmol, 1 equiv.), PdCl2(PPh3)2 (0.0225 mmol, 0.9 equiv.), K2CO3 (0.25 mmol, 1 equiv.), DMF (3 mL), 90 °C, 2 h. Isolated yields based on three aryl couplings from BiAr3. Homo-coupled biaryls formed in minor amounts. All products were characterized by 1H, 13C-NMR, EI/ESI HRMS and IR spectroscopy. | ||
|---|---|---|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
It is to be highlighted that the established cross-coupling protocols using triarylbismuths are synthetically useful in the arylations of 2-bromo-1,4-naphthoquines to access a variety of 2-aryl-1,4-naphthoquinones and 2,3-disubstituted 1,4-naphthoquinones in high yields in short times. Triarylbismuths as threefold arylating agents demonstrated three C–C couplings with 2-bromo-1,4-quinones in a one-pot operation. The same is not possible with literature known methods using boron and tin reagents. Synthetically, 2-aryl-1,4-naphthoquinones can also be obtained by other means such as (i) Dotz benzannulation involving chromium carbene complexes,28 (ii) oxidations using cerium(IV) and manganese(III) of the corresponding aromatic substrates.29 However, the present general method is advantageous and outweighs other methods, as the process involves threefold arylations from triarylbismuths in a one-pot operation and delivers a plethora of differently functionalized 2-aryl-1,4-naphthoquinones in high yields.
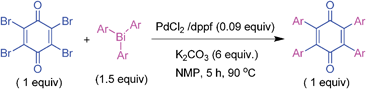
At this stage, we turned our attention to 2,3,5,6-tetrabromo-1,4-benzoquinone (bromanil) or tetrabromo-p-benzoquinone which is an easily accessible and well utilized substrate in organic synthesis.6–8,30 It was of interest to explore its coupling reactivity with triarylbismuth reagents. At first, the investigation was carried out with tetrabromo-p-benzoquinone and tris(4-methylphenyl)bismuth as given in Table 4. The reaction of 1 equivalent of tetrabromo-p-benzoquinone was tested with 1.5 equivalent of bismuth reagent under the earlier optimized conditions (Table 4, entry 1). This reaction furnished the desired tetraphenyl-p-quinone in 44% yield. To improve further, screening was conducted with different catalysts (Table 4, entries 2 and 3). However, the desired product was not formed in high yield. At this stage, it was decided to employ a dppf ligand to check its efficacy in tetrabromide couplings (Table 4, entry 4). Encouragingly, this reaction afforded product in 72% yield. Hence, screening was carried out in other solvents. Of various solvents studied, NMP proved to be a better choice with 78% yield (Table 4, entry 5). Tetrahydrofuran, 1,2-dimethoxyethane (DME) and DMA solvents furnished poor yields (Table 4, entries 6–8). Screening with different bases was conducted using Cs2CO3, K3PO4 and Na2CO3 in NMP solvent, and this effort provided lower yields (Table 4, entries 9–11). The cross-coupling was also found to be ineffective with lower loadings of the catalyst, ligand or base, or at lower temperature (Table 4, entries 12–16). Very poor or no conversion was obtained without the addition of base or catalyst (Table 4, entries 17 and 18). Finally, the protocol with PdCl2/dppf with K2CO3 (6 equiv.) in NMP at 90 °C for 5 h was chosen as the optimized conditions for tetrabromide couplings (Table 4, entry 5).
| Entry | Catalyst/ligand | Base | Solvent | Temperature (°C) | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: Tetrabromo-p-benzoquinone (0.25 mmol, 1 equiv.), tri(4-methylphenyl)bismuth (0.375 mmol, 1.5 equiv.), Pd catalyst (0.0225 mmol, 0.09 equiv.), PPh3 (0.9 mmol, 0.36 equiv.) or dppf (0.0225 mmol, 0.09 equiv.), base (1.5 mmol, 6.0 equiv.), solvent (3 mL), 5 h. Isolated yields based on tetrabromo-p-benzoquinone. Homo-coupled biaryls were formed in minor amounts. b Tetrabromo-p-benzoquinone (0.25 mmol, 1 equiv.), tri(4-methylphenyl)bismuth (0.375 mmol, 1.5 equiv.), PdCl2(PPh3)2 (0.0225 mmol, 0.09 quiv), K2CO3 (0.25 mmol, 1 equiv.), DMF (3 mL), 90 °C, 2 h. c With PdCl2/dppf (0.0025 mmol, 0.01 equiv.). d K2CO3(1.00 mmol, 4 equiv.). e K2CO3(1.25 mmol, 5 equiv.). f Control reaction without base. g Control reaction without catalyst. | |||||
| 1. | PdCl2(PPh3)2 | K2CO3 | DMF | 90 | 44b |
| 2. | Pd(OAc)2/4 PPh3 | K2CO3 | DMF | 90 | 30 |
| 3. | PdCl2/4 PPh3 | K2CO3 | DMF | 90 | 62 |
| 4. | PdCl2/dppf | K2CO3 | DMF | 90 | 72 |
| 5. | PdCl2/dppf | K2CO3 | NMP | 90 | 78 |
| 6. | PdCl2/dppf | K2CO3 | THF | 90 | 35 |
| 7. | PdCl2/dppf | K2CO3 | DME | 90 | 48 |
| 8. | PdCl2/dppf | K2CO3 | DMA | 90 | 44 |
| 9. | PdCl2/dppf | Cs3CO3 | NMP | 90 | 36 |
| 10. | PdCl2/dppf | K3PO4 | NMP | 90 | 38 |
| 11. | PdCl2/dppf | Na2CO3 | NMP | 90 | 55 |
| 12. | PdCl2/dppf | K2CO3 | NMP | 90 | 27c |
| 13. | PdCl2/dppf | K2CO3 | DMF | 60 | 35 |
| 14. | PdCl2/dppf | K2CO3 | DMF | 80 | 55 |
| 15. | PdCl2/dppf | K2CO3 | DMF | 90 | 60d |
| 16. | PdCl2/dppf | K2CO3 | DMF | 90 | 64e |
| 17. | PdCl2/dppf | — | DMF | 90 | 26f |
| 18. | — | K2CO3 | DMF | 90 | —g |
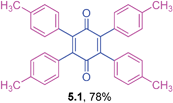
The general coupling ability of tetrabromo-p-benzoquinone with different triarylbismuth reagents is summarized in Table 5. The couplings with different triarylbismuth reagents comprising electron-rich and -poor aryl groups participated appreciably well and the corresponding tetraaryl-p-quinones were obtained in moderate to high yields. We have also obtained crystals for one of the products, 5.1, whose X-ray analysis unequivocally confirmed that tetraaryl coupling had occurred. The couplings of tetrabromo-p-benzoquinone and triarylbismuth reagents exemplifies the multi-coupling reactivity of both the organic electrophile (tetrabromo-p-benzoquinone) and nucleophilic partner (triarylbismuth) in a one-pot operation under palladium-catalyzed conditions. Thus, four bromide couplings of tetrabromo-p-benzoquinone with three aryl groups of the bismuth reagent underwent smoothly to afford the desired tetraaryl substituted 1,4-quinones in a facile manner. Importantly, under the reported palladium protocol involving the Suzuki type couplings, dihydroquinone was formed as a minor side product.25a This was treated with an additional DDQ oxidation to obtain the pure p-quinone product. However, the present protocol condition obviates this additional oxidation.
| a Reaction conditions: Tetrabromo-p-benzoquinone (0.25 mmol, 1 equiv.), BiAr3 (0.375 mmol, 1.5 equiv.), PdCl2 (0.0225 mmol, 0.09 equiv.), dppf (0.0225 mmol, 0.09 equiv.), K2CO3 (1.5 mmol, 6.0 equiv.), NMP (3 mL), 90 °C, 5 h. Isolated yields based on tetrabromo-p-benzoquinone (bromanil). Homo-coupled biaryls were formed in minor amounts. All products were characterized by 1H, 13C-NMR, EI/ESI HRMS and IR spectroscopy. | |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
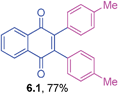
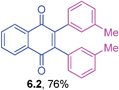
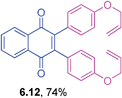
It is to be mentioned that 2,3-dihalo-1,4-naphthoquinones are useful building blocks in synthetic organic chemistry for the preparation of medicinally important compounds in addition to their use in other transformations.31 The cross-couplings of these substrates would generate 2,3-diaryl-1,4-naphthoquinones directly in a one-pot operation. However, this chemistry has not been widely explored so far in the literature.32 Hence, the cross-coupling reaction of 2,3-dibromo-1,4-naphthoquinone with triarylbismuth reagents was studied under the conditions established for tetrabromide couplings.
Encouragingly, these bis-coupling reactions demonstrated a facile reactivity and the corresponding functionalized 2,3-diaryl-1,4-naphthoquinones were formed in good to high yields (Table 6). The cross-coupling reactivity of 2,3-dibromo-1,4-naphthoquinone was not reported so far, whereas the couplings of the corresponding dichloro derivative was reported with one example using azabismocines.32 Another highlight is that there are not many methods available for the preparation of 2,3-diaryl-1,4-naphthoquinones in the literature. For example, Dotz benzannulations of chromium carbene complexes with disubstituted alkynes gives 2,3-diaryl-1,4-naphthoquinones.33 Another two step process involves stepwise synthesis of 2-phenyl-benzoquinone boronic ester through Dotz benzannulation/oxidation protocol followed by cross-coupling with phenyl bromide or triflates under palladium catalysis.24 Reaction of phthaloyliron complexes with disubstituted acetylenes was also reported to give these products.34 Hence, this study is an excellent demonstration of an elaborative bis-coupling with different triarylbismuth reagents involving threefold arylations.
| a Reaction conditions: 2,3-dibromo-1,4-naphthoquinone (0.25 mmol, 1 equiv.), BiAr3 (0.1875 mmol, 0.75 equiv.), K2CO3 (1.5 mmol, 6 equiv.), PdCl2 (0.0225 mmol, 0.09 equiv.), dppf (0.0225 mmol, 0.09 equiv.), NMP (3 mL), 90 °C, 5 h. Isolated yields based on 2,3-dibromo-1,4-naphthoquinone. Homo-coupled biaryls were formed in minor amounts. All the products were identified by 1H, 13C-NMR, EI/ESI HRMS and IR spectroscopic analyses. | ||
|---|---|---|
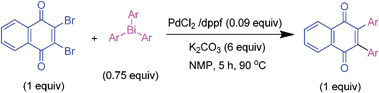
|
||
|
|
|
|
|
|
|
|
|
|
|
|
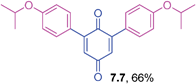
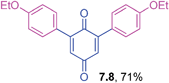
2,6-Dihalo-1,4-benzoquinones are useful precursors in the preparation of 2-halonaphthoquinones, anthraquinones and other derivatives in organic synthesis.35 However, the coupling reactivity of these substrates has not often been reported with organometallic reagents under metal catalysis. Hence, the coupling reactions of 2,6-dibromo-1,4-benzoquinone with triarylbismuths were undertaken to generate 2,6-diaryl-1,4-benzoquiones using the protocol standardized for tetrabromide couplings (Table 7). These reactions also delivered good yields of the corresponding 2,6-diaryl-1,4-benzoquinones with electronically different triarylbismuth regents. It is surprising to see that the methods available for their synthesis of 2,6-diaryl-1,4-benzoquinones are scarce. In that context, the present method embedded with several advantages provides a ready access to 2,6-diaryl-1,4-benzoquinones through cross-couplings of 2,6-dibromo-1,4-benzoquinone with bismuth reagents.
| a Reaction conditions: 2,6-Dibromo-1,4-benzoquinone (0.25 mmol, 1 equiv.), BiAr3 (0.1875 mmol, 0.75 equiv.), K2CO3 (1.5 mmol, 6 equiv.), PdCl2 (0.0225 mmol, 0.09 equiv.), dppf (0.0225 mmol, 0.09 equiv.), NMP (3 mL), 90 °C, 5 h. Isolated yields based on 2,6-dibromo1,4-benzoquinone. Homo-coupled biaryls were formed in minor amounts. All the products were identified by 1H, 13C-NMR, EI/ESI HRMS and IR spectroscopic analyses. | |
|---|---|
|
|
|
|
|
|
|
|
|
|
Conclusions
We have disclosed an elaborate cross-coupling study of different bromo substituted 1,4-quinones with triarylbismuths under palladium catalysis. A few highlights of this study are (i) diverse bromo-1,4-quinones were demonstrated to couple triarylbismuth reagents with equal ease, (ii) triarylbismuths which are non-toxic and air stable performed excellently with threefold arylating capabilities, (iii) the protocol conditions are operationally simple and employ non-toxic reagents, unlike tin reagents which generate toxic wastes, (iv) coupling reactions proved to be fast with short times involving three fold arylations under the mild conditions employed, (v) electronically different triarylbismuths either with electon-rich or -poor aryl groups participated well under the established conditions. Thus, the coupling reactions of substituted 2-bromo-1,4-naphthoquinones, 2,3,5,6-tetrabromo-p-quinone, 2,3-dibromo-1,4-naphthoquinone and 2,6-dibromo-p-quinone with different triarylbismuths showed excellent coupling reactivity under palladium-catalyzed conditions to furnish a wide variety of arylated 1,4-quinone derivatives in a one-pot operation.Experimental
General
All bromoquinones were synthesized according to the reported procedures: 2-bromo-1,4-naphthoquinone,2d 2-bromo-8-methoxy-1,4 naphthoquinone,36 methyl and ethyl 2-(3-bromo-1,4-dioxo-1,4-dihydronaphthalen-2-yl)acrylates,37 2-benzyl-3-bromo-1,4-naphthoquinone derivatives,38 Bromanil,39 2,3-dibromo-1,4-naphthoquinone40 and 2,6-dibromo-1,4-benzoquinone.20b Triarylbismuth compounds were prepared following literature methods.41 Organic solvents were purified by the standard methods. High resolution mass spectra were recorded in ESI-Q-TOF or EI-TOF mode.Representative procedure for cross-coupling reaction of 2-bromo-1,4-naphthoquinone (Table 2 and 3)
A hot oven dried Schlenk tube was charged with tris(3-methoxyphenyl)bismuth (0.25 mmol, 131 mg, 1.0 equiv.), 2-bromo-1,4-naphthoquinone (0.825 mmol, 195.5 mg, 3.3 equiv.), K2CO3 (0.25 mmol, 34.5 mg, 1.0 equiv.), PdCl2(PPh3)2 (0.0225 mmol, 15.8 mg, 0.09 equiv.) and DMF (3 mL) solvent under N2 atmosphere. The mixture was stirred at 90 °C for 2 h. After the work-up,27a the crude product was subjected to silica gel column chromatography (100–200 mesh) using 20% EtOAc/petroleum ether as eluent and the product 2.11 was obtained (0.175 g, 88%) as an orange solid.The above procedure was followed for tetra- and bis-couplings with the following conditions.
Representative conditions for tetra couplings (for Table 5)
Tetrabromo-p-benzoquinone (0.25 mmol, 105.7 mg, 1.0 equiv.), tris-p-tolylbismuth (0.375 mmol, 181.7 mg, 1.5 equiv.), K2CO3 (1.5 mmol, 207.5 mg, 1.0 equiv.), PdCl2 (0.0225 mmol, 3.9 mg, 0.09 equiv.), dppf (0.0025 mmol, 12.4 mg, 0.09 equiv.), NMP (3 mL), 90 °C, 5 h.Representative conditions for bis-couplings (Table 6 and 7)
2,3-dibromo-1,4-napththoquinone (0.25 mmol, 78.9 mg, 1.0 equiv.), tris-p-tolylbismuth (0.1875 mmol, 90.8 mg, 0.75 equiv.), K2CO3 (1.5 mmol, 207.5 mg, 1.0 equiv.), PdCl2 (0.0225 mmol, 3.9 mg, 0.09 equiv.), dppf (0.0025 mmol, 12.4 mg, 0.09 equiv.), NMP (3 mL), 90 °C, 5 h.2-Phenyl-1,4-naphthoquinone (2.1)42
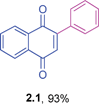
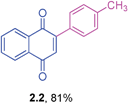
Yellow solid (0.164 g, 93%), mp 107–109 °C (lit. 110–112 °C); IR (KBr): ν = 1664, 1592, 1308, 1248, 1206, 795, 570 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.17–8.19 (m, 1H, CHar), 8.10–8.12 (m, 1H, CHar), 7.76–7.79 (m, 2H, CHar), 7.46–7.58 (m, 5H, CHar), 7.08 (s, 1H, CH) ppm; 13C NMR (125 MHz, CDCl3):24dδ = 185.1, 184.3, 148.0, 135.2, 133.9, 133.8, 133.3, 132.4, 132.0, 130.0, 129.4, 128.4, 127.0, 125.9 ppm; HRMS (EI): [M]+ calcd for C16H10O2 234.0681; found 234.0680.2-p-Tolyl-1,4-naphthoquinone (2.2)
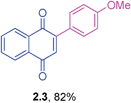
Yellow solid (0.151 g, 81%), mp 114–116 °C; IR (KBr): ν = 1659, 1595, 1303, 1250, 1157, 904, 892, 527 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.16–8.18 (m, 1H, CHar), 8.10–8.12 (m, 1H, CHar), 7.74–7.77 (m, 2H, CHar), 7.48 (d, 2H, J = 8.2 Hz, CHar), 7.27–7.29 (m, 2H, CHar), 7.06 (s, 1H, CH), 2.41 (s, 3H, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.2, 184.5, 147.9, 140.4, 134.5, 133.8, 133.7, 132.4, 132.0, 129.3, 129.2, 126.9, 125.8, 21.4 ppm; HRMS (EI): [M]+ calcd for C17H12O2 248.0837; found 248.0834.2-(4-Methoxyphenyl)-1,4-naphthoquinone (2.3)
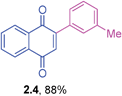
Orange solid (0.175 g, 82%), mp 133–134 °C; IR (KBr): ν = 1660, 1594, 1509, 1327, 1304, 1247, 1207, 1118, 906, 836, 778, 552 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.16–8.18 (m, 1H, CHar), 8.09–8.11 (m, 1H, CHar), 7.75–7.77 (m, 2H, CHar), 7.56–7.59 (m, 2H, CHar), 7.04 (s, 1H, CH), 6.98–7.00 (m, 2H, CHar), 3.86 (s, 3H, OCH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.1, 184.7, 161.2, 147.3, 133.7, 133.6, 132.5, 132.0, 131.0, 126.9, 125.8, 125.6, 113.9, 55.3 ppm; HRMS (EI): [M]+ calcd for C17H12O3 264.0786; found 264.0787.2-m-Tolylphenyl-1,4-naphthoquinone (2.4)
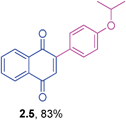
Yellow solid (0.164 g, 88%), mp 116–118 °C; IR (KBr): ν = 1666, 1592, 1482, 1309, 1262, 1246, 781, 696, 575 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.17–8.18 (m, 1H, CHar), 8.10–8.12 (m, 1H, CHar), 7.76–7.78 (m, 2H, CHar), 7.29–7.37 (m, 4H, CHar), 7.06 (s, 1H, CH), 2.42 (s, 3H, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.1, 184.4, 148.2, 138.1, 135.0, 133.8, 133.7, 133.2, 132.4, 132.0, 130.8, 129.9, 128.3, 127.0, 126.5, 125.9, 21.4 ppm; HRMS (ESI): [M+H]+ calcd for C17H12O2 249.0916; found 249.0913.2-(4-Isopropoxyphenyl)-1,4-naphthoquinone (2.5)
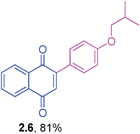
Orange solid (0.181 g, 83%), mp 54–56 °C; IR (KBr): ν = 1664, 1596, 1508, 1210, 1164, 1108, 1050, 906, 864, 780, 574 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.16–8.18 (m, 1H, CHar), 8.09–8.11 (m, 1H, CHar), 7.75–7.76 (m, 2H, CHar), 7.55–7.56 (m, 2H, CHar), 7.04 (s, 1H, CH), 6.95–6.97 (m, 2H, CHar), 4.59–4.66 (m, 1H, OCH), 1.37 (d, 6H, J = 6.1 Hz, C(CH3)2) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.2, 184.8, 159.7, 147.4, 133.7, 133.5, 132.5, 132.0, 131.0, 126.9, 125.8, 125.1, 115.5, 69.9, 21.9 ppm; HRMS (ESI): [M+H]+ calcd for C19H16O3 293.1178; found 293.1175.2-(4-Isobutoxyphenyl)-1,4-naphthoquinone (2.6)
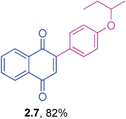
Orange liquid (0.188 g, 81%), mp 60–62 °C IR (KBr): ν = 1662, 1596, 1510, 1470, 1309, 1247, 1209, 1158, 1030, 837, 778, 568, 537 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.16–8.17 (m, 1H, CHar), 8.09–8.11 (m, 1H, CHar), 7.74–7.76 (m, 2H, CHar), 7.55–7.56 (m, 2H, CHar), 7.03 (s, 1H, CH), 6.96–6.98 (m, 2H, CHar), 3.77 (d, 2H, J = 6.3 Hz, OCH2), 2.08–2.13 (m, 1H. CH), 1.03 (d, 6H, J = 6.6 Hz, C(CH3)2) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.2, 184.8, 161.0, 147.4, 133.7, 133.5, 132.5, 132.1, 131.0, 126.9, 125.8, 125.3, 114.5, 74.4, 28.1, 19.2 ppm; HRMS (ESI): [M+H]+ calcd for C20H18O3 307.1334; found 307.1333.2-(4-Sec-butoxyphenyl)-1,4-naphthoquinone (2.7)
Orange liquid (0.187 g, 82%); IR (KBr): ν = 1663, 1598, 1507, 1250, 1209, 573 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.16–8.18 (m, 1H, CHar), 8.09–8.11 (m, 1H, CHar), 7.75–7.77 (m, 2H, CHar), 7.54–7.57 (m, 2H, CHar), 7.04 (s, 1H, CH), 6.95–6.98 (m, 2H, CHar), 4.35–4.41 (m, 1H, OCH), 1.73–1.82 (m, 1H, OCH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 1.60–1.69 (m, 1H, OCH
), 1.60–1.69 (m, 1H, OCH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 1.33 (d, 3H, J = 6.3 Hz, CH3), 0.99 (t, 3H, J = 7.4 Hz, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.2, 184.8, 160.1, 147.4, 133.6, 133.4, 132.5, 132.1, 131.0, 126.9, 125.8, 125.1, 115.5, 75.0, 29.1, 19.2, 9.7 ppm; HRMS (ESI): [M+H]+ calcd for C20H18O3 307.1334; found 307.1332.
), 1.33 (d, 3H, J = 6.3 Hz, CH3), 0.99 (t, 3H, J = 7.4 Hz, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.2, 184.8, 160.1, 147.4, 133.6, 133.4, 132.5, 132.1, 131.0, 126.9, 125.8, 125.1, 115.5, 75.0, 29.1, 19.2, 9.7 ppm; HRMS (ESI): [M+H]+ calcd for C20H18O3 307.1334; found 307.1332.
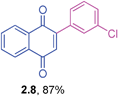
2-(3-Chlorophenyl)-1,4-naphthoquinone (2.8)
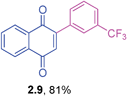
Yellow solid (0.176 g, 87%), mp 134–136 °C; IR (KBr): ν = 1664, 1593, 1342, 1309, 1251, 1163, 1098, 1055, 1023, 771, 576 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.11–8.19 (m, 2H, CHar), 7.77–7.81 (m, 2H, CHar), 7.57–7.58 (m, 1H, CHar), 7.39–7.46 (m, 3H, CHar), 7.07 (s, 1H, CH) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.8, 183.9, 146.7, 135.6, 135.0, 134.4, 134.0, 132.2, 131.9, 130.0, 129.7, 129.4, 127.5, 127.1, 126.0 ppm; HRMS (ESI): [M+H]+ calcd for C16H9ClO2 269.0369; found 269.0362.2-(3-(Trifluoromethyl)phenyl)-1,4-naphthoquinone (2.9)
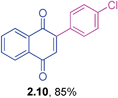
Yellow solid (0.184 g, 81%), mp 118–120 °C; IR (KBr): ν = 1666, 1591, 1326, 1255, 1172, 1110, 743, 716 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.18–8.20 (m, 1H, CHar), 8.11–8.14 (m, 1H, CHar), 7.73–7.84 (m, 5H, CHar), 7.59–7.62 (m, 1H, CHar), 7.11 (s, 1H, CH) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.7, 183.8, 146.7, 135.9, 134.1, 132.6, 132.1, 132.0, 131.1, 130.8, 128.9, 127.1, 126.6, 126.5, 126.3, 126.2, 126.1 ppm; HRMS (EI): [M]+ calcd for C17H9F3O2 302.0555; found 302.0556.2-(4-Chlorophenyl)-1,4-naphthoquinone (2.10)
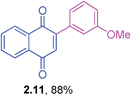
Yellow solid (0.172 g, 85%), mp 156–158 °C; IR (KBr): ν = 1658, 1593, 1490, 1248, 1307,1253, 1091, 1021, 924, 855, 527 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.10–8.18 (m, 2H, CHar), 7.76–7.79 (m, 2H, CHar), 7.43–7.53 (m, 4H, CHar), 7.06 (s, 1H, CH) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.9, 184.1, 146.8, 136.3, 135.1, 133.9, 132.2, 131.9, 131.6, 130.7, 128.7, 127.0, 126.0, ppm; HRMS (ESI): [M+H]+ calcd for C16H9ClO2 269.0369; found 269.0367.2-(3-Methoxyphenyl)-1,4-naphthoquinone (2.11)
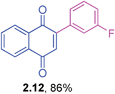
Orange solid (0.175 g, 88%), mp 110–112 °C; IR (KBr): ν = 1666, 1596, 1490, 1498, 1292,1268, 1231, 1060, 773, 577 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.17–8.19 (m, 1H, CHar), 8.11–8.12 (m, 1H, CHar), 7.77–7.79 (m, 2H, CHar), 7.36–7.40 (m, 1H, CHar), 7.10–7.15 (m, 1H, CHar), 7.07(s, 1H, CH), 7.01–7.03 (m, 2H, CHar), 3.86 (s, 3H, OCH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.1, 184.2, 159.4, 147.9, 135.3, 134.6, 133.9, 133.8, 132.4, 132.0, 129.5, 127.0, 125.9, 121.8, 115.8, 114.8, 55.3 ppm; HRMS (ESI): [M+H]+ calcd for C17H12O3 265.0865; found 265.0865.2-(3-Fluorophenyl)-1,4-naphthoquinone (2.12)
Yellow solid (0.162 g, 86%), mp 114–116 °C; IR (KBr): ν = 1666, 1588, 1486, 1349, 1329, 1249, 1221, 1174, 1161, 773, 688, 576 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.11–8.19 (m, 2H, CHar), 7.77–7.81 (m, 2H, CHar), 7.42–7.46 (m, 1H, CHar), 7.30–7.35 (m, 2H, CHar), 7.16–7.19 (m, 1H, CHar), 7.07 (s, 1H, CH) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.9, 183.9, 162.4 (d, J = 244.4 Hz), 146.7, 135.6, 135.2, 134.0 (d, J = 7.1 Hz), 132.2, 131.9, 130.0 (d, J = 8.3 Hz), 127.1, 126.0, 125.0, 117.0, 116.9 (d, J = 20.2 Hz), 116.6 (d, J = 22.6 Hz) ppm; HRMS (ESI): [M+Na]+ calcd for C16H9FO2Na 275.0484; found 275.0482.8-Methoxy-2-p-tolyl-1,4-naphthoquinone (3.1)
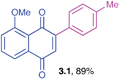
Yellow solid (0.186 g, 89%), mp 136–138 °C; IR (KBr): ν = 1664, 1584, 1303, 1270, 1052, 1026, 826 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.66–7.75 (m, 2H, CHar), 7.45–7.46 (m, 2H, CHar), 7.23–7.32 (m, 3H, CHar), 6.97 (s, 1H, CH), 4.00 (s, 3H, OCH3), 2.39 (s, 3H, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.3, 184.0, 159.8, 149.7, 140.2, 134.8, 134.2, 132.1, 130.8, 129.5, 129.0, 120.5, 118.5, 117.8, 56.5, 21.4 ppm; HRMS (EI): [M]+ calcd for C18H14O3 278.0943; found 278.0944.8-Methoxy-2-phenyl-1,4-naphthoquinone (3.2)
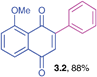
Orange solid (0.174 g, 88%), mp 78–80 °C; IR (KBr): ν = 1653, 1584, 1470, 1443, 1304, 1273, 756, 694 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.67–7.76 (m, 2H, CHar), 7.53–7.55 (m, 2H, CHar), 7.43–7.44 (m, 2H, CHar), 7.31–7.35 (m, 2H, CHar), 6.98 (s, 1H, CH), 4.01 (s, 3H, OCH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.2, 183.7, 159.8, 149.8, 134.8, 134.2, 133.7, 132.8, 129.8, 129.5, 128.2, 120.3, 118.6, 117.9, 56.4 ppm; HRMS (EI): [M]+ calcd for C17H12O3 264.0786; found 264.0787.2-Benzyl-3-phenyl-1,4-naphthoquinone (3.3)
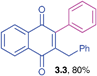
Yellow solid (0.195 g, 80%), mp 119–121 °C; ; IR (KBr): ν = 1663, 1594, 1491, 1294, 787, 764, 707, 549 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.10–8.13 (m, 2H, CHar), 7.73–7.75 (m, 2H, CHar), 7.44–7.46 (m, 3H, CHar), 7.13–7.21 (m, 5H, CHar), 7.00–7.01 (m, 2H, CHar), 3.91 (s, 2H, CH2) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.3, 184.7, 147.0, 145.8, 138.5, 133.7, 133.6, 133.2, 132.1, 132.0, 129.1, 128.6, 128.4, 128.2, 126.5, 126.4, 126.2, 33.4 ppm HRMS (EI): [M]+ calcd for C23H16O2 324.1150; found 324.1154.2-Benzyl-3-(4-ethoxyphenyl)-1,4-naphthoquinone (3.4)
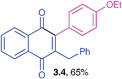
Orange solid (0.179 g, 65%), mp 108–110 °C; IR (KBr): ν = 1665, 1661, 1594, 1510, 1288, 1265, 1224, 744, 731, 558 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.10–8.12 (m, 2H, CHar), 7.72–7.73 (m, 2H, CHar), 6.96–7.21 (m, 9H, CHar), 4.07 (q, 2H, J = 7.1 Hz,![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH3), 3.95 (s, 2H, CH2), 1.45 (t, 3H, J = 7.0 Hz, OCH2
CH3), 3.95 (s, 2H, CH2), 1.45 (t, 3H, J = 7.0 Hz, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.3, 184.9, 159.2, 146.8, 145.5, 138.8, 133.6, 133.5, 132.1, 132.0, 130.7, 128.6, 128.3, 126.5, 126.3, 126.2, 125.2, 114.1, 63.4, 33.4, 14.8 ppm; HRMS (EI): [M]+ calcd for C25H20O3 368.1412; found 368.1412.
ppm; 13C NMR (125 MHz, CDCl3): δ = 185.3, 184.9, 159.2, 146.8, 145.5, 138.8, 133.6, 133.5, 132.1, 132.0, 130.7, 128.6, 128.3, 126.5, 126.3, 126.2, 125.2, 114.1, 63.4, 33.4, 14.8 ppm; HRMS (EI): [M]+ calcd for C25H20O3 368.1412; found 368.1412.
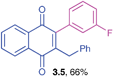
2-Benzyl-3-(3-fluorophenyl)-1,4-naphthoquinone (3.5)
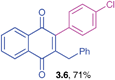
Yellow solid (0.171 g, 66%), mp 110–112 °C; IR (KBr): ν = 1665, 1649, 1580, 1490, 1448, 1226, 1239, 788, 739, 744, 559 cm1; 1H NMR (500 MHz, CDCl3): δ = 8.10–8.14 (m, 2H, CHar), 7.74–7.76 (m, 2H, CHar), 7.40–7.45 (m, 1H, CHar), 7.13–7.21 (m, 4H, CHar), 6.91–7.00 (m, 4H, CHar), 3.90 (s, 2H, CH2), ppm; 13C NMR (125 MHz, CDCl3): δ = 185.0, 184.3, 162.4 (d, J = 245.6 Hz), 146.2, 145.7, 138.1, 135.2, 133.8 (d, J = 9.5 Hz), 132.0, 131.8, 129.9, 128.6, 128.4, 126.6, 126.5, 126.4, 124.9, 116.5 (d, J = 22.6 Hz), 115.6 (d, J = 21.4 Hz), 33.3 ppm; HRMS (EI): [M]+ calcd for C23H15FO2 342.1056; found 342.1052.2-Benzyl-3-(4-chlorophenyl)-1,4-naphthoquinone (3.6)
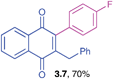
Yellow solid (0.191 g, 71%), mp 98–100 °C; IR (KBr): ν = 1659, 1592, 1489, 1267, 798, 717, 513 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.10–8.14 (m, 2H, CHar), 7.74–7.76 (m, 2H, CHar), 7.42–7.44 (m, 2H, CHar), 7.18–7.21 (m, 5H, CHar), 6.99–7.16 (m, 2H, CHar), 3.90 (s, 2H, CH2) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.0, 184.4, 146.1, 145.9, 138.2, 134.8, 133.9, 133.8, 132.0, 131.8, 131.6, 130.6, 128.6, 128.5, 126.6, 126.5, 126.4, 33.3 ppm; HRMS (EI): [M]+ calcd for C23H15ClO2 358.0761; found 358.0760.2-Benzyl-3-(4-fluorophenyl)-1,4-naphthoquinone (3.7)
Yellow solid (0.181 g, 70%), mp 106–108 °C; IR (KBr): ν = 1659, 1593, 1506, 1157, 1078, 1013, 972, 790, 730, 720 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.10–8.14 (m, 2H, CHar), 7.74–7.76 (m, 2H, CHar), 7.12–7.21 (m, 7H, CHar), 6.98–6.99 (m, 2H, CHar), 3.91 (s, 2H,![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) ), ppm; 13C NMR (125 MHz, CDCl3): δ = 185.1, 184.6, 162.8 (d, J = 246.8 Hz), 146.1, 138.3, 133.8 (d, J = 7.1 Hz), 132.0, 131.9, 131.2, 131.1, 129.1, 128.5, 128.4, 126.6, 126.5, 126.3, 115.4 (d, J = 21.4 Hz), 33.3 ppm; HRMS (EI): [M]+ calcd for C23H15FO2 342.1056; found 342.1053.
), ppm; 13C NMR (125 MHz, CDCl3): δ = 185.1, 184.6, 162.8 (d, J = 246.8 Hz), 146.1, 138.3, 133.8 (d, J = 7.1 Hz), 132.0, 131.9, 131.2, 131.1, 129.1, 128.5, 128.4, 126.6, 126.5, 126.3, 115.4 (d, J = 21.4 Hz), 33.3 ppm; HRMS (EI): [M]+ calcd for C23H15FO2 342.1056; found 342.1053.
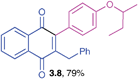
2-Benzyl-3-(4-sec-butoxyphenyl)-1,4-naphthoquinone (3.8)
Yellow solid (0.235 g, 79%), mp 62–64 °C; IR (KBr): ν = 1662, 1604, 1507, 1443, 1286, 1266, 784, 732, 716, 535 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.10–8.13 (m, 2H, CHar), 7.71–7.74 (m, 2H, CHar), 6.93–7.20 (m, 9H, CHar), 4.33–4.37 (m, 1H, OCH), 3.96 (s, 2H, CH2), 1.75–1.81(m, 1H, OCH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 1.62–1.68 (m, 1H, OCH
), 1.62–1.68 (m, 1H, OCH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 1.33–1.34 (m, 3H, CH3), 0.98–1.01 (m, 3H, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.3, 184.9, 158.6, 146.8, 145.5, 138.8, 133.6, 133.5, 132.2, 132.1, 130.8, 128.6, 128.3, 126.5, 126.3, 126.1, 124.9, 115.3, 75.0, 33.5, 29.1, 19.2, 9.8 ppm; HRMS (ESI): [M+H]+ calcd for C27H24O3 397.1804; found 397.1800.
), 1.33–1.34 (m, 3H, CH3), 0.98–1.01 (m, 3H, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.3, 184.9, 158.6, 146.8, 145.5, 138.8, 133.6, 133.5, 132.2, 132.1, 130.8, 128.6, 128.3, 126.5, 126.3, 126.1, 124.9, 115.3, 75.0, 33.5, 29.1, 19.2, 9.8 ppm; HRMS (ESI): [M+H]+ calcd for C27H24O3 397.1804; found 397.1800.
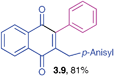
2-(4-Methoxybenzyl)-3-phenyl-1,4-naphthoquinone (3.9)
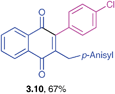
Orange solid (0.215 g, 81%), mp 92–94 °C; IR (KBr): ν = 1663, 1592, 1509, 1442, 1326, 1296, 1240, 1032, 826, 852, 829, 808, 549 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.09–8.13 (m, 2H, CHar), 7.71–7.73 (m, 2H, CHar), 7.45–7.49 (m, 3H, CHar), 7.22–7.23 (m, 2H, CHar), 6.93 (d, 2H, J = 8.8 Hz, CHar), 6.73 (d, 2H, J = 8.8 Hz, CHar), 3.86 (s, 2H, CH2), 3.73 (s, 3H, OCH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.3, 184.7, 157.9, 146.6, 146.0, 133.6, 133.5, 133.3, 132.0, 131.9, 130.4, 129.6, 129.2, 128.5, 128.2, 126.4, 126.3, 113.7, 55.1, 32.4 ppm; HRMS (ESI): [M+H]+ calcd for C24H18O3 355.1334; found 355.1339.2-(4-Chlorophenyl)-3-(4-methoxybenzyl)-1,4-naphthoquinone (3.10)
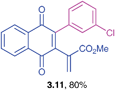
Yellow solid (0.194 g, 67%), mp 70–72 °C; IR (KBr): ν = 1661, 1610, 1510, 1489, 1326, 1291, 1177, 1090, 1032, 1015, 823, 791, 756, 716, 597 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.09–8.13 (m, 2H, CHar), 7.73–7.75 (m, 2H, CHar), 7.43–7.44 (m, 2H, CHar), 7.13 (d, 2H, J = 8 Hz, CHar), 6.90 (d, 2H, J = 8.5 Hz, CHar), 6.71 (d, 2H, J = 8.5 Hz, CHar), 3.83 (s, 2H, CH2), 3.73 (s, 3H, OCH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.1, 184.5, 158.1, 146.3, 145.5, 134.8, 133.8, 132.0, 131.8, 131.7, 130.7, 130.2, 129.6, 128.5, 126.6, 126.5, 113.8, 55.1, 32.4 ppm; HRMS (ESI): [M+H]+ calcd for C24H17ClO3 389.0944; found 389.0947.Methyl-2-(3-(3-chlorophenyl)-1,4-dioxo-1,4-dihydronaphthalen-2-yl)acrylate (3.11)
Orange solid (0.211 g, 80%), mp 86–88 °C; IR (KBr): ν = 1737, 1659, 1592, 1296, 1127, 729 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.13–8.15 (m, 2H, CHar), 7.78–7.79 (m, 2H, CHar), 7.22–7.34 (m, 3H, CHar), 7.08–7.10 (m, 1H, CHar), 6.41 (br s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.49 (br s, 1H, C
CH2), 5.49 (br s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 3.73 (s, 3H, OCH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 183.8, 183.7, 165.6, 145.1, 143.3, 134.7, 134.4, 134.1, 132.2, 131.8, 131.7, 129.5, 129.3, 128.9, 127.6, 127.3, 126.8, 126.6, 52.4 ppm; HRMS (EI): [M]+ calcd for C20H13ClO4 352.0502; found 352.0501.
CH2), 3.73 (s, 3H, OCH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 183.8, 183.7, 165.6, 145.1, 143.3, 134.7, 134.4, 134.1, 132.2, 131.8, 131.7, 129.5, 129.3, 128.9, 127.6, 127.3, 126.8, 126.6, 52.4 ppm; HRMS (EI): [M]+ calcd for C20H13ClO4 352.0502; found 352.0501.
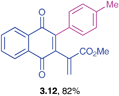
Methyl 2-(1,4-dioxo-3-p-tolyl)-1,4-dihydronaphthalen-2-yl)-acrylate (3.12)
Orange solid (0.206 g, 82%), mp 102–104 °C; IR (KBr): ν = 1729, 1665, 1594, 1290, 1129, 725 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.12–8.16 (m, 2H, CHar), 7.75–7.78 (m, 2H, CHar), 7.10–7.20 (m, 4H, CHar), 6.40 (br s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.48 (br s, 1H, C
CH2), 5.48 (br s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 3.72 (s, 3H, OCH3), 2.37 (s, 3H, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.3, 184.0, 165.9, 146.6, 142.5, 138.8, 135.1, 133.9, 133.8, 132.0, 131.7, 131.5, 129.7, 129.4, 128.7, 126.7, 126.4, 52.3, 21.3 ppm; HRMS (EI): [M]+ calcd for C21H16O4 332.1049; found 332.1042.
CH2), 3.72 (s, 3H, OCH3), 2.37 (s, 3H, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.3, 184.0, 165.9, 146.6, 142.5, 138.8, 135.1, 133.9, 133.8, 132.0, 131.7, 131.5, 129.7, 129.4, 128.7, 126.7, 126.4, 52.3, 21.3 ppm; HRMS (EI): [M]+ calcd for C21H16O4 332.1049; found 332.1042.
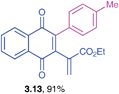
Ethyl 2-(1,4-dioxo-3-p-tolyl)-1,4-dihydronaphthalen-2-yl)-acrylate (3.13)
Brown liquid (0.235 g, 91%); IR (KBr): ν = 1726, 1666, 1594, 1324, 1292, 1260, 1050, 1024, 725 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.12–8.14 (m, 2H, CHar), 7.74–7.76 (m, 2H, CHar), 7.11–7.18 (m, 4H, CHar), 6.38 (br s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.47 (br s, 1H, C
CH2), 5.47 (br s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.17 (q, 2H, J = 6.8 Hz,
CH2), 4.17 (q, 2H, J = 6.8 Hz, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH3), 2.35 (s, 3H, CH3), 1.22 (t, 3H, J = 6.8 Hz, OCH2
CH3), 2.35 (s, 3H, CH3), 1.22 (t, 3H, J = 6.8 Hz, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.3, 183.9, 165.3, 146.4, 142.6, 138.7, 135.4, 133.8, 131.9, 131.7, 131.2, 129.7, 129.4, 128.6, 126.6, 126.3, 61.2, 21.3, 14.0 ppm; HRMS (EI): [M]+ calcd for C22H18O4 346.1205; found 346.1209.
) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.3, 183.9, 165.3, 146.4, 142.6, 138.7, 135.4, 133.8, 131.9, 131.7, 131.2, 129.7, 129.4, 128.6, 126.6, 126.3, 61.2, 21.3, 14.0 ppm; HRMS (EI): [M]+ calcd for C22H18O4 346.1205; found 346.1209.
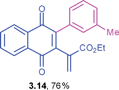
Ethyl 2-(1,4-dioxo-3-m-tolyl-1,4-dihydronaphthalen-2-yl)-acrylate (3.14)
Orange solid (0.197 g, 76%), mp 100–102 °C; IR (KBr): ν = 1724, 1660, 1593, 1296, 1273, 784 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.12–8.15 (m, 2H, CHar), 7.75–7.78 (m, 2H, CHar), 7.16–7.27 (m, 2H, CHar), 7.00–7.03 (m, 2H, CHar), 6.38 (br s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.46 (br s, 1H, C
CH2), 5.46 (br s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.18 (q, 2H, J = 7.1 Hz,
CH2), 4.18 (q, 2H, J = 7.1 Hz, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH3), 2.34 (s, 3H, CH3), 1.21–1.24 (m, 3H, OCH2
CH3), 2.34 (s, 3H, CH3), 1.21–1.24 (m, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.4, 184.0, 165.3, 146.6, 142.9, 137.5, 135.4, 133.9, 133.8, 132.7, 132.0, 131.8, 131.1, 130.0, 129.5, 127.9, 126.7, 126.5, 126.4, 61.2, 21.4, 14.0 ppm; HRMS (EI): [M+H]+ calcd for C22H18O4 347.1283; found 347.1282.
) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.4, 184.0, 165.3, 146.6, 142.9, 137.5, 135.4, 133.9, 133.8, 132.7, 132.0, 131.8, 131.1, 130.0, 129.5, 127.9, 126.7, 126.5, 126.4, 61.2, 21.4, 14.0 ppm; HRMS (EI): [M+H]+ calcd for C22H18O4 347.1283; found 347.1282.
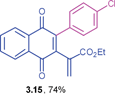
Ethyl 2-(3-(4-chlorophenyl)-1,4-dioxo-1,4-dihydronaphthalen-2-yl)acrylate (3.15)
Brown liquid (0.204 g, 74%); IR (KBr): ν = 1727, 1667, 1594, 1290, 1264, 736 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.13–8.16 (m, 2H, CHar), 7.77–7.80 (m, 2H, CHar), 7.23–7.35 (m, 3H, CHar), 7.10–7.12 (m, 1H, CHar), 6.41 (br s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.48 (br s, 1H, C
CH2), 5.48 (br s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.19 (q, 2H, J = 7.1 Hz,
CH2), 4.19 (q, 2H, J = 7.1 Hz, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH3), 1.24 (t, 3H, J = 7.1 Hz, OCH2
CH3), 1.24 (t, 3H, J = 7.1 Hz, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ) ppm; 13C NMR (125 MHz, CDCl3): δ = 183.8, 183.7, 165.0, 145.0, 143.4, 135.1, 134.5, 134.1, 131.5, 129.5, 129.3, 128.9, 127.6, 126.8, 126.6, 61.4, 14.0 ppm; HRMS (ESI): [M+H]+ calcd for C21H15ClO4 367.0737; found 367.0735.
) ppm; 13C NMR (125 MHz, CDCl3): δ = 183.8, 183.7, 165.0, 145.0, 143.4, 135.1, 134.5, 134.1, 131.5, 129.5, 129.3, 128.9, 127.6, 126.8, 126.6, 61.4, 14.0 ppm; HRMS (ESI): [M+H]+ calcd for C21H15ClO4 367.0737; found 367.0735.
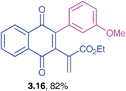
Ethyl 2-(3-(3-methoxyphenyl)-1,4-dioxo-1,4-dihydronaphthalen-2-yl)acrylate (3.16)
Orange solid (0.223 g, 82%), mp 68–70 °C; IR (KBr): ν = 1711, 1662, 1593, 1242, 1200, 1091, 1070, 994, 961, 733 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.13–8.16 (m, 2H, CHar), 7.76–7.78 (m, 2H, CHar), 7.25–7.30 (m, 1H, CHar), 6.77–6.91 (m, 3H, CHar), 6.37 (br s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.48 (br s, 1H, C
CH2), 5.48 (br s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.17 (q, 2H, J = 7.3 Hz,
CH2), 4.17 (q, 2H, J = 7.3 Hz, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH3), 3.77 (s, 3H, OCH3), 1.23 (t, 3H, J = 7.3 Hz, OCH2
CH3), 3.77 (s, 3H, OCH3), 1.23 (t, 3H, J = 7.3 Hz, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.2, 184.0, 165.3, 159.0, 146.3, 143.0, 135.4, 134.0, 133.9, 131.9, 131.7, 131.1, 129.1, 126.7, 126.4, 121.8, 114.9, 114.4, 61.3, 55.2, 14.0 ppm; HRMS (EI): [M]+ calcd for C22H18O5 362.1154; found 362.1151.
) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.2, 184.0, 165.3, 159.0, 146.3, 143.0, 135.4, 134.0, 133.9, 131.9, 131.7, 131.1, 129.1, 126.7, 126.4, 121.8, 114.9, 114.4, 61.3, 55.2, 14.0 ppm; HRMS (EI): [M]+ calcd for C22H18O5 362.1154; found 362.1151.
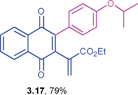
Ethyl 2-(3-(4-isopropoxyphenyl)-1,4-dioxo-1,4-dihydronaphthalen-2-yl)acrylate (3.17)
Orange solid (0.213 g, 79%), mp 50–52 °C; IR (KBr): ν = 1727, 1666, 1606, 1507, 1293, 1247, 1184, 796 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.12–8.16 (m, 2H, CHar), 7.74–7.78 (m, 2H, CHar), 7.16–7.18 (m, 2H, CHar), 6.87 (d, 2H, J = 8.5 Hz, CHar), 6.41 (br s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.49 (br s, 1H, C
CH2), 5.49 (br s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.51–4.61 (m, 1H, OCH), 4.17 (q, 2H, J = 7.0 Hz,
CH2), 4.51–4.61 (m, 1H, OCH), 4.17 (q, 2H, J = 7.0 Hz, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH3), 1.35 (d, 6H, J = 6.1 Hz, C(CH3)2), 1.22 (t, 3H, J = 7.1 Hz, OCH2
CH3), 1.35 (d, 6H, J = 6.1 Hz, C(CH3)2), 1.22 (t, 3H, J = 7.1 Hz, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.6, 184.0, 165.5, 158.5, 146.1, 142.2, 135.7, 133.8, 132.1, 131.9, 131.4, 131.1, 126.7, 126.4, 124.6, 115.0, 69.8, 61.2, 22.0, 14.0 ppm; HRMS (EI): [M]+ calcd for C24H22O5 390.1467; found 390.1462.
) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.6, 184.0, 165.5, 158.5, 146.1, 142.2, 135.7, 133.8, 132.1, 131.9, 131.4, 131.1, 126.7, 126.4, 124.6, 115.0, 69.8, 61.2, 22.0, 14.0 ppm; HRMS (EI): [M]+ calcd for C24H22O5 390.1467; found 390.1462.
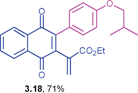
Ethyl 2-(3-(4-isobutoxyphenyl)-1,4-dioxo-1,4-dihydronaphthalen-2-yl)acrylate (3.18)
Orange solid (0.215 g, 71%), mp 140–142 °C; IR (KBr): ν = 1723, 1665, 1604, 1510, 1288, 1177, 1129, 1027, 726 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.11–8.14 (m, 2H, CHar), 7.73–7.76 (m, 2H, CHar), 7.16–7.18 (m, 2H, CHar), 6.88–6.90 (m, 2H, CHar), 6.40 (br s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.49 (br s, 1H, C
CH2), 5.49 (br s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.17 (q, 2H, J = 7.1 Hz,
CH2), 4.17 (q, 2H, J = 7.1 Hz, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH3), 3.73 (d, 2H, J = 6.5 Hz, OCH2), 2.04–2.12 (m, 1H, CH), 1.22 (t, 3H, J = 7.0 Hz, OCH2
CH3), 3.73 (d, 2H, J = 6.5 Hz, OCH2), 2.04–2.12 (m, 1H, CH), 1.22 (t, 3H, J = 7.0 Hz, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ), 1.02 (d, 6H, J = 6.6 Hz, C(CH3)2) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.5, 183.9, 165.4, 159.7, 146.1, 142.2, 135.7, 133.7, 132.0, 131.8, 131.2, 131.0, 126.6, 126.3, 124.6, 113.9, 74.2, 61.1, 28.1, 19.1, 14.0 ppm; HRMS (ESI): [M+H]+ calcd for C25H24O5 405.1702; found 405.1708.
), 1.02 (d, 6H, J = 6.6 Hz, C(CH3)2) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.5, 183.9, 165.4, 159.7, 146.1, 142.2, 135.7, 133.7, 132.0, 131.8, 131.2, 131.0, 126.6, 126.3, 124.6, 113.9, 74.2, 61.1, 28.1, 19.1, 14.0 ppm; HRMS (ESI): [M+H]+ calcd for C25H24O5 405.1702; found 405.1708.
2,3,5,6-Tetra-p-tolylcyclohexa-2,5-diene-1,4-dione (5.1)
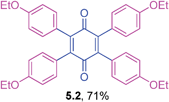
Orange solid (0.0922 g, 78%), mp 262–264 °C; IR (KBr): ν = 1649, 1607, 1503, 1317, 1136, 804 cm−1; 1H NMR (500 MHz, CDCl3): δ = 6.97–7.03 (m, 4 × 4H, CHar), 2.29 (s, 4 × 3H, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 187.1, 142.6, 138.0, 130.7, 130.0, 128.3, 21.3 ppm; HRMS (ESI): [M+H]+ calcd for C34H28O2 469.2168, found 469.2167.2,3,5,6-Tetrakis(4-ethoxyphenyl)cyclohexa-2,5-diene-1,4-dione (5.2)
Orange solid (0.1054 g, 71%), mp 122–124 °C; IR (KBr): ν = 1648, 1603, 1505, 1251, 1176, 1135, 1042 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.02 (d, 4 × 2H, J = 8.9 Hz, CHar), 6.73 (d, 4 × 2H, J = 8.9 Hz, CHar), 3.98 (q, 4 × 2H, J = 7.0 Hz,![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH3), 1.37 (t, 4 × 3H, J = 7.0 Hz, OCH2
CH3), 1.37 (t, 4 × 3H, J = 7.0 Hz, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ) ppm; 13C NMR (125 MHz, CDCl3): δ = 187.4, 158.7, 141.9, 132.4, 125.3, 113.6, 63.2, 14.7 ppm; HRMS (ESI): [M+H]+ calcd for C38H36O6 589.2590, found 589.2590.
) ppm; 13C NMR (125 MHz, CDCl3): δ = 187.4, 158.7, 141.9, 132.4, 125.3, 113.6, 63.2, 14.7 ppm; HRMS (ESI): [M+H]+ calcd for C38H36O6 589.2590, found 589.2590.
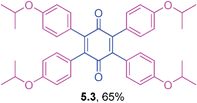
2,3,5,6-Tetrakis(4-isopropoxyphenyl)cyclohexa-2,5-diene-1,4-dione (5.3)
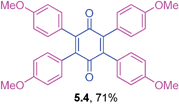
Orange solid (0.1064 g, 65%), mp 202–204 °C; IR (KBr): ν = 2975, 1648, 1603, 1503, 1283, 1247, 1186, 1137, 1111, 950, 824 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.01 (d, 4 × 2H, J = 8.5 Hz, CHar), 6.72 (d, 4 × 2H, J = 8.8 Hz, CHar), 4.46–4.54 (m, 4 × 1H, OCH), 1.30 (d, 4 × 6H, J = 5.8 Hz, C(CH3)2) ppm; 13C NMR (125 MHz, CDCl3): δ = 187.5, 157.7, 141.9, 132.5, 125.2, 114.8, 69.7, 21.9 ppm; HRMS (ESI): [M+H]+ calcd for C42H44O6 645.3216, found 645.3215.2,3,5,6-Tetrakis(4-methoxyphenyl)cyclohexa-2,5-diene-1,4-dione (5.4)
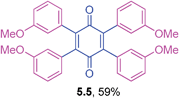
Orange solid (0.0949 g, 71%), mp 176–178 °C; IR (KBr): ν = 1697, 1649, 1606, 1506, 1283, 1247, 1139, 1024, 823 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.02–7.05 (m, 4 × 2H, CHar), 6.73–6.76 (m, 4 × 2H, CHar), 3.76 (s, 4 × 3H, OCH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 187.3, 159.3, 141.9, 132.4, 125.3, 113.1, 55.1 ppm; HRMS (ESI): [M+H]+ calcd for C34H28O6 533.1964, found 533.1967.2,3,5,6-Tetrakis(3-methoxyphenyl)cyclohexa-2,5-diene-1,4-dione (5.5)
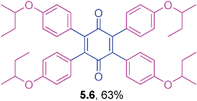
Orange solid (0.0784 g, 59%), mp 167–169 °C; IR (KBr): ν = 2935, 1652, 1592, 1465, 1305, 1181, 1033, 792, 712, 696 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.12–7.15 (m, 4 × 1H, CHar), 6.63–6.79 (m, 4 × 3H, CHar), 3.63 (s, 4 × 3H, OCH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 186.4, 158.8, 142.9, 133.9, 128.6, 123.1, 115.8, 114.6, 55.1 ppm; HRMS (ESI): [M+H]+ calcd for C34H28O6 533.1964, found 533.1965.2,3,5,6-Tetrakis(4-sec-butoxyphenyl)cyclohexa-2,5-diene-1,4-dione (5.6)
Orange solid (0.1104 g, 63%), mp 159–161 °C; IR (KBr): ν = 2933, 1647, 1603, 1503, 1284, 1245, 1182, 1138, 995, 824 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.00–7.02 (m, 4 × 2H, CHar), 6.71–6.73 (m, 4 × 2H, CHar), 4.22–4.28 (m, 4 × 1H, OCH), 1.68–1.72 (m, 4 × 1H, OCH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 1.56–1.61 (m, 4 × 1H, OCH
), 1.56–1.61 (m, 4 × 1H, OCH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 1.25 (d, 4 × 3H, J = 6.1 Hz, CH3), 0.94 (t, 4 × 3H, J = 7.4 Hz, CH3) ppm; 13C NMR (125 MHz, CDCl3) δ = 187.5, 158.0, 141.8, 132.5, 125.0, 114.8, 74.8, 29.0, 19.1, 9.7 ppm; HRMS (ESI): [M+H]+ calcd for C46H52O6 701.3842, found 701.3848.
), 1.25 (d, 4 × 3H, J = 6.1 Hz, CH3), 0.94 (t, 4 × 3H, J = 7.4 Hz, CH3) ppm; 13C NMR (125 MHz, CDCl3) δ = 187.5, 158.0, 141.8, 132.5, 125.0, 114.8, 74.8, 29.0, 19.1, 9.7 ppm; HRMS (ESI): [M+H]+ calcd for C46H52O6 701.3842, found 701.3848.
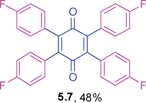
2,3,5,6-Tetrakis(4-fluorophenyl)cyclohexa-2,5-diene-1,4-dione (5.7)
Orange solid (0.0584 g, 48%), mp 252–256 °C; IR (KBr): ν = 1650, 1598, 1504, 1312, 1224, 1158, 1139, 833 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.04–7.07 (m, 4 × 2H, CHar), 6.93–6.96 (m, 4 × 2H, CHar) ppm; 13C NMR (125 MHz, CDCl3): δ = 186.2, 162.6 (d, J = 248 Hz), 142.3, 132.6 (d, J = 8.4 Hz), 127.4, 115.1 (d, J = 21.6 Hz) ppm; HRMS (ESI): [M+Cl]+ calcd for C30H16ClF4O2 519.0775, found 519.0771.2,3,5,6-Tetra-3-tolylcyclohexa-2,5-diene-1,4-dione (5.8)
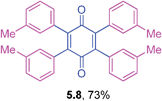
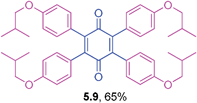
Orange solid (0.0854 g, 73%), mp 208–210 °C; IR (KBr): ν = 1650, 1582, 1308, 1292, 1215, 1129, 776, 694 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.30–7.43 (m, 4 × 3H, CHar), 7.18–7.19 (m, 4 × 1H, CHar), 2.57 (s, 4 × 3H, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 187.1, 142.9, 136.9, 132.7, 131.4, 128.9, 127.7, 127.4, 21.3 ppm; HRMS (ESI): [M+H]+ calcd for C34H28O2 469.2168, found 469.2169.2,3,5,6-Tetrakis(4-isobutoxyphenyl)cyclohexa-2,5-diene-1,4-dione (5.9)
Orange solid (0.1142 g, 65%), mp 152–154 °C; IR (KBr): ν = 2918, 1649, 1604, 1504, 1468, 1284, 1248, 1174, 1135, 1027 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.02 (d, 4 × 2H, J = 8.6 Hz, CHar), 6.74 (d, 4 × 2H, J = 8.6 Hz, CHar), 3.67 (d, 4 × 2H, J = 6.3 Hz, OCH2), 2.00–2.07 (m, 4 × 1H, CH), 1.00 (d, 4 × 6H, J = 6.8 Hz, C(CH3)2) ppm; 13C NMR (125 MHz, CDCl3): δ = 187.5, 159.0, 141.8, 132.4, 125.2, 113.7, 74.2, 28.2, 19.2 ppm; HRMS (ESI): [M+H]+ calcd for C46H52O6 701.3842, found 701.3840.2,3-Di-4-tolyl-1,4-naphthoquinone (6.1)
Yellow solid (0.0651 g, 77%), mp 154–156 °C; IR (KBr): ν = 1659, 1592, 1506, 1290, 808, 792, 721, 512 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.17–8.18 (m, 2H, CHar), 7.76–7.78 (m, 2H, CHar), 7.04 (d, 4H, J = 8.2 Hz, CHar), 6.97 (d, 4H, 7.9 Hz, CHar), 2.29 (s, 2 × 3H, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.9, 145.3, 138.0, 133.7, 132.1, 130.4, 130.3, 128.3, 126.5, 21.3 ppm; HRMS (ESI): [M+H]+ calcd. for C24H18O2 339.1385, found 339.1380.2,3-Di-3-tolyl-1,4-naphthoquinone (6.2)
Yellow solid (0.0646 g, 76%), mp 177–179 °C; IR (KBr): ν = 1660, 1595, 1292, 1244, 774, 717 cm−1; 1HNMR (500 MHz, CDCl3): δ = 8.18–8.19 (m, 2H, CHar), 7.78–7.79 (m, 2H, CHar), 7.03–7.11 (m, 4H, CHar), 6.91 (s, 2 × 1H, CHar), 6.83 (d, 2H, J = 7.6 Hz), 2.25 (s, 2 × 3H, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.9, 145.8, 137.0, 133.7, 133.1, 132.1, 131.0, 128.9, 127.4, 126.5, 21.3 ppm; HRMS (ESI): [M+H]+ calcd. for C24H18O2 339.1385, found 339.1382.2,3-Bis(4-fluorophenyl)-1,4-naphthoquinone (6.3)
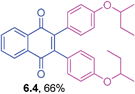
Yellow solid (0.0578 g, 67%), mp 172–174 °C; IR (KBr): ν = 1661, 1599, 1504, 1478, 1273, 1227, 1159, 1113, 824, 794, 737 cm−1; 1HNMR (500 MHz, CDCl3): δ = 8.18–8.20 (m, 2H, CHar), 7.79–7.81 (m, 2H, CHar), 7.04–7.06 (m, 4H, CHar), 6.93–6.97 (m, 4H, CHar) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.4, 162.5 (d, J = 247.9 Hz), 144.8, 134.0, 132.5 (d, J = 8.3 Hz), 131.9, 128.9, 126.6, 115.0 (d, J = 22.6) ppm; HRMS (ESI): [M+H]+ calcd. for C22H12F2O2 346.0805, found 346.0808.2,3-Bis(4-sec-butoxyphenyl)-1,4-naphthoquinone (6.4)
Orange solid (0.0754 g, 66%), mp 94–96 °C; IR (KBr): ν = 1657, 1603, 1556, 1504, 1286, 1249, 1179, 804, 720 cm−1; 1HNMR (500 MHz, CDCl3): δ = 8.16–8.18 (m, 2H, CHar), 7.75–7.77 (m, 2H, CHar), 7.00 (d, 4H, J = 8.6 Hz, CHar), 6.75 (d, 4H, J = 9.1 Hz, CHar), 4.24–4.30 (m, 2 × 1H, OCH), 1.68–1.77 (m, 2 × 1H, OCH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 1.55–1.63 (m, 2 × 1H, OCH
), 1.55–1.63 (m, 2 × 1H, OCH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 1.27 (d, 2 × 3H, J = 6.1 Hz, CH3), 0.95 (t, 2 × 3H, J = 7.3 Hz, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.1, 158.0, 144.6, 133.6, 132.3, 126.4, 125.3, 114.9, 74.9, 29.0, 19.1, 9.7 ppm; HRMS (ESI): [M+H]+ calcd. for C30H30O4 455.2222, found 455.2224.
), 1.27 (d, 2 × 3H, J = 6.1 Hz, CH3), 0.95 (t, 2 × 3H, J = 7.3 Hz, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.1, 158.0, 144.6, 133.6, 132.3, 126.4, 125.3, 114.9, 74.9, 29.0, 19.1, 9.7 ppm; HRMS (ESI): [M+H]+ calcd. for C30H30O4 455.2222, found 455.2224.
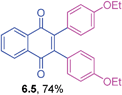
2,3-Bis(4-ethoxyphenyl)-1,4-naphthoquinone (6.5)
Orange solid (0.0741 g, 74%), mp 162–164 °C; IR (KBr): ν = 1659, 1604, 1506, 1288, 1245, 1177, 1059, 1047, 797, 738 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.17–8.18 (m, 2H, CHar), 7.76–7.78 (m, 2H, CHar), 7.00–7.03 (m, 4H, CHar) , 6.74–6.77 (m, 4H, CHar), 4.00 (q, 2 × 2H, J = 7.0 Hz,![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH3), 1.39 (t, 2 × 3H, J = 7.0 Hz, OCH2
CH3), 1.39 (t, 2 × 3H, J = 7.0 Hz, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.0, 158.7, 144.6, 133.6, 132.2, 128.3, 126.5, 125.4, 113.6, 63.3, 14.7 ppm; HRMS (ESI): [M+H]+ calcd. for C26H22O4 399.1596, found 399.1592.
) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.0, 158.7, 144.6, 133.6, 132.2, 128.3, 126.5, 125.4, 113.6, 63.3, 14.7 ppm; HRMS (ESI): [M+H]+ calcd. for C26H22O4 399.1596, found 399.1592.
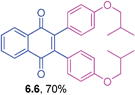
2,3-Bis(4-isobutoxyphenyl)-1,4-naphthoquinone (6.6)
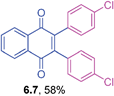
Orange solid (0.0794 g, 70%), mp 119–121 °C; IR (KBr): ν = 1665, 1605, 1508, 1472, 1283, 1242, 1174, 1033, 814, 788, 533 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.15–8.18 (m, 2H, CHar), 7.76–7.78 (m, 2H, CHar), 6.99–7.03 (m, 4H, CHar), 6.75–6.78 (m, 4H, CHar), 3.68 (d, 2 × 2H, J = 6.4 Hz, OCH2), 2.02–2.09 (m, 2 × 1H, CH), 1.00 (d, 2 × 6H, J = 6.7 Hz, C(CH3)2) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.1, 159.0, 144.6, 133.6, 132.2, 131.1, 126.5, 125.3, 113.7, 74.2, 28.2, 19.2 ppm; HRMS (ESI): [M+H]+ calcd. for C30H30O4, 455.2222, found 455.2227.2,3-Bis(4-chlorophenyl)-1,4-naphthoquinone (6.7)
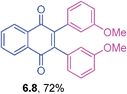
Yellow solid (0.0554 g, 58%), mp 157–159 °C; IR (KBr): ν = 1660, 1591, 1487, 1292, 1092, 1015, 805, 796 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.07–8.09 (m, 2H, CHar), 7.69–7.72 (m, 2H, CHar), 7.11–7.15 (m, 4H, CHar), 6.89–6.92 (m, 4H, CHar) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.2, 144.7, 134.7, 134.1, 132.0, 131.9, 131.3, 128.2, 126.7 ppm; HRMS (ESI): [M]+ calcd. for C22H12Cl2O2, 378.0214, found 378.0218.2,3-Bis(3-methoxyphenyl)-1,4-naphthoquinone (6.8)
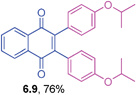
Orange solid (0.0671 g, 72%), mp 116–118 °C; IR (KBr): ν = 1660, 1596, 1576, 1339, 1248, 1256, 1061, 1046, 753, 718, 707 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.18–8.20 (m, 2H, CHar), 7.78–7.80 (m, 2H, CHar), 7.16 (t, 2H, J = 7.9 Hz, CHar) , 6.78–6.80 (m, 2H, CHar), 6.69–6.71 (m, 2H, CHar), 6.60–6.61 (m, 2H, CHar), 3.64 (s, 2 × 3H, OCH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 184.6, 158.8, 145.6, 134.4, 133.8, 132.0, 128.6, 126.6, 122.9, 115.7, 114.3, 55.1 ppm; HRMS (ESI): [M+H]+ calcd. for C24H18O4, 371.1283, found 371.1284.2,3-Bis(4-isopropoxyphenyl)-1,4-naphthoquinone (6.9)
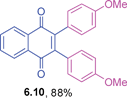
Orange solid (0.0819 g, 76%), mp 148–149 °C; IR (KBr): ν = 1664, 1603, 1504, 1287, 1246, 1184, 1104, 1017, 950, 823, 720, 581 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.16–8.18 (m, 2H, CHar), 7.75–7.77 (m, 2H, CHar), 6.99–7.02 (m, 4H, CHar), 6.73–6.76 (m, 4H, CHar), 4.48–4.55 (m, 2 × 1H, OCH), 1.31 (d, 2 × 6H, J = 5.8 Hz, C(CH3)2) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.1, 157.7, 144.6, 133.6, 132.3, 132.1, 126.4, 125.3, 114.8, 69.6, 21.9 ppm; HRMS (ESI): [M+H]+ calcd. for C28H26O4 427.1909, found 427.1902.2,3-Bis(4-methoxyphenyl)-1,4-naphthoquinone (6.10)
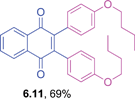
Orange solid (0.0814 g, 88%), mp 152–154 °C; IR (KBr): ν = 1660, 1604, 1505, 1287, 1248, 1182, 1057, 1027, 823, 796, 720 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.17–8.19 (m, 2H, CHar), 7.76–7.78 (m, 2H, CHar), 7.01–7.04 (m, 4H, CHar) , 6.77–6.79 (m, 4H, CHar), 3.78 (s, 2 × 3H, OCH3) ppm; 13C NMR (125 MHz, CDCl3): δ =185.0, 159.3, 144.7, 133.6, 132.2, 126.5, 125.6, 113.2, 55.1 ppm; HRMS (ESI): [M+H]+ calcd. for C24H18O4 371.1283, found 371.1287.2,3-Bis(4-butoxyphenyl)-1,4-naphthoquinone (6.11)
Orange solid (0.0781 g, 69%), mp 86–88 °C; IR (KBr): ν = 1664, 1603, 1508, 1304, 1284, 1242, 1178, 813, 788, 716 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.16–8.18 (m, 2H, CHar), 7.75–7.77 (m, 2H, CHar), 7.00–7.02 (m, 4H, CHar), 6.75–6.77 (m, 4H, CHar), 3.93 (t, 2 × 2H, J = 6.4 Hz,![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH2CH3), 1.71–1.77 (m, 2 × 2H, OCH2
CH2CH2CH3), 1.71–1.77 (m, 2 × 2H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH2CH3), 1.42–1.51 (m, 2 × 2H, OCH2CH2
CH2CH3), 1.42–1.51 (m, 2 × 2H, OCH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH3), 0.96 (t, 2 × 3H, J = 7.5 Hz, OCH2CH2CH2
CH3), 0.96 (t, 2 × 3H, J = 7.5 Hz, OCH2CH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.0, 158.9, 144.6, 133.6, 132.2, 126.4, 125.4, 113.7, 67.5, 31.2, 19.1, 13.8 ppm; HRMS (ESI): [M+H]+ calcd. for C30H30O4 455.2222, found 455.2226.
) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.0, 158.9, 144.6, 133.6, 132.2, 126.4, 125.4, 113.7, 67.5, 31.2, 19.1, 13.8 ppm; HRMS (ESI): [M+H]+ calcd. for C30H30O4 455.2222, found 455.2226.
2,3-Bis(4-(allyloxy)phenyl-1,4-naphthoquinone (6.12)
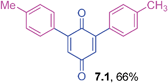
Viscous liquid (0.0781 g, 74%); IR (KBr): ν = 1660, 1604, 1505, 1287, 1247, 1179, 1059, 1021, 996, 820, 719 cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.16–8.19 (m, 2H, CHar), 7.75–7.78 (m, 2H, CHar), 7.00–7.02 (m, 4H, CHar), 6.77–6.79 (m, 4H, CHar), 5.99–6.07 (m, 2 × 1H, CHvinyl), 5.40 (dd, 2 × 1H, J = 17.2 Hz, 1.3 Hz, CHvinyl), 5.28 (dd, 2 × 1H, J = 10.7 Hz, 1.2 Hz, CHvinyl), 4.50 (d, 2 × 2H, J = 5.1 Hz, OCH2) ppm; 13C NMR (125 MHz, CDCl3): δ = 185.0, 158.4, 144.7, 133.6, 132.9, 132.2, 132.1, 126.5, 125.7, 117.8, 113.9, 68.6 ppm; HRMS (ESI): [M+H]+ calcd. for C28H22O4 423.1596, found 423.1595.2,6-Di-(4-methylphenyl)-1,4-benzoquinone (7.1)43
Yellow solid (0.0481 g, 66%), mp 142–144 °C (lit. 150–155 °C); IR (KBr): ν = 1662, 1641, 1607, 1585, 1303, 1106, 834, 514 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.41 (d, 4H, J = 8.2 Hz, CHar), 7.26 (d, 4H, J = 7.9 Hz, CHar), 6.88 (s, 2 × 1H, CHvinyl), 2.40 (s, 2 × 3H, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 187.7, 186.4, 146.4, 140.3, 131.9, 130.3, 129.3, 129.2, 21.3 ppm; HRMS (ESI): [M+H]+ calcd for C20H16O2 289.1229; found 289.1228.2,6-Bis(3-methoxyphenyl)-1,4-benzoquinone (7.2)
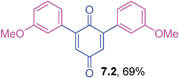
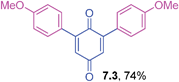
Orange liquid (0.0555 g, 69%); IR (neat): ν = 1646, 1598, 1483, 1309, 1209, 1177, 1048, 852 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.35–7.38 (m, 2H, CHar), 7.01–7.09 (m, 6H, CHar), 6.91 (s, 2 × 1H, CHvinyl), 3.84 (s, 2 × 3H, OCH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 187.5, 185.8, 159.4, 146.3, 134.4, 132.7, 129.5, 121.7, 115.8, 114.8, 55.3 ppm; HRMS (ESI): [M+H]+ calcd for C20H16O4 321.1127; found 321.1124.2,6-Bis(4-methoxyphenyl)-1,4-benzoquinone (7.3)
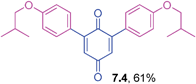
Orange solid (0.0594 g, 74%), mp 175–177 °C (lit. 180–182 °C); IR (KBr): ν = 1664, 1644, 1604, 1510, 1320, 1306, 1292, 1249, 1178, 1110, 1030, 995, 829 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.50 (d, 4H, J = 8.8 Hz, CHar), 6.97 (d, 4H, J = 8.8 Hz, CHar), 6.85 (s, 2 × 1H, CHvinyl), 3.86 (s, 2 × 3H, OCH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 187.7, 186.9, 161.2, 145.8, 131.1, 130.9, 125.5, 114.0, 55.3 ppm; HRMS (ESI): [M+H]+ calcd for C20H16O4 321.1127; found 321.1129.2,6-Bis(4-isobutoxyphenyl)-1,4-benzoquinone (7.4)
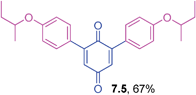
Orange solid (0.0624 g, 61%), mp 132–134 °C; IR (KBr): ν = 1667, 1649, 1602, 1569, 1506, 1326, 1284, 1255, 1176, 1113, 963, 913, 885, 839 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.47–7.49 (m, 4H, CHar), 6.95–6.97 (m, 4H, CHar), 6.84 (s, 2 × 1H, CHvinyl), 3.76 (d, 2 × 2H, J = 6.6 Hz, OCH2), 2.06–2.14 (m, 2 × 1H, CH), 1.03 (d, 2 × 6H, J = 6.9 Hz, C(CH3)2) ppm; 13C NMR (125 MHz, CDCl3): δ = 187.7, 187.0, 160.9, 145.8, 130.9, 125.3, 114.5, 74.4, 28.1, 19.2, ppm; HRMS (ESI): [M+H]+ calcd for C26H28O4 405.2066; found 405.2065.2,6-Bis(4-sec-butoxyphenyl)-1,4-benzoquinone (7.5)
Orange liquid (0.0681 g, 67%); IR (neat): ν = 2972, 1662, 1642, 1602, 1506, 1249, 1183, 1107, 992, 922, 838 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.46–7.49 (m, 4H, CHar), 6.93–6.97 (m, 4H, CHar), 6.84 (s, 2 × 1H, CHvinyl), 4.34–4.40 (m, 2 × 1H, OCH), 1.73–1.81 (m, 2 × 1H, OCH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 1.60–1.69 (m, 2 × 1H, OCH
), 1.60–1.69 (m, 2 × 1H, OCH![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 1.33 (d, 2 × 3H, J = 6.1 Hz, CH3), 0.98 (t, 2 × 3H, J = 7.4 Hz, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 187.7, 187.0, 160.0, 145.8, 130.9, 130.8, 125.1, 115.5, 75.0, 29.1, 19.1, 9.7 ppm; HRMS (ESI): [M+H]+ calcd for C26H28O4 405.2066; found 405.2062.
), 1.33 (d, 2 × 3H, J = 6.1 Hz, CH3), 0.98 (t, 2 × 3H, J = 7.4 Hz, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 187.7, 187.0, 160.0, 145.8, 130.9, 130.8, 125.1, 115.5, 75.0, 29.1, 19.1, 9.7 ppm; HRMS (ESI): [M+H]+ calcd for C26H28O4 405.2066; found 405.2062.
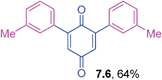
2,6-Di-(3-methylphenyl)-1,4-benzoquinone (7.6)
Yellow solid (0.0461 g, 64%), mp 102–104 °C; IR (KBr): ν = 1643, 1583, 1482, 1304, 1112, 1031, 794, 735 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.26–7.35 (m, 8H, CHar), 6.89 (s, 2 × 1H, CHvinyl), 2.40 (s, 2 × 3H, CH3) ppm; 13C NMR (125 MHz, CDCl3): δ = 187.7, 186.2, 146.6, 138.1, 133.1, 132.4, 130.7, 128.3, 126.4, 21.4 ppm; HRMS (ESI): [M+H]+ calcd for C20H16O2 289.1229; found 289.1227.2,6-Bis(4-isopropoxyphenyl)-1,4-benzoquinone (7.7)
Orange solid (0.0625 g, 66%), mp 68–70 °C; IR (KBr): ν = 1668, 1645, 1602, 1567, 1283, 1237, 995, 958, 834 cm−1; 1H NMR (500 MHz, CDCl3): 7.48 (d, 4H, J = 8.8 Hz, CHar), 6.94 (d, 4H, J = 8.8 Hz, CHar), 6.84 (s, 2 × 1H, CHvinyl), 4.58–4.65 (m, 2 × 1H, OCH), 1.37 (d, 2 × 6H, J = 6.0 Hz, C(CH3)2); 13C NMR (125 MHz, CDCl3): 187.7, 187.0, 159.6, 145.8, 130.9, 125.1, 115.5, 69.9, 21.9 ppm; HRMS (ESI): [M+H]+ calcd for C24H24O4 377.1753; found 377.1758.2,6-Bis(4-ethoxyphenyl)-1,4-benzoquinone (7.8)
Orange solid (0.0621 g, 71%), mp 142–144 °C; IR (KBr): ν = 1656, 1642, 1606, 1512, 1309, 1278, 1260, 1183, 1112, 1049, 828, 783 cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.47–7.49 (m, 4H, CHar), 6.94–6.97 (m, 4H, CHar), 6.85 (s, 2 × 1H, CHvinyl), 4.08 (q, 2 × 2H, J = 7 Hz,![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH3), 1.44 (t, 2 × 3H, J = 7 Hz, OCH2
CH3), 1.44 (t, 2 × 3H, J = 7 Hz, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ) ppm; 13C NMR (125 MHz, CDCl3): δ = 187.7, 187.0, 160.6, 145.8, 130.9, 125.3, 114.4, 63.5, 14.7 ppm; HRMS (ESI): [M+H]+ calcd for C22H20O4 349.1440; found 349.1447.
) ppm; 13C NMR (125 MHz, CDCl3): δ = 187.7, 187.0, 160.6, 145.8, 130.9, 125.3, 114.4, 63.5, 14.7 ppm; HRMS (ESI): [M+H]+ calcd for C22H20O4 349.1440; found 349.1447.
Acknowledgements
We thank the Department of Science and Technology (DST), New Delhi for funding this work through Green Chemistry programme (DST No. SR/S5/GC-11/2008). S. G. thanks the Council of Scientific and Industrial Research (CSIR), New Delhi for a research fellowship.References
- (a) I. Abraham, R. Joshi, P. Pardasani and R.T. Pardasani, J. Braz. Chem. Soc., 2011, 22, 385–421 CrossRef CAS; (b) Y. Kumagai, Y. Shinkai, T. Miura and A. K. Cho, Annu. Rev. Pharmacol. Toxicol., 2012, 52, 221–247 CrossRef CAS; (c) A. V. Pinto and S. L. Castro, Molecules, 2009, 14, 4570–4590 CrossRef.
- (a) D. C. K. Rathwell, S.-H. Yang, K. Y. Tsang and M. A. Brimble, Angew. Chem., Int. Ed., 2009, 48, 7996–8000 CrossRef CAS; (b) B. Kesteleyn, N. De Kimpe and L. V. Puyvelde, J. Org. Chem., 1999, 64, 1173–1179 CrossRef CAS; (c) W. R. Roush and D. S. Coffey, J. Org. Chem., 1995, 60, 4412–4418 CrossRef CAS; (d) P. Bachu, J. Sperry and M. A. Brimble, Tetrahedron, 2008, 64, 4827–4834 CrossRef CAS; (e) O. Hoffman-Ostenhof, Science, 1947, 105, 549–550 CAS; (f) A. M. Echavarren, N. Tamayo and D. J. Cardenas, J. Org. Chem., 1994, 59, 6075–6083 CrossRef CAS.
- (a) V. Ambrogi, D. Artini, I. Carneri, S. Castellino, E. Dradi, W. Logemann, G. Meinardi, M. D. Somma and G. Tosolini, Br. J. Pharmacol., 1970, 40, 871–880 CrossRef CAS; (b) T. B. Machado, A. V. Pinto, M. C. F. R. Pinto, I. C. R. Leal, M. G. Silva, A. C. F. Amaral, R. M. Kuster and K. R. Netto-dosSantos, Int. J. Antimicrob. Agents, 2003, 21, 279–284 CrossRef CAS; (c) V. K. Tandon, R. B. Chhor, R. V.Singh, S. Rai and D. B. Yadav, Bioorg. Med. Chem. Lett., 2004, 14, 1079–1083 CrossRef CAS; (d) G. Meazza, F. E. Dayan and D. E. Wedge, J. Agric. Food Chem., 2003, 51, 3824–3828 CrossRef CAS; (e) N. Islam and A. H. Pandith, J. Pharm. Res., 2012, 5, 1846–1853 CAS; (f) A. A. Khan, M. Nasr and F. G. Araujo, Antimicrob. Agents Chemother., 1998, 42, 2284–2289 CAS; (g) B. P. S. Khambay, D. Batty, M. Cahill and I. Denholm, J. Agric. Food Chem., 1999, 47, 770–775 CrossRef CAS; (h) E. Perez-Sacau, R. G. Diaz-Penate, A. Estevez-Braun, A. G. Ravelo, J. M. Garcia-Castellano, L. Pardo and M. Campillo, J. Med. Chem., 2007, 50, 696–706 CrossRef CAS; (i) A. E. Mathew, R. K.-Y. Zee-Cheng and C. C. Cheng, J. Med. Chem., 1986, 29, 1792–1795 CrossRef CAS.
- (a) K.-L. Wu, E. V. Mercado and T. R. R. Pettus, J. Am. Chem. Soc., 2011, 133, 6114–6117 CrossRef CAS; (b) L. S. Hegedus, T. A. Mulhern and A. Mori, J. Org. Chem., 1985, 50, 4282–4288 CrossRef CAS.
- H. N. A. Tran, S. Bongarzone, P. Carloni and G. Legname, Bioorg. Med. Chem. Lett., 2010, 20, 1866–1868 CrossRef CAS.
- B. C. Tlach, A. L. Tomlinson, A. Bhuwalka and M. Jeffries-EL, J. Org. Chem., 2011, 76, 8670–8681 CrossRef CAS.
- A. K. Machocho, T. Win, S. Grinberg and S. Bittner, Tetrahedron Lett., 2003, 44, 5531–5534 CrossRef CAS.
- B. VanVeller, K. Miki and T. M. Swager, Org. Lett., 2010, 12, 1292–1295 CrossRef CAS.
- (a) S. Witayakran, A. Zettili and A. J. Ragauskas, Tetrahedron Lett., 2007, 48, 2983–2987 CrossRef CAS; (b) D. Rutolo, S. Lee, R. Sheldon and H. W. Moore, J. Org. Chem., 1978, 43, 2304–2306 CrossRef CAS; (c) D. H. Ryu, G. Zhou and E. J. Corey, J. Am. Chem. Soc., 2004, 126, 4800–4802 CrossRef CAS.
- Y. Liu and J.-W. Sun, J. Org. Chem., 2012, 77, 1191–1197 CrossRef CAS.
- J. Magano and J. R. Dunetz, Chem. Rev., 2011, 111, 2177–2250 CrossRef CAS.
- T. Itahara, J. Org. Chem., 1985, 50, 5546–5550 CrossRef CAS.
- P. K. Singh, B. K. Rohtagi and R. N. Khanna, Synth. Commun., 1992, 22, 987–993 CrossRef CAS.
- O. M. Demchuk and K. M. Pietrusiewicz, Synlett, 2009, 1149–1153 CrossRef CAS.
- L. S. Liebeskind and S. W. Riesinger, Tetrahedron Lett., 1991, 32, 5681–5682 CrossRef CAS.
- A. Ortega, Á. Rincón, K. L. Jiménez-Aliaga, P. Bermejo-Bescós, S. Martín-Aragón, M. T. Molina and A. G. Csákÿ, Bioorg. Med. Chem. Lett., 2011, 21, 2183–2187 CrossRef CAS.
- S. Azuma, K. Nishio, K. Kubo, T. Sasamori, N. Tokitoh, K. Kuramochi and K. Tsubaki, J. Org. Chem., 2012, 77, 4812–4820 CrossRef CAS.
- Y. Tamura, M. Sasho, H. Ohe, S. Akai and Y. Kita, Tetrahedron Lett., 1985, 26, 1549–1552 CrossRef CAS.
- (a) M. S. Shvartsberg, E. A. Kolodina, N. I. Lebedeva and L. G. Fedenok, Tetrahedron Lett., 2009, 50, 6769–6771 CrossRef CAS; (b) M. Inman and C. J. Moody, J. Org. Chem., 2010, 75, 6023–6026 CrossRef CAS.
- (a) P. A. Suryavanshi, V. Sridharan and J. C. Menendez, Org. Biomol. Chem., 2010, 8, 3426–3436 RSC; (b) S. Laugraud, A. Guingant, C. Chassagnard and J. d'Angelo, J. Org. Chem., 1988, 53, 1557–1560 CrossRef CAS.
- W. S. Phutdhawong, W. Ruensamran, W. Phutdhawong and T. Taechowisan, Bioorg. Med. Chem. Lett., 2009, 19, 5753–5756 CrossRef CAS.
- T. N. Van and N. De Kimpe, Tetrahedron, 2003, 59, 5941–5946 CrossRef.
- M. Sakakibara, Y. Watanabe, T. Toru and Y. Ueno, J. Chem. Soc., Perkin Trans. 1, 1991, 1231–1234 RSC.
- M. W. Davies, C. N. Johnson and J. P. A. Harrity, J. Org. Chem., 2001, 66, 3525–3532 CrossRef CAS.
- (a) I. Ullah, R. A. Khera, M. Hussain, A. Villinger and P. Langer, Tetrahedron Lett., 2009, 50, 4651–4653 CrossRef CAS; (b) M. K. Hadden, S. A. Hill, J. Davenport, R. L. Matts and B. S. J. Blagg, Bioorg. Med. Chem., 2009, 17, 634–640 CrossRef CAS; (c) X. Gan, W. Jiang, W. Wang and L. Hu, Org. Lett., 2009, 11, 589–592 CrossRef CAS; (d) K. M. Dawood, Tetrahedron, 2007, 63, 9642–9651 CrossRef CAS; (e) D. J. Wallace and C. Chen, Tetrahedron Lett., 2002, 43, 6987–6990 CrossRef CAS; (f) O. M. Demchuk, B. Yoruk, T. Blackburn and V. Snieckus, Synlett, 2006, 2908–2913 CrossRef CAS.
- (a) A. M. Echavarren, O. Frutos, N. Tamayo, P. Noheda and P. Calle, J. Org. Chem., 1997, 62, 4524–4527 CrossRef CAS; (b) N. Tamayo, A. M. Echavarren and M. C. Paredes, J. Org. Chem., 1991, 56, 6488–6491 CrossRef CAS; (c) K. W. Stagliano and H. C. Malinakova, J. Org. Chem., 2001, 66, 7530–7534 CrossRef CAS; (d) K. W. Stagliano and H. C. Malinakova, Tetrahedron Lett., 1997, 38, 6617–6620 CrossRef CAS; (e) K. W. Stagliano and H. C. Malinakova, J. Org. Chem., 1999, 64, 8034–8040 CrossRef CAS.
- (a) M. L. N. Rao, D. N. Jadav and P. Dasgupta, Org. Lett., 2010, 12, 2048–2051 CrossRef CAS and the references cited therein; (b) M. L. N. Rao, V. Venkatesh and D. Banerjee, Synfacts, 2008, 406–406 CrossRef; (c) M. L. N. Rao, D. Banerjee and R. J. Dhanorkar, Synfacts, 2010, 928–928 CrossRef; (d) M. L. N. Rao and S. Giri, Eur. J. Org. Chem., 2012, 4580–4589 CrossRef CAS; (e) M. L. N. Rao, D. K. Awasthi and J. B. Talode, Tetrahedron Lett., 2012, 53, 2662–2666 CrossRef CAS; (f) M. L. N. Rao and P. Dasgupta, Tetrahedron Lett., 2012, 53, 162–165 CrossRef CAS.
- (a) E. J. Hutchinson, W. J. Kerr and E. J. Magennis, Chem. Commun., 2002, 2262–2263 RSC; (b) C. Wu, D. O. Berbasov and W. D. Wulff, J. Org. Chem., 2010, 75, 4441–4452 CrossRef CAS.
- (a) M. V. Bhatt and M. Periasamy, Tetrahedron, 1994, 50, 3575–3586 CrossRef CAS; (b) M. Periasamy and M. V. Bhatt, Tetrahedron Lett., 1977, 18, 2357–2360 CrossRef.
- (a) M. A.-M. Gomaa, Tetrahedron Lett., 2003, 44, 3493–3496 CrossRef CAS; (b) A. S. Hammam, M. S. K. Youssef, F. M. Atta and T. A. Mohamed, Chem. Pap., 2007, 61, 292–299 CrossRef CAS.
- (a) C. M. Woo, L. Lu, S. L. Gholap, D. R. Smith and S. B. Herzon, J. Am. Chem. Soc., 2010, 132, 2540–2541 CrossRef CAS; (b) M. M. M. Santos, N. Faria, J. Iley, S. J. Coles, M. B. Hursthouse, M. L. Martins and R. Moreira, Bioorg. Med. Chem. Lett., 2010, 20, 193–195 CrossRef CAS; (c) K. Maruyama, T. Otsuki and S. Tai, J. Org. Chem., 1985, 50, 52–60 CrossRef CAS; (d) B. Horowska, Z. Mazerska, A. Ledochowski, G. Cristalli, P. Franchetti and S. Martelli, Eur. J. Med. Chem., 1988, 23, 91–96 CrossRef CAS; (e) L. B. Nielsen, R. Slamet and D. Wege, Tetrahedron, 2009, 65, 4569–4577 CrossRef CAS; (f) M. Hussain, D. S. Zinad, G. A. Salman, M. Sharif, A. Villinger and P. Langer, Synlett, 2010, 276–278 CAS; (g) D. L. J. Clive, A. Khodabocus, P. G. Vernon, A. G. Angoh, L. Bordeleau, D. S. Middleton, C. Lowe and D. Kellner, J. Chem. Soc., Perkin Trans. 1, 1991, 1433–1444 RSC.
- O. Yamazaki, T. Tanaka, S. Shimada, Y. Suzuki and M. Tanaka, Synlett, 2004, 1921–1924 CAS.
- M. Shanmugasundaram, I. Garcia-Martinez, Q. Li, A. Estrada, N. E. Martinez and L. E. Martinez, Tetrahedron Lett., 2005, 46, 7545–7548 CrossRef CAS.
- L. S. Liebeskind, S. L. Baysdon, M. S. South, S. Iyer and J. P. Leeds, Tetrahedron, 1985, 41, 5839–5853 CrossRef CAS.
- (a) J.-L. Grandmaison and P. Brassard, Tetrahedron, 1977, 33, 2047–2053 CrossRef CAS; (b) J. Padwal, W. Lewis and C. J. Moody, Synlett., 2010, 514–516 CAS; (c) T. R. Kelly, A. Garcia, F. Lang, J. J. Walsh, K. V. Bhaskar, M. R. Boyd, R. Götz, P. A. Keller, R. Walter and G. Bringmann, Tetrahedron Lett., 1994, 35, 7621–7624 CrossRef CAS; (d) D. Barker, M. A. Brimble, P. Do and P. Turner, Tetrahedron, 2003, 59, 2441–2449 CrossRef CAS; (e) A. V. R. Rao, K. L. Reddy and A. S. Rao, Tetrahedron Lett., 1994, 35, 5047–5050 CrossRef CAS.
- L. F. Tietze, R. R. Singidi and K. M. Gericke, Chem.–Eur. J., 2007, 13, 9939–9947 CrossRef CAS.
- S. W. Lee, C. H. Lee and K.-J. Lee, Bull. Korean Chem. Soc., 2006, 27, 769–772 CrossRef CAS.
- (a) A. Bunge, H.-J. Hamann, E. McCalmont and J. Liebscher, Tetrahedron Lett., 2009, 50, 4629–4632 CrossRef CAS; (b) S. C. Sharma and K. Torssell, Acta Chem. Scand., Ser. B, 1978, 32, 347–53 CrossRef.
- H. A. Torrey and W. H. Hunter, J. Am. Chem. Soc., 1912, 34, 702–716 CrossRef CAS.
- I. T. Crosby, M. L. Rose, M. P. Collis, P. J. Bruyn, P. L. C. Keep and A. D. Robertson, Aust. J. Chem., 2008, 61, 768–784 CrossRef CAS.
- H. Suzuki and Y. Matano, (ed.), Organobismuth chemistry, Elsevier, Amsterdam, 2001 Search PubMed.
- (a) C. Wu, D. O. Berbasov and W. D. Wulff, J. Org. Chem., 2010, 75, 4441–4452 CrossRef CAS; (b) S. Irvine, W. J. Kerr, A. R. McPherson and C. M. Pearson, Tetrahedron, 2008, 64, 926–935 CrossRef CAS.
- (a) K. Maruyama, T. Shio and Y. Yamamoto, Bull. Chem. Soc. Jpn., 1979, 52, 1877–1878 CrossRef CAS; (b) E. C. S. Jones and J. Kenner, J. Chem. Soc., 1931, 1842–1857 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra22058j |
| This journal is © The Royal Society of Chemistry 2012 |