Green and recyclable glycine nitrate (GlyNO3) ionic liquid triggered multicomponent Biginelli reaction for the efficient synthesis of dihydropyrimidinones†
Nandini
Sharma
,
Upendra
Kumar Sharma
,
Rajesh
Kumar
,
Richa
and
Arun
Kumar Sinha
*
Natural Plant Products Division, CSIR-Institute of Himalayan Bioresource Technology, Post Box # 6, Palampur, 176061 (H.P), India. E-mail: aksinha08@rediffmail.com; Fax: +91-1894-230433
First published on 11th September 2012
Abstract
Herein we report the use of an amino acid ionic liquid as a green catalyst for the multicomponent synthesis of 3,4-dihydropyrimidin-2(1H)-ones in excellent yields and short reaction time. The ionic liquid is inexpensive and biodegradable and can be reused for more than ten consecutive reactions. The potential of the protocol for gram scale synthesis adds to its practical applicability.
Introduction
From an environmental and economic perspective, multicomponent reactions (MCRs) have emerged as valuable tools for the preparation of structurally diverse drug-like compounds.1 One prominent MCR that produces an interesting class of nitrogen heterocycles is the venerable Biginelli reaction providing 3,4-dihydropyrimidin-2-(1H)-ones (DHPMs).2 DHPM derivatives exhibit a wide range of biological and pharmacological properties such as antiviral, antimitotic, anticarcinogenic, antihypertensive and most importantly, as calcium channel modulators.3 Additionally, the biological activity of the potent HIV gp-120-CD4 inhibitors Batzelladine A and B have also been attributed to the DHPM moiety.4 The classical Biginelli synthesis involves the three component condensation of an aldehyde, urea and a β-ketoester under acidic conditions in ethanol5 which suffers from the drawbacks like low to moderate yields besides harsh conditions and long reaction times.6 Consequently, several modifications have been developed for the classical Biginelli approach.7 However, in spite of their potential utility many of these methods involve expensive and/or toxic catalysts, stoichiometric amount of catalyst, unsatisfactory yields, cumbersome product isolation procedures and incompatibility with certain functional groups, and thus are not closer to the principles of green chemistry. Hence, the challenge for a sustainable environment calls for more general and viable routes employing recyclable catalysts in Biginelli synthesis which would be of great relevance to both synthetic and medicinal chemists.In this context, ionic liquids (ILs) have offered the potential for path-breaking changes to synthetic routes as “green” substitutes for organic solvents or catalysts8 due to their particular properties such as undetectable vapour pressure, ease of recovery and reuse.9 So far, ILs with cations derived from alkylammonium, dialkylimidazolium and pyridinium based ILs have been used as catalysts for Biginelli reaction.10 Though these ILs help reduce the risk of air pollution, concerns are being raised over their potential toxicity to aquatic environments and inaccessible biodegradability.11 Therefore, the development of bio-renewable ILs based on amino acids and their derivatives to replace the above cations is another promising approach because amino acids and their derivatives are the most abundant natural source of quaternary nitrogen precursors.12 Moreover, low cost, easy preparation, biodegradability, non or lesser toxicity and properties of amino acids to act as both anions and cations are added advantage of these amino acid ionic liquids (AAILs).11a,12b,h,13 Apart from this, in an endeavour to design truly environmental benign processes green technologies such as microwave-assisted synthesis are being combined with ILs and/or organocatalysts.14
In our search for inexpensive ionic liquids with reduced toxicity and better biodegradability, herein we report the catalytic efficiency of highly recyclable amino acid ionic liquids, particularly glycine nitrate, under microwave irradiation (MW) for the one-pot three-component Biginelli condensation (Scheme 1). To the best of our knowledge, this is the first report on the use of AAIL in a multicomponent reaction.
 | ||
| Scheme 1 Amino acid based ionic liquid catalyzed one-pot three-component Biginelli reaction. | ||
Results and discussion
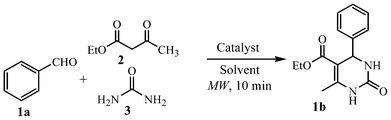
Initially, glycine was examined as a simple and cheap organocatalyst for the Biginelli condensation with a mixture of benzaldehyde (1a, 0.25 mmol), ethyl acetoacetate (0.25 mmol), and urea (0.25 to 0.75 mmol) in EtOH under various conditions. Optimum results were obtained when the reaction was performed using 3 equiv. of glycine and 0.75 mmol urea affording dihydropyrimidinone 1b in 74% yield after 8 h at 80 °C. Decreasing the reaction temperature (40 °C) adversely affected the yield of 1b (58%) besides a longer time up to 144 h under conventional conditions. Interestingly, a positive effect in terms of significant reduction of the reaction time (10 min) was observed when the reaction was carried out under microwave irradiation (Table 1, entry 1). Products could be isolated after pouring the reaction mixture in ice-cold water, filtration and recrystallization from water–alcohol mixture. However, in our study recovery of glycine catalyst from water posed a problem for its reusability.| Entry | Amount of Glycine | Time | T/°C | Yieldb % |
|---|---|---|---|---|
| a Reaction conditions: Benzaldehyde (0.25 mmol), dicarbonyl compound (1 equiv), urea (3 equiv), solvent (3 mL). b Isolated yield. c MW irradiation at P = 100 W. | ||||
| 1 | 3 equiv. | 10 min | MWc | 74% |
| 2 | 3 equiv. | 5 min | MWc | 35% |
| 3 | 3 equiv. | 15 min | MWc | 69% |
| 4 | 2 equiv. | 10 min | MWc | 63% |
In view of an environmental friendly procedure, the reuse of a catalyst is quite preferable, therefore, to identify a more recyclable organocatalyst, amino acid ionic liquids comprising of glycine as cation in combination with suitable inorganic anions were synthesized12g,h (glycine nitrate; GlyNO3, glycine sulphate; GlySO4, and glycine chloride; GlyCl). Inorganic anions used i.e. NO3−, SO42− and Cl− are non-toxic and pharmaceutically acceptable;15 thus making these ionic liquids fully green ones.12g To evaluate the ability of above AAILs as catalysts in the Biginelli reaction, the three component condensation reaction of benzaldehyde, ethyl acetoacetate and urea was performed at 1 equiv. of catalyst amount in EtOH (Table 2, entries 1–3).
| Entry | Catalyst | Catalyst amount | Solvent | Yieldb % |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.25 mmol), ethyl acetoacetate, urea, catalyst in 3 mL of solvent under MW at P = 100 W. b Isolated yield of 1b. | ||||
| 1 | GlyNO3 | 1 equiv. | EtOH | 90% |
| 2 | GlySO4 | 1 equiv. | EtOH | 78% |
| 3 | GlyCl | 1 equiv. | EtOH | 82% |
| 4 | GlyNO3 | 1 equiv. | Water | 22% |
| 5 | GlyNO3 | 0.5 equiv. | EtOH | 90% |
| 6 | GlyNO3 | 0.4 equiv. | EtOH | 92% |
| 7 | GlyNO3 | 0.3 equiv. | EtOH | 79% |
From the results, it was noticed that all the three ionic liquids could promote the reaction, with GlyNO3 (Table 2, entry 1) providing superior results. As a clean and inexpensive solvent, water was also employed as reaction medium; however it failed to produce any significant yield (Table 2, entry 4). Interestingly, in comparison to 3 equiv. glycine (amino acid, Table 1), the use of only 0.4 equiv. of GlyNO3 (amino acid ionic liquid) could give a yield up to 92% under the microwave power (P) of 100 W and the irradiation time of 10 min (Table 2, entries 5–7).
To investigate the reusability of GlyNO3, recycling studies were carried out. GlyNO3 was easily collected from the reaction medium by cooling the mixture at 0 °C and filtering the contents. The catalyst was regenerated by washing with ethanol, followed by drying at room temperature and used for Biginelli condensation studies of 1a for another ten cycles. No appreciable loss of catalytic activity was noticed16 (Scheme 2), thereby suggesting the robustness AAIL as an catalyst.
 | ||
| Scheme 2 Recyclability study of glycine nitrate for the synthesis of 1b. | ||
As per sustainable chemistry principles, aromatic aldehydes 2a–5a were generated from abundantly available phenylpropenes as per our previous reports17 and subjected to Biginelli condensation under the optimized reaction conditions (Table 3, entries 2–5). Further, to expand the practical scope of the developed method, commercial benzaldehydes 6a–15a were treated with urea and 1,3 dicarbonyl compounds for the synthesis of dihydropyrimidone derivatives 6b–15b. Thiourea was also used with similar success to provide the corresponding dihydropyrimidine-(2H)-thiones (Table 3, entries 16–19), which are also of much interest with regard to biological activity (e.g. Monastrol3g18a, Table 3). An important aspect of the established protocol is the survival of a variety of functional groups such as NO2, Cl, OH and OCH3 under the reaction conditions. All the obtained products (Table 3, entries 1–19) were characterized by NMR and HRMS data (see ESI†).
| Entry | R | R′ | X | Time (min) | Yieldb % |
|---|---|---|---|---|---|
| a Reaction conditions: Subsituted benzaladehyde (0.25 mmol), dicarbonyl compound (1 equiv.), urea or thiourea (3 equiv.), MW at P = 100 W in 3 mL of EtOH. b Isolated yield (after recrystallization). All compounds have been characterized by NMR and HRMS data (available in the ESI†). | |||||
| 1 | C6H5 | Et | O | 10 | 92 |
| 2 | 4-OMeC6H4 | Et | O | 10 | 88 |
| 3 | 2,4,5-(OMe)3C6H2 | Et | O | 10 | 84 |
| 4 | 3,4-(-OCH2O-)C6H3 | Et | O | 10 | 80 |
| 5 | 4-OH, 3-OMeC6H3 | Et | O | 10 | 80 |
| 6 | 4-OHC6H4 | Et | O | 10 | 75 |
| 7 | 3-OHC6H4 | Et | O | 10 | 76 |
| 8 | 4-ClC6H4 | Et | O | 10 | 86 |
| 9 | 3-BrC6H4 | Et | O | 10 | 88 |
| 10 | 4-NO2C6H4 | Et | O | 10 | 85 |
| 11 | C10H7 | Et | O | 10 | 85 |
| 12 | 4-N,N(CH3)2C6H4 | Et | O | 10 | 84 |
| 13 | 4-MeC6H4 | Et | O | 10 | 87 |
| 14 | C6H5 | CH3 | O | 10 | 84 |
| 15 | C6H5 | t-Bu | O | 10 | 89 |
| 16 | C6H5 | Et | S | 20 | 80 |
| 17 | 4-OH, 3-OMeC6H3 | Et | S | 20 | 80 |
| 18 | 3-OHC6H4 | Et | S | 20 | 74 |
| 19 | 4-ClC6H4 | Et | S | 20 | 83 |
Reaction of 2-hydroxybenzaldehyde (20a), ethyl acetoacetate and urea provided a product (20c) instead of the expected product (20b). The mass of both 20b and 20c was similar; however the NMR spectral data of 20c was different from 20b (Scheme S1; see ESI†). Based on 1H and 13C NMR values and previous reports, structure of the compound 20c was confirmed to be oxygen-bridged instead of the classical Biginelli structure (Scheme S1; see ESI†).
To probe the exact mechanism of GlyNO3 mediated Biginelli reaction, three routes18 were investigated: (i) involving carbenium ion pathway (A),18a (ii) iminium ion pathway (B)18b and (iii) bis-urea pathway (C).18c When 4-chlorobenzaldehyde (8a, 0.25 mmol), ethyl acetoacetate (2, 0.25 mmol) and GlyNO3 were subjected to MW irradiation for 10 min, no product was observed which rules out the possibility of carbenium ion pathway (A).18a Next, to investigate the possibility of iminium ion pathway (B), 8a (0.25 mmol) and urea (3, 0.25 mmol) were subjected to MW irradiation for 10 min in GlyNO3, afterwards ethyl acetoacetate (0.25 mmol) was added and again MW for 10 min, however lack of desired product indicated that iminium ion pathway was not followed.18b Consequently, when urea (0.75 mmol, 3 equiv.) was reacted with 8a (0.25 mmol), bis-urea derivative was obtained which subsequently condensed with ethyl acetoacetate (0.25 mmol) generating the desired product18c8b in 90% yield under MW irradiation for 10 min. Thus, the reaction mechanism is proposed to follow the bis-urea path (C) as shown in Scheme 3.
 | ||
| Scheme 3 Proposed reaction mechanism for Biginelli reaction. | ||
After successfully accomplishing the synthesis of compounds 1b–19b (Table 3) under focused monomode microwave (CEM, P = 100 W, 10 min), we ventured to increase the generality of the developed protocol by conducting the conversion of 1a into 1b under multimode domestic microwave oven (due to its easy access in almost every laboratory). Though some concern has been raised on the reproducibility of domestic microwave ovens due to unevenness of the microwave energy inside,14d we however, got reproducible yields (89 ± 1%) for above conversion (repeated thrice) under multimode domestic microwave (Kenstar 2450 MHz, 900 ± 10% Watts, 10 min), which implied that our method worked efficiently in both multimode and monomode microwave conditions.
Further, to demonstrate the practical applicability of the developed method, preparative scale reaction (1 g batch) of 3-hydroxybenzaldehyde (18a) with ethyl acetoacetate/thiourea was effectively accomplished using glycine nitrate as catalyst under multimode domestic microwave leading to the formation of a potent mitotic kinesin Eg5 inhibitor, Monastrol3g (18b) in good yield (Scheme 4).
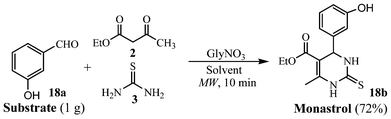 | ||
| Scheme 4 Gram scale synthesis of bioactive Monastrol 18b under microwave. | ||
Conclusion
In conclusion, we have successfully developed a novel amino acid ionic liquid (GlyNO3) based green approach for the synthesis of bioactive 3,4-dihydropyrimidin-2(1H)-ones. The developed method not only preserved the simplicity of Biginelli's one-pot condensation but also provided excellent to good yields of dihydropyrimidinones in shorter reaction times under microwave irradiation. Moreover, the inexpensive AAIL is biodegradable and recyclable and is reused for more than ten cycles without showing any significant loss in its catalytic activity.Experimental section
General procedure for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones (1b–20b) from substituted benzaldehydes (Table 3, entries 1–19 and Scheme S1†)
Substituted benzaldehyde (1a–20a, 0.25 mmol), ethyl acetoacetate (1 equiv.), urea (3 equiv.) and glycine nitrate (0.4 equiv.) were taken in 3 mL ethanol in a round bottom flask and the reaction mixture was subjected to microwave irradiation using CEM monomode microwave at Power = 100 W for 10 min. After completion of reaction, solvent was removed under vacuum and recrystallized with MeOH and analyzed with HPLC which showed a conversion yield of 90–98%. Further, the crude product was purified by recrystallization from water–ethanol mixture giving an isolated yield of 1b–20b in the range of 74–92%. Products were identified and confirmed by their 1H, 13C NMR spectra and HRMS/MS values.Acknowledgements
NS, UKS, RK are indebted to CSIR, New Delhi, for the award of research fellowships. The authors gratefully acknowledge the Director, IHBT Palampur, for his kind cooperation and encouragement as well as project MLP0025 for financial assistance. Thanks to Mr. Shiv Kumar for help regarding HRMS and NMR analysis.References
- (a) I. Ugi, A. Dömling and W. Hörl, Endeavour, 1994, 18, 115–122 CrossRef CAS; (b) L. F. Tietze and A. Modi, Med. Res. Rev., 2000, 20, 304–322 CrossRef CAS; (c) R. V. A. Orru and M. De Greef, Synthesis, 2003, 1471–1499 CrossRef CAS.
- (a) C. O. Kappe, Curr. Opin. Chem. Biol., 2002, 6, 314–320 CrossRef CAS; (b) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168–3210 CrossRef; (c) Multicomponent Reactions, ed., J. Zhu, H. Bienayme, Wiley, Weinheim, Germany, 2005 Search PubMed.
- (a) C. O. Kappe, Molecules, 1998, 3, 1–9 CrossRef CAS; (b) C. O. Kappe, Eur. J. Med. Chem., 2000, 35, 1043–1052 CrossRef CAS; (c) M. Yarim, S. Saraç, F. S. Kiliç and K. Erol, Farmaco, 2003, 58, 17–24 CrossRef CAS; (d) M. Kidwai, S. Saxena, M. K. R. Khan and S. S. Thukral, Eur. J. Med. Chem., 2005, 40, 816–818 CrossRef CAS; (e) P. Kumar, B. R. Sankar, G. Nasir, R. B. Baig and S. Chandrashekaran, Eur. J. Med. Chem., 2009, 44, 4192–4198 CrossRef; (f) K. S. Jain, J. B. Bariwal, M. K. Kathiravan, M. S. Phoujdar, R. S. Sahne, B. S. Chauhan, A. K. Shah and M. R. Yadav, Bioorg. Med. Chem., 2008, 16, 4759–4800 CrossRef CAS; (g) T. U. Mayer, T. M. Kapoor, S. J. Haggarty, R. W. King, S. L. Schreiber and T. J. Mitchison, Science, 1999, 286, 971–974 CrossRef CAS.
- S. F. Hojati, M. Gholizadeh, M. Haghdoust and F. Shafiezadeh, Bull. Korean Chem. Soc., 2010, 31, 3238–3240 CrossRef CAS.
- P. Biginelli, Gazz. Chim. Ital., 1893, 23, 360–413 Search PubMed.
- (a) K. S. Atwal, G. C. Rovnyak, B. C. O'Reilly and J. Schwartz, J. Org. Chem., 1989, 54, 5898–5907 CrossRef CAS; (b) J. Barluenga, M. Tomas, A. Ballesteros and L. A. Lopez, Tetrahedron Lett., 1989, 30, 4573–4576 CrossRef CAS; (c) D. Russowsky, F. A. Lopesa, V. S. S. da Silvaa, K. F. S. Cantoa, M. G. Montes D'Ocab and M. N. Godoi, J. Braz. Chem. Soc., 2004, 15, 165–169 CrossRef CAS.
- (a) S. Gore, S. Baskaran and B. Koenig, Green Chem., 2011, 13, 1009–1013 RSC; (b) C. Ramalingan, S.-J. Park, I.-S. Lee and Y.-W. Kwak, Tetrahedron, 2010, 66, 2987–2994 CrossRef CAS; (c) Z. L. Shen, X. P. Xu and S. J. Ji, J. Org. Chem., 2010, 75, 1162–1167 CrossRef CAS; (d) F. Tamaddon, Z. Razmi and A. A. Jafari, Tetrahedron Lett., 2010, 51, 1187–1189 CrossRef CAS; (e) S. Chitra and K. Pandiarajan, Tetrahedron Lett., 2009, 50, 2222–2224 CrossRef CAS; (f) M. I. Lannou, F. Hélion and J. L. Namy, Synlett, 2008, 105–107 CAS; (g) M. A. Chari, A. Mano, S. T. Selvan, K. Mukkanti and A. Vinu, Tetrahedron, 2009, 65, 10608–10611 CrossRef; (h) I. Suzuki, Y. Iwata and K. Takeda, Tetrahedron Lett., 2008, 49, 3238–3241 CrossRef CAS; (i) A. Debache, M. Amimour, A. Belfaitah, S. Rhouati and B. Carboni, Tetrahedron Lett., 2008, 49, 6119–6121 CrossRef CAS; (j) V. Polshettiwar and R. S. Varma, Tetrahedron Lett., 2007, 48, 7343–7346 CrossRef CAS; (k) Y. Yu, C. Liu and G. Luo, Bioorg. Med. Chem. Lett., 2007, 17, 3508–3510 CrossRef CAS; (l) A. Debache, B. Boumoud, M. Amimour, A. Belfaitah, S. Rhouati and B. Carboni, Tetrahedron Lett., 2006, 47, 5697–5699 CrossRef CAS; (m) R. S. Bhosale, S. V. Bhosale, S. V. Bhosale, T. Wang and P. K. Zubaidha, Tetrahedron Lett., 2004, 45, 9111–9113 CrossRef CAS; (n) D. Shobha, E. Rafiee and H. Jafari, Bioorg. Med. Chem. Lett., 2006, 16, 2463–2466 CrossRef; (o) B. K. Banik, A. T. Reddy, A. Datta and C. Mukhopadhyay, Tetrahedron Lett., 2007, 48, 7392–7394 CrossRef CAS.
- (a) R. Sheldon, Chem. Commun., 2001, 2399–2407 RSC; (b) J. Dupont, R. F. de Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667–3692 CrossRef CAS.
- (a) P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef CAS; (b) J. S. Wilkes, J. Mol. Catal. A: Chem., 2004, 214, 11–17 CrossRef CAS; (c) C. K. Z. Andrade and L. M. Alves, Curr. Org. Chem., 2005, 9, 195–218 CrossRef CAS; (d) D. R. Macfarlane, J. M. Pringle, K. M. Johansson, S. A. Forsyth and M. Forsyth, Chem. Commun., 2006, 1905–1917 RSC; (e) A. C. Cole, J. L. Jensen, I. Ntai, T. Tran, K. J. Weaver, D. C. Forbes and J. H. Davis Jr, J. Am. Chem. Soc., 2002, 124, 5962–5963 CrossRef CAS.
- (a) J. J. Peng and Y. Q. Deng, Tetrahedron Lett., 2001, 42, 5917–5919 CrossRef CAS; (b) A. Shaabani and A. Rahmati, Catal. Lett., 2005, 100, 177–179 CrossRef CAS; (c) M. Li, W. S. Guo, L. R. Wen, Y. F. Li and H. Z. Yang, J. Mol. Catal. A: Chem., 2006, 258, 133–138 CrossRef CAS; (d) R. Zheng, X. Wang, H. Xu and J. Du, Synth. Commun., 2006, 36, 1503–1513 CrossRef CAS; (e) P. Wasserscheid, R. Van Hal and A. Bösmann, Green Chem., 2002, 4, 400–404 RSC; (f) J. Fraga-Dubreuil, K. Bourahla, M. Rahmouni, J. P. Bazureau and J. Hamelin, Catal. Commun., 2002, 3, 185–190 CrossRef CAS; (g) F. Dong, L. Jun, Z. Xinli, Y. Zhiwen and L. Zuliang, J. Mol. Catal. A: Chem., 2007, 274, 208–211 CrossRef CAS; (h) X. Liu, M. Lu, T. Lu and C. Mai, J. Sci., 2011, 38, 263–269 CAS; (i) S. R. Roy, P. S. Jadhavar, K. Seth, K. K. Sharma and A. K. Chakraborti, Synthesis, 2011, 2261–2267 CAS.
- (a) D. Zhao, Y. Liao and Z. Zhang, Clean, 2007, 35, 42–48 CAS; (b) J. Ranke and B. Jastorff, Environ. Sci. Pollut. Res., 2000, 7, 105–114 CrossRef CAS; (c) A. Latala, P. Stepnowski, M. Nedzi and W. Mrozik, Aquat. Toxicol., 2005, 73, 91–98 CrossRef CAS; (d) M. A. P. Martins, C. P. Frizzo, D. N. Moreira, N. Zanatta and H. G. Bonacorso, Chem. Rev., 2008, 108, 2015–2050 CrossRef CAS; (e) A. Romeroa, A. Santos, J. Tojob and A. Rodriguez, J. Hazard. Mater., 2008, 151, 268–273 CrossRef.
- (a) W. Bao, Z. Wang and Y. Li, J. Org. Chem., 2003, 68, 591–593 CrossRef CAS; (b) K. Fukumoto and H. Ohno, Chem. Commun., 2006, 3081–3083 RSC; (c) K. Fukumoto, M. Yoshizawa and H. Ohno, J. Am. Chem. Soc., 2005, 127, 2398–2399 CrossRef CAS; (d) L. C. Branco, P. M. P. Gois, N. M. T. Lourenço, V. B. Kurteva and C. A. M. Afonso, Chem. Commun., 2006, 2371–2372 RSC; (e) F. Guillen, D. Brégeon and J. C. Plaquevent, Tetrahedron Lett., 2006, 47, 1245–1248 CrossRef CAS; (f) S. Luo, D. Zu, H. Yue, L. Wang, W. Yang and Z. Xu, Tetrahedron: Asymmetry, 2006, 17, 2028–2033 CrossRef CAS; (g) G. H. Tao, L. He, N. Sun and Y. Kou, Chem. Commun., 2005, 3562–3564 RSC; (h) G. H. Tao, L. He, W. S. Liu, L. Xu, W. Xiong, T. Wang and Y. Kou, Green Chem., 2006, 8, 639–646 RSC.
- H. Ohno and K. Fukumoto, Acc. Chem. Res., 2007, 40, 1122–1129 CrossRef CAS.
- (a) J. M. Levenque and G. Cravotto, Chimia, 2006, 60, 613–620 Search PubMed; (b) H. Hoffmann, M. Nuchter, B. Ondruschka and P. Wasserscheid, Green Chem., 2003, 5, 296–299 RSC; (c) S. K. Singh and K. N. Singh, J. Heterocyclic Chem., 2010, 47, 194–198 CAS; (d) B. L. Hayes, Microwave Synthesis: Chemistry at the Speed of Light, CEM Publishing, Matthews, NC, USA, 2002 Search PubMed.
- R. P. Swatloski, J. D. Holbrey and R. D. Rogers, Green Chem., 2003, 5, 361–363 RSC.
- After ten cycles, yield of isolated product was 85%. The difference in the yields for the first and tenth cycle was found to be 7%.
- (a) N. Sharma, U. K. Sharma, R. Salwan, R. C. Kasana and A. K. Sinha, Catal. Lett., 2011, 141, 616–622 CrossRef CAS; (b) R. C. Kasana, U. K. Sharma, N. Sharma and A. K. Sinha, Curr. Microbiol., 2007, 54, 457–461 CrossRef CAS.
- (a) C. O. Kappe, J. Org. Chem., 1997, 62, 7201–7204 CrossRef CAS; (b) R. O. M. A. De Souza, E. T. da Penha, H. M. S. Milagre, S. J. Garden, P. M. Esteves, M. N. Eberlin and O. A. C. Antunes, Chem.–Eur. J., 2009, 15, 9799–9804 CrossRef CAS; (c) Z. L. Shen, X. P. Xu and S. J. Ji, J. Org. Chem., 2010, 75, 1162–1167 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Complete experimental details and spectroscopic data of compounds. |
| This journal is © The Royal Society of Chemistry 2012 |