Solar powered lithium-ion battery incorporating high performing electrode materials†
S.
Gopukumar
*a,
C.
Nithya
a,
P. H.
Maheshwari
b,
R.
Ravikumar
a,
R.
Thirunakaran
a,
A.
Sivashanmugam
a,
S. K.
Dhawan
b and
R. B.
Mathur
b
aCSIR-Network Institutes of Solar Energy (CSIR-NISE), CSIR-Central Electrochemical Research Institute, Karaikudi, 630 006, India. E-mail: deepika_41@rediffmail.com; Fax: 914565 227779
bCSIR-National Physical Laboratory, New Delhi, 110 007, India
First published on 1st October 2012
Abstract
The development of portable electronic communities requires high performing and high power lithium rechargeable batteries. Herein, we explore a new lithium ion battery combined with a new carbon based anode and cobalt based cathode which delivers an energy output of 280 Wh kg−1 and cycling efficiency of 97% over the investigated 500 cycles (1 C rate) of the lithium ion cell.
Introduction
The commercialization of lithium ion cells was achieved in the early 1990′s by the Sony Corporation. Almost 90% of commercial lithium ion batteries consist of a lithium cobalt oxide cathode1 and a graphite anode, which is separated by a lithium ion conducting electrolyte, for example 1 M LiPF6 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ethylene carbonate
1 (ethylene carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diethylene carbonate). Only 5–10% of the lithium ion cells are made by using LiFePO4 or LiNi1/3Co1/3Mn1/3O2 based cathodes and carbon based anodes. The commercial success of lithium ion batteries is still limited at high voltages and high rates because of the lack of suitable electrode materials for this application. Tin based amorphous oxide anode materials exhibit a high capacity for storing Li+ ions as reported by Y. Yu et al.2 Recently TiO23 and carbon nanotube4 based anode materials exhibiting high capacities have been reported by various research groups. However, a large volume change during cycling can destroy the anode structure, resulting in poor retention capacity for prolonged cycling. With this in mind, researchers still focus on graphite based anode materials like graphene,5,6 which exhibits excellent capacity and cyclability.
diethylene carbonate). Only 5–10% of the lithium ion cells are made by using LiFePO4 or LiNi1/3Co1/3Mn1/3O2 based cathodes and carbon based anodes. The commercial success of lithium ion batteries is still limited at high voltages and high rates because of the lack of suitable electrode materials for this application. Tin based amorphous oxide anode materials exhibit a high capacity for storing Li+ ions as reported by Y. Yu et al.2 Recently TiO23 and carbon nanotube4 based anode materials exhibiting high capacities have been reported by various research groups. However, a large volume change during cycling can destroy the anode structure, resulting in poor retention capacity for prolonged cycling. With this in mind, researchers still focus on graphite based anode materials like graphene,5,6 which exhibits excellent capacity and cyclability.
Besides this, the cathode material also limits the storage capacity of rechargeable lithium batteries. K. Kang et al.7 reported that the safe, inexpensive LiNi0.5Mn0.5O2 cathode material delivers a discharge capacity of 125 mAhg−1 at 1 C rate. Recently Recham et al.8 reported LiFeSO4F cathode materials exhibiting good capacity retention, however, the voltage (3.6 vs. Li) is lower than for LiCoO2. Iron and manganese based phosphate materials such as LiFePO49–12 and LiCoxMn1−xPO413 have drawn attention for use in lithium ion batteries; however, these materials have problems like conductivity and lower voltage, which is overcome by carbon coating and nano-sized particles. LiNi0.8Mn0.1Co0.1O2 surrounded by a concentration gradient outer layer exhibits high capacity, but after 50 cycles the capacity fades very rapidly as reported by Y. K. Sun et al.14 Due to the above shortcomings, researchers still focus on LiCoO2 cathode materials because it has a high theoretical capacity and high voltages compared to the above mentioned materials; however, it delivers half of the theoretical capacity, and is also expensive. Various methods to overcome these shortcomings, such as coating with inactive metal oxides15 and doping with metal cations,16,17 improve the capacity in the limited voltage regions (∼4.3 V), but this strategy fails at the deep discharge states as reported by many researchers. Deepa et al.18 and M. Zou et al.19 reported that the conductivity of LiCoO2 was enhanced by Cu and operates well at 4.5 V delivering high capacity retention. Increasing the potential to >4.5 V requires a suitable dopant to increase the structural stability as well as cycling stability at high voltages. Mg is the most promising dopant to stabilize the layered structure of LiCoO2 as reported by many authors.20–23 The presently used lithium ion cells deliver an energy output of 100–250 Wh kg−1 and a cycling efficiency only 80–90%.24 To overcome the above problems in electrode materials and lithium ion cells, we have developed a new lithium ion cell comprising of a graphite paper anode and Cu, Mg doped LiCoO2 cathode.
Results and discussion
Fig. 1 shows the XRD patterns of the carbon paper and LiMg0.025Cu0.175Co0.8O2 materials. XRD patterns of the carbon paper indicate that the presence of a graphitic structure, and the cathode material clearly shows a layered structure in the R3m space group. The clear splitting of the (006) (102) (Fig. 1c) and (108) (110) (Fig. 1d) peak doublets indicates that an ordered distribution of lithium and transition metal ions exist in the structure.25 Unit cell parameters of the synthesized materials are calculated by using X‘Pert’ high score plus software. Lattice parameters ‘a’ (2.816), ‘c’ (14.057) and unit cell volume (96.087) increase after doping with Cu and Mg in LiCoO2. The c/a (4.991) ratio > 4.9, I003/I104 > 1 and ‘R’ value (0.34) suggest the formation of a well developed layered structure. Fig. 2 depicts the SEM images of carbon paper and LiMg0.025Cu0.175Co0.8O2 materials. The anode material exhibits a microfibre type morphology and the cathode material appears like a bunch of flakes. This kind of anode and cathode morphology could easily extract and insert the lithium ions during charge and discharge. The size of the flakes is around 0.5 μm.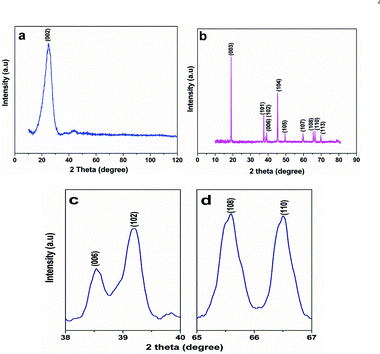 | ||
| Fig. 1 a) XRD patterns of carbon paper and b) LiMg0.025Cu0.175Co0.8O2 materials. Magnified patterns of c) (006) (102) and d) (108) (110) peak doublets. | ||
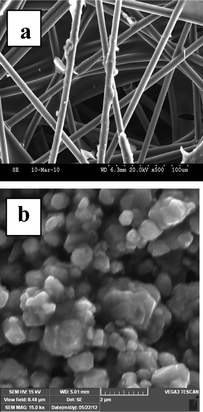 | ||
| Fig. 2 SEM images of a) carbon paper and b) LiMg0.025Cu0.175Co0.8O2 materials. | ||
Fig. 3a shows the first charge/discharge curves and cycling performance of carbon paper (vs. Li/Li+) at 0.2 and 1 C rates cycled between 0.01–1.5 V. The carbon paper delivers a discharge capacity of 225 and 100 mAh g−1 at a rate of 0.2 and 1 C respectively. A very low irreversible capacity (<5 mAh g−1) is obtained in both cases, which is due to the formation of a very thin SEI layer, which consumes a small amount of Li+ ions. This charge/discharge process is coupled with intercalation and deintercalation of lithium into the layers of carbon. Fig. 3b presents the cycling performance of carbon paper; at the end of the 100th cycle it retains 95 and 96% of the initial capacity at a rate of 0.2 and 1 C respectively. This excellent cycling stability is due to the formation of a thin SEI layer on the initial cycle, which assists the movement of Li+ ions in the subsequent cycles. This shows excellent cycling stability at high rates in comparison to previous works.3–6
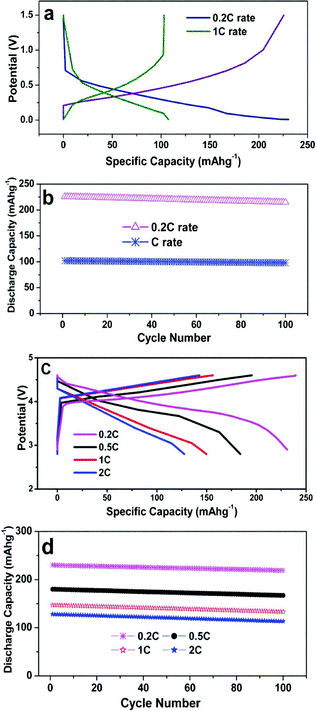 | ||
| Fig. 3 a) Initial charge/discharge curves of carbon paper at a rate of 1 C and 0.2 C cycled between 0.01–1.5 V b) Cycling performance of carbon paper c) Initial charge/discharge curves of LiMg0.025Cu0.175Co0.8O2 at different rates cycled between 2.9–4.6 V d) Cycling performances of LiMg0.025Cu0.175Co0.8O2 materials at different C rates. | ||
The charge/discharge curves and cycling performance of the LiMg0.025Cu0.175Co0.8O2 material (vs. Li/Li+) at different C rates cycled between the potential limits 2.9–4.6 V are shown in Fig. 3c. This material delivers the initial discharge capacities of 230, 180, 147 and 128 mAhg−1 at 0.2 C, 0.5 C, 1 C and 2 C rates respectively. In this material, 0.826 Li+ and 0.798 Li+ ions are extracted and re-inserted in the first cycle, after prolonged cycling the extraction and insertion amount of Li+ ions decreases very slightly. The highest capacity of this material is ascribed to the increase of structural stability and conductivity of Mg2+ ions (Fig. 5). Furthermore, the dopant Cu2+ ions also completely participate in the redox reactions (Fig. 6). The larger ionic radii of Cu2+ and Mg2+ ions compared to Co3+ increases the lattice volume, which is favorable for intercalation and de-intercalation of Li+ ions. In general, when the cell was cycled at high voltages, it could extract and insert a large amount of Li+ ions and also the redox event takes place completely. Fig. 3d depicts the cycling performance of this material at different C rates; the capacity retention obtained at 0.2 , 0.5 C, 1 C and 2 C rates are 95.2, 92.3, 90.1, and 89.32 respectively over the investigated 100 cycles. It is interesting to note that the discharge capacities are higher and the capacity fade is very minimal at high voltage and high discharge rates as compared to the previous reports.8,9,12–14,18–23,26–31 This excellent retention capacity of Cu and Mg doped materials is due to the structural stability of the layered LiMg0.025Cu0.175Co0.8O2 materials enhanced by Mg2+ addition. The Mg2+ ions are electrochemically inactive, however, Mg2+ ions act as pillars to prevent the collapse of the CoO2 interslab and also do not hinder the lithium ion diffusion because it has a similar ionic radii to Li+ ions. Furthermore, Mg2+ suppresses Co4+ ion dissolution in the electrolyte due to the stronger Mg–O bond than Co–O bond. This kind of pillar effect affords more space for Li+ ions during cycling and offers excellent cycling stability.
To investigate the performance of the above-mentioned two electrode materials in a lithium ion cell we assembled the coin cell using carbon paper as the anode, LiMg0.025Cu0.175Co0.8O2 as the cathode and 1 M LiPF6 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EC/DEC as the electrolyte. Fig. 4a presents the charge/discharge behaviour of the lithium ion cell at 1 C and 2 C rates. The discharge capacities are 38 (specific discharge capacity 77 mAhg−1) and 27 mAh (specific discharge capacity 55 mAhg−1) at 1 C and 2 C rates when cycled between 2.9–4.2 V. Fig. 4b shows the cycling performance of the lithium ion cell over the 500 cycles and the inset presents the cycling behaviour at 1 C rate. The capacity retention over the investigated 500 cycles is 96 and 97.5% at 1 C and 2 C rates respectively. The highest capacity retention is due to the pillaring effect of dopant ions in the cathode materials and the structure of the carbon paper also supports repeated lithium insertion and de-insertion. It delivers an energy output of 280 Wh kg−1 (average specific capacity per kilogram x average cell voltage = 75.5 Ah kg−1 × 3.7 V= 280 Wh kg−1), the charge/discharge efficiency is 97% at both C rates. Fig. 4c shows glowing LED lights (16 LEDs) using this assembled lithium ion cell powered by solar power. The assembled lithium ion cell can be charged either by means of solar or normal charging. This excellent performance is superior to the presently used lithium ion cells,24 which have an energy output of 100–250 Wh kg−1 and the charge/discharge efficiency is 80–90%. The fade in capacity of this lithium ion cell after 500 cycles is only 4% at 1 C rate, which is superior compared to previously reported work incorporating Sn–C anode and Li[Ni0.45Co0.1Mn1.45]O4 as cathode, which exhibits a capacity fade of 14%.31
1 EC/DEC as the electrolyte. Fig. 4a presents the charge/discharge behaviour of the lithium ion cell at 1 C and 2 C rates. The discharge capacities are 38 (specific discharge capacity 77 mAhg−1) and 27 mAh (specific discharge capacity 55 mAhg−1) at 1 C and 2 C rates when cycled between 2.9–4.2 V. Fig. 4b shows the cycling performance of the lithium ion cell over the 500 cycles and the inset presents the cycling behaviour at 1 C rate. The capacity retention over the investigated 500 cycles is 96 and 97.5% at 1 C and 2 C rates respectively. The highest capacity retention is due to the pillaring effect of dopant ions in the cathode materials and the structure of the carbon paper also supports repeated lithium insertion and de-insertion. It delivers an energy output of 280 Wh kg−1 (average specific capacity per kilogram x average cell voltage = 75.5 Ah kg−1 × 3.7 V= 280 Wh kg−1), the charge/discharge efficiency is 97% at both C rates. Fig. 4c shows glowing LED lights (16 LEDs) using this assembled lithium ion cell powered by solar power. The assembled lithium ion cell can be charged either by means of solar or normal charging. This excellent performance is superior to the presently used lithium ion cells,24 which have an energy output of 100–250 Wh kg−1 and the charge/discharge efficiency is 80–90%. The fade in capacity of this lithium ion cell after 500 cycles is only 4% at 1 C rate, which is superior compared to previously reported work incorporating Sn–C anode and Li[Ni0.45Co0.1Mn1.45]O4 as cathode, which exhibits a capacity fade of 14%.31
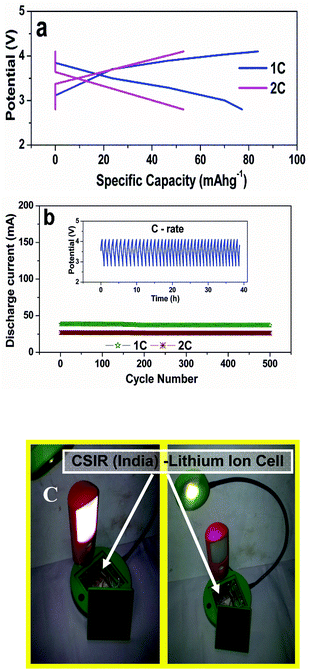 | ||
| Fig. 4 a) Charge/discharge curves of the lithium ion cell at 1 C and 2 C rates. b) Cycling performance of the lithium ion cell at 1 C and 2 C rates over 500 cycles. Inset shows the cycling behaviour of the lithium ion cell at 1 C rate. c) Assembled lithium ion pouch cell charged by solar power used to activate LED lights. | ||
To further confirm the highest discharge capacity of the LiMg0.025Cu0.175Co0.8O2 cathode materials in the lithium ion battery we carried out conductivity measurements and cyclic voltammetry studies. The electronic conductivity of the cathode material is very important for a better charge transfer process during lithium intercalation/de-intercalation processes in the lithium ion cell. The electrical conductivity of the synthesized materials was determined by using a four-probe DC method from a set of voltage–current values using a power source controlled by a PC. The values were obtained by taking σ = 1/ρ, ρ = RA/L where L is the distance between voltage contacts and A is the sample cross section. Fig. 5 shows the electronic conductivities of the LiCoO2, LiCu0.2Co0.8O2 and LiMg0.025Cu0.175O2 materials at different temperatures. While the temperature increases from 25 to 150 °C, the conductivity of material increases and hence the conductivity of both Cu and Mg doped materials is two orders of magnitude higher than the pristine LiCoO2 material. Fig. 6 shows the cyclic voltammogram of LiMg0.025Cu0.175O2 material carried out between the potential limits 2.9 and 4.6 V. Two pairs of redox peaks observed for LiMg0.025Cu0.175O2 material which is the characteristic behaviour of Li+ ion intercalation and de-intercalation. Redox peaks are observed for Cu2+/Cu+ ions at 3.6 and 3.59 V, which is in agreement with previous works.32 The above obtained results confirm that the highest capacity of LiMg0.025Cu0.175O2 material is due to the highest conductivity of Mg2+ ions (which also enhance the structural stability by the pillaring effect) and Cu2+ ions which completely participate in the redox reactions.
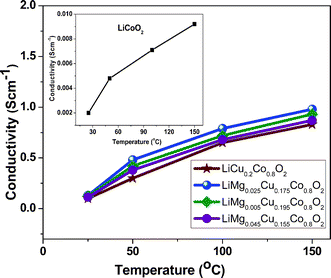 | ||
| Fig. 5 Conductivity measurements of LiMgxCuyCo1−x−yO2 materials. | ||
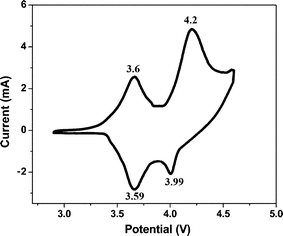 | ||
| Fig. 6 Cyclic voltammogram of LiMg0.025Cu0.175Co0.8O2 material. | ||
Conclusions
In conclusion, we have developed a new high performing lithium ion battery combined with new anode and cathode materials which can be charged by solar power. These materials are very suitable for making solar powered lithium ion batteries. Anode and cathode materials (vs. Li) deliver a capacity retention of 96% (100 cycles) and 90.1% (100 cycles) at 1 C rate respectively. The lithium ion cell exhibits an energy output of 270 Wh kg−1, where the charge/discharge efficiency is 97%, and 97.5% capacity retention could be obtained even at high rates (2 C) over the investigated 500 cycles. Suitable anode and cathode materials enhance power and cycling life of the rechargeable lithium batteries.Acknowledgements
The authors thank the Council of Scientific and Industrial Research (CSIR), India for support of this work under the TAPSUN project of CSIR.References
- M. S. Whittingham, Science, 1976, 192, 1126 CAS.
- Y. Yu, L. Gu, C. Zhu, P. A. VanAken and J. Maier, J. Am. Chem. Soc., 2009, 131, 15984 CrossRef CAS.
- K. Park, J. Kang, Y. J. Choi, S. Lee, D. Kim and J. Park, Energy Environ. Sci., 2011, 4, 1796 CAS.
- B. J. Landi, M. J. Ganter, C. D. Cress, R. A. DiLeo and R. P. Raeffaelle, Energy Environ. Sci., 2009, 2, 638 CAS.
- Y. Sun, Q. Wu and G. Shi, Energy Environ. Sci., 2011, 4, 1113 CAS.
- M. Pumera, Energy Environ. Sci., 2011, 4, 668 CAS.
- K. Kang, Y. S. Meng, J. Breger, C. P. Grey and G. Ceder, Science, 2006, 311, 977 CrossRef CAS.
- N. Recham, J. N. Chotord, L. Dupont, C. Delacourt, W. Walker, M. Armand and J. M. Tarascon, Nat. Mater., 2009, 9, 68 CrossRef.
- J. Liu, T. E. Conry, X. Song, M. M. Doeff and T. J. Richardson, Energy Environ. Sci., 2011, 4, 855 Search PubMed.
- L. Gu, C. Zhu, H. Li, Y. Yu, C. Li, S. Tsukimoto, J. Maier and Y. J. JIkuhara, J. Am. Chem. Soc., 2011, 133, 4661 CrossRef CAS.
- B. Ellis, L. K. Perry, D. H. Ryan and L. F. Nazar, J. Am. Chem. Soc., 2006, 128, 11416 CrossRef CAS.
- J. Zhao, J. He, J. Zhou, Y. Guo, T. Wang, S. Wu, X. Ding, R. Huang and H. J. Xue, J. Phys. Chem. C, 2011, 115, 2888 CAS.
- C. Nithya, R. Thirunakaran, A. Sivashanmugam and S. Gopukumar, Chem.–Asian J., 2012, 7, 163 CrossRef CAS.
- Y. K. Sun, S. T. Myung, B. Park, J. Prakash, I. Belharouak and K. Amine, Nat. Mater., 2008, 8, 320 CrossRef.
- J. Cho, Y. J. Kim and B. Park, Angew. Chem., Int. Ed., 2001, 40, 3367 CrossRef CAS.
- S. Gopukumar, Y. Jeong and K. B. Kim, Solid State Ionics, 2003, 159, 223 CrossRef CAS.
- C. N. Zaheena, C. Nithya, R. Thirunakaran, A. Sivashanmugam and S. Gopukumar, Electrochim. Acta, 2009, 54, 2877 CrossRef CAS.
- S. Deepa, N. S. Arvindan, C. Sugadev, R. Tamilselvi, M. Sakthivel, A. Sivashanmugam and S. Gopukumar, Bull. Electrochem., 1999, 15, 381 CAS.
- M. Zou, M. Yoshio, S. Gopukumar and J. Yamaki, Chem. Mater., 2003, 15, 4699 CrossRef CAS.
- I. Saadoune and C. Delmas, J. Solid State Chem., 1998, 136, 8 CrossRef CAS.
- P. Elumalai, H. N. Vasan and N. Munichandraiah, J. Power Sources, 2004, 125, 77 CrossRef CAS.
- W. Luo, F. Zhou, X. Zhao, Z. Lu, X. Li and J. R. Dahn, Chem. Mater., 2010, 22, 1164 CrossRef CAS.
- R. Vasanthi, I. Ruthmangani and S. Selladurai, Inorg. Chem. Commun., 2003, 6, 953 CrossRef CAS.
- Rechargeable lithium ion battery products, http://www.panasonic.com.
- S. Venkatraman, J. Choi and A. Manthiram, Electrochem. Commun., 2004, 6, 832 CrossRef CAS.
- H. Kim, Y. K. Jeong, J. H. Lee and J. J. Kim, J. Power Sources, 2006, 156, 233 CrossRef.
- S. A. Needham, G. Wang, H. K. Liu, V. A. Drozd and R. S. Liu, J. Power Sources, 2007, 174, 828 CrossRef CAS.
- H. Y. Xu, S. Xie, C. P. Zhang and C. H. Chen, J. Power Sources, 2005, 148, 90 CrossRef CAS.
- Y. Bai, H. Shi, Z. Wang and L. Chen, J. Power Sources, 2007, 167, 504 CrossRef CAS.
- J. Thomas, Nat. Mater., 2003, 2, 705 CrossRef CAS.
- J. Hassoun, K. S. Lee, Y. K. Sun and B. Scrosati, J. Am. Chem. Soc., 2011, 133, 3139 CrossRef CAS.
- A. Cabellero, M. C. Yusta, J. Morales, J. S. Pena and E. R. Castellan, Eur. J. Inorg. Chem., 2006, 1, 1758 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c2ra21999a |
| This journal is © The Royal Society of Chemistry 2012 |
