Cyclodextrin cavity size induced formation of superstructures with embedded gold nanoclusters†
Tarasankar
Das
a,
Prasun
Ghosh
a,
M. S.
Shanavas
b,
Arnab
Maity
a,
Somen
Mondal
a and
Pradipta
Purkayastha
*a
aDepartment of Chemical Sciences, Indian Institute of Science Education and Research, Mohanpur 741252, India. E-mail: pradiptp@gmail.com; Fax: 91 33 2587 3020; Tel: 91 33 2587 3121
bSchool of Chemical Sciences, Mahatma Gandhi University, Kottayam, Kerala 686560, India
First published on 5th October 2012
Abstract
L-cysteine double layer protected gold nanoclusters (Au NCs) have projected thiol groups that induce hydrophobicity around the NCs attracting the relatively hydrophobic cavities of cyclodextrins (CDs) to accumulate around. The different sizes of the CDs result into different accumulating patterns to form spherical to cuboid aggregates with embedded Au NCs.
Introduction
Fluorescent nanomaterials have been of great interest for quite some time now and intensively studied since they have unique optical and photophysical properties and can be potentially used in optical cell imaging.1,2 The ultra-small size of these nanoclusters induces quantum confinement resulting in discrete electronic structure and molecule-like properties, such as HOMO–LUMO electronic transition and enhanced photoluminescence.3–6 The strongly fluorescent metal nanoclusters have drawn special attention of researchers in the fields of chemistry, biology, and materials.7–10 Owing to their ultra-small size, biocompatibility, and highly fluorescent properties, applications of fluorescent gold nanoclusters (Au NCs) has received special attention. The ultrafine sized fluorescent Au NCs do not disturb biological functions of the labelled bioentities, and therefore show their prospect to be applied as luminescent marker.7–11Synthesis of fluorescent Au NCs in protein template is being practised regularly to produce biocompatible nanoclusters.12–15 However, in most of the cases bigger Au NCs have been synthesised that emit at higher wavelengths (red emitting). In the search to produce blue emitting, smaller sized stable Au NCs with much less compact capping unlike thiolates and proteins, some groups have synthesised amino acid capped Au NCs.16–18L-cysteinyl-l-cysteine coated Au NCs have been reported to have very high fluorescence quantum yield and are used to sense As(III).16L-cysteine coated Au NCs are also proved to be potent glucose biosensor.18 The nature of coating created by L-cysteine on the Au NCs forms an amino acid bilayer due to the charged nature of the molecules.19–22 A possible way to chemisorb proteins on gold surfaces is to use L-cysteine to anchor the protein to the gold surface via cysteine thiol groups. Theoretical and experimental studies on the L-cysteine coating of Au surface propose two different models.19–22 These models are schematically represented by Scheme 1.
 | ||
| Scheme 1 Probable modes of attachment of the thiol group of zwitterionic L-cysteine on Au surface with a second layer of the same molecule neutralising the system. The blue inverted buckets at the top represent cyclodextrin (CD) molecules (see text). | ||
Irrespective of the models shown in Scheme 1, the arrangement of L-cysteine on the surface of the Au NCs shows that the outer surface of the amino acid bilayer is rich in thiol group. Thus, in contrast to the water soluble Au NCs prepared through protein templates, the L-cysteine coated Au NCs will be hydrophobic to form a colloidal suspension in water.16,19 Interestingly, the hydrophobic nature of the thiol groups can be applied in host–guest chemistry to solubilise the L-cysteine coated Au NCs.
Although some efforts have been made in using cyclodextrin (CD) as host in protecting Au NCs and Au NPs,23–29 there is hardly any report on the CD-cavity size induced structural morphology with the Au NCs inside. α-, β-, and γ-CDs have been shown to protect the glutathione induced core etched Au NCs inside their cores.23 CD-Au nanoparticle combination has been successfully used in catalysis,24 targeted drug delivery,25 and as sensors.26 In some early works, Au nanoparticles were protected by thiolated CDs to control particle aggregation and to provide multisite hosts for binding guests in solution.27–29
In the present work, we have adopted a different host–guest concept and shown that the surface thiol groups of the L-cysteine bilayer over the Au NCs can induce protection by the CD molecules through hydrophobic interactions (Scheme 1). In addition to this, it is also demonstrated that the different sizes of the α-, β-, and γ-variants of the CDs can aggregate with embedded Au NCs through hydrogen bonding between their external hydroxyl groups. Depending on the nature and number of CD molecules around the Au NCs, either necklace type bead arrangement (for α-CD) or regular cuboid arrangement (for γ-CD) can be obtained. We have characterised the Au NCs and their CD conjugates by steady state and time-resolved fluorescence spectroscopy, as also by atomic force, transmission electron, and confocal laser microscopy techniques. The cavity size of α-CDs tentatively induces to form a spherical aggregate with one Au NC embedded into it, which in turn forms larger entity through intersphere hydrogen bonding. These bigger spheres accumulate into cylindrical necklace-like arrangement. As the size of the CDs increases, the distribution of the CDs around one Au NC is supposed to change in terms of number thus changing the structural morphology. The largest among the three CDs (γ-CD) finds only six of them to arrange around one Au NC. This can be presumed by calculating the difference in diameter between the Au NCs and the rim of the CDs. Thus, in case of γ-CD, we observed formation of nano-cuboids with embedded Au NCs whereas β-CD could not produce any regular shape.
In several reports, gold nanoparticles (Au NPs) have been protected by CDs to be used for different purposes. In most of the studies thiolated CDs have been used to synthesise CD-coated Au NPs.25,30–32 Since thiols are prone toward Au NP surface, hence anchoring thiolated CDs to protect the Au NPs has been a very good technique as used in various applications. Protecting Au NCs by non-thiolated CDs has been hardly attempted although this could be achieved very easily. As discussed previously, we have protected the Au NC surface with L-cysteine that forms a bilayer on the substrate and as a result keeps the thiol group of the amino acid protruding toward the bulk environment. Thus, to surround the gold substrates with CDs, we do not need to functionalise the CDs with thiol groups. The hydrophobic nature of the thiols of the cysteine molecules induces accumulation of CDs around the Au NCs. Hence, instead of anchoring the thiolated CDs to the gold surface covalently, we have devised a way to protect Au NCs through hydrophobic interaction. In one recent report the authors have described facile synthesis of CD-capped Au NPs without functionalising either the CDs or the Au NPs.33 However, the enthalpy of Au–O chemisorption is remarkably low.34 Wei et al. showed in some of their reports that resorcinarenes having peripheral hydroxyl groups can protect large Au NPs.35,36 However, probably due to weak interaction between the O-atom of the hydroxyl groups and the gold surface they opted the thiol derivatives of resorcinarenes in their later works.37 Thus, better interaction between S-atom and Au surface seems to be an important factor to consider. However, we stressed upon a novel concept by exploiting the hydrophobic nature of the thiol groups to attract the hydrophobic cavities of CDs. In our findings we have worked at neutral pH so that the system remains biologically significant and the superstructure formation has been explained from the aspect of hydrogen bonding between the CD coatings, which is more scientifically feasible.
Experimental
Materials
All the chemicals were bought from Sigma-Aldrich and were used as received without further purification. Triple distilled water was used in all the experiments. The water was checked to be devoid of any other fluorescing impurities.Synthesis of L-cysteine coated gold nanoclusters
L-Cysteine coated Au NCs have been synthesised following a previous report.17,18 Briefly, 25 ml aqueous solution of 2 mM L-cysteine was prepared by stirring for 30 min. To this solution of L-cysteine, 250 μL of 0.05 M stock solution HAuCl4 was added under constant stirring for 30 min. 350 μL of 0.01 M NaBH4 solution was subsequently added to it. The solution was then stirred for further 2 h for complete reduction and formation of L-cysteine capped Au NCs at 300 K. The product was washed and centrifuged to remove any unreacted precursors. The concentration of gold in resultant solution became 0.5 mM. This solution is stable when refrigerated at 4 °C.Characterisation
The absorption spectra were recorded by a Varian Cary Bio 300 UV-Visible spectrophotometer. The steady state fluorescence measurements were carried out using a PerkinElmer LS 55 spectrofluorimeter. The samples were excited at 280 nm since the fluorescence emission from the Au NCs was best obtained at that wavelength. The time-resolved fluorescence studies were performed with a Horiba Jobin Yvon time correlated single photon counting (TCSPC) set-up with picoseconds resolution. The excitation source was a 280 nm NanoLED with 70 ps detection time resolution. Fluorescence decay was monitored at 415 nm.The Dynamic Light Scattering (DLS) measurements were carried out using a Malvern Zetasizer Nano equipped with a 4.0 mW HeNe laser operating at λ = 633 nm. All samples were measured in aqueous system at room temperature with a scattering angle of 173°. The size distribution was calculated using Nano software based on a non-negative least square analysis (NNLS) method.
The Atomic Force Microscopic (AFM) studies were made using an NT-MDT NTEGRA instrument procured from NT-MDT, CA, USA.
Transmission Electron Microscopy (TEM) measurements of the samples were performed in a Hitachi H-9000 NAR. The samples were prepared by drop-casting on copper grids precoated with carbon film followed by solvent evaporation under vacuum.
Mass spectrometry (MS) was performed with a matrix assisted laser desorption ionization time-of-flight (MALDI-TOF/TOF) Mass Spectrometer (Applied Biosystems 4800 Proteomics Analyzer). All spectra were collected in the linear positive mode using a-cyano-4-hydroxycinnamic acid (CHCA) as the matrix. Confocal Microscope images were taken in an LSM 710 with microscope Axio Observer Z.1, Carl Zeiss. The confocal laser scanning micrographs (CLSM) were taken with the excitation laser of 405 nm in the emission range of 410–460 nm with 100× optical magnification. Despite the limited resolution of the confocal microscope, compared to AFM and TEM, the ability to detect fluorescence makes it possible to visualise nanotubes or nanocubes with suboptical resolution.
Thermogravimetric analysis (TGA) was performed on a Mettler-Toledo TGA/SDTA851e instrument. Approximately 1–2 mg of the sample was added to an aluminium crucible and heated from 30° to 700 °C at a rate of 10 °C min−1 under continuous nitrogen purge.
Results and discussion
Steady state spectral descriptions
The absorption spectrum of the Au NCs does not show any sharp peak at 500 nm, which indicates absence of any Au NP formation. The normalised absorption spectra of the L-cysteine coated Au NCs (Fig. 1) with addition of CDs of different cavity sizes show that the main peak due to the nanoclusters at ∼360 nm shifts toward lower wavelength by different degrees. The extent of shift in the peak increases from α- to γ-CD. This is accompanied by the evolution of a very broad shoulder spanning 400–600 nm indicates aggregation of the CD coated Au NCs. The enhancement for the 500 nm band is highest for α-CD and lowest for γ-CD. These spectral features indicate changes in polarity around the Au NCs. Remembering that the L-cysteine coated Au NCs are colloidal in aqueous medium and addition of CDs tend to solubilise them, it can be stated that the stabilisation of the highest occupied molecular orbital (HOMO) of the Au NCs due to solvation is greatest in case of γ-CD and lowest for α-CD.38,39 Whereas, the largest enhancement in the aforesaid broad band intensity indicates that the Au NCs aggregate most with α-CD. So far, there is hardly any effort in synthesising and characterising protected Au NCs by all the three different CDs. This subject is interesting, since the three CDs vary in their cavity sizes due to addition of one extra sugar moiety in each case and thus differ in their inherent polarity and hydrophobicity of the cavity. | ||
| Fig. 1 Normalised absorption spectra of Au NC with increase in (A) α-, (B) β-, and (C) γ-CD concentration. Shifts in the peak at ∼360 nm and enhancement in absorbance at ∼500 nm are labelled in the figures. | ||
The synthesised L-cysteine coated Au NCs emit in the blue region of the spectrum at ∼415 nm (Fig. 2) indicating a diameter of around 1–2 nm.16–18 The size of the L-cysteine coated Au NCs has been evidenced by images taken through transmission electron microscope (TEM), dynamic light scattering (DLS) and matrix-assisted laser desorption/ionisation-time of flight mass spectrometry (MALDI-TOF ms) (Fig. 3). Generally, Au NPs of 1–2 nm diameter range are toxic to the biological system,40–42 whereas similar sized Au NCs are potentially non-toxic.43,44 Thiolated CD-capped Au NPs have been used as targeted drug delivery agents to cancer cells.25 However, to avoid toxicity the diameter of the synthesised Au NPs needs to be kept above 10 nm that, in turn, may limit the accessibility of the Au NP drug carriers to many sites. Since smaller Au NCs are non-toxic unlike Au NPs, thus CD covered Au NCs may serve as better carriers for targeted drugs. The Au NCs that we used in the present work are coated with L-cysteine, which is an amino acid and thus better than polymer coatings as far as toxicity is considered.25
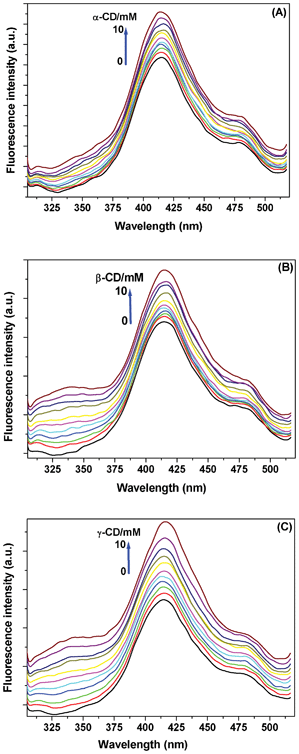 | ||
| Fig. 2 Fluorescence spectra of Au NC with increase in concentration of (A) α-, (B) β-, and (C) γ-CD. The enhancement in fluorescence intensity is labelled in the figures. The excitation wavelength was 280 nm. | ||
 | ||
| Fig. 3 Clockwise from the top: TEM image, DLS histogram and MALDI data of L-cysteine coated Au NCs revealing the actual size of the Au NCs. | ||
The fluorescence spectrum of the Au NCs shows a peak at ∼415 nm, but on gradual addition of the three CDs, a new broad emission evolves at ∼340 nm. We attribute this to the Au NC-CD aggregates (Fig. 2). This behaviour is in conformity with the absorption spectral changes. The ground state aggregate formation was predicted to be highest with α-CD that, probably, fluoresces less; whereas, the relatively open aggregates of Au NCs due to β- and γ-CDs fluoresce more at ∼340 nm. The enhancement in intensity of the main band at 415 nm may be due to change in polarity of the medium of the non-aggregated counterparts.
Time-resolved fluorescence spectral analysis
The steady state fluorescence data show that the α-CD-Au NC aggregate results into less fluorescence emission from the nanoclusters. This trend is followed by the β- and the γ-CD aggregates. Time-resolved fluorescence spectroscopy with these aggregates shows that the L-cysteine coated Au NCs emit slowest when aggregated with α-CD compared to β- and γ-CD (Fig. 4). Thus, it is pertinent that the Au NCs seem to be fluorimetrically more stable when surrounded by α-CD. The lifetimes represented by τ2 in the picosecond regime are attributed to charge transfer between the intermediate Au(I) to the ligand.12–15 This is the first report of its kind in demonstrating that template synthesised Au NCs are protected differently by CDs varying in the size of their cavity. Steady-state and time-resolved fluorescence studies clarify the differential characteristics of the protected species. | ||
| Fig. 4 Time resolved fluorescence decay data of L-cysteine coated Au NCs (red) and the respective aggregates with α- (green), β- (blue), and γ-CD (cyan). The excitation of the sample was made at 280 nm and the emissions were monitored at 415 nm. The black data points are for the prompt. The table in the inset shows the lifetime data (τ) for the species, figures in parentheses represent the percentage contribution of the fluorescence decay and χ2 represents the fitting suitability. | ||
Analysis of the motifs of encapsulation of the Au NCs by CDs
Since the L-cysteine molecules surround the Au NCs forming a bilayer, thus, the hydrophobic nature of the thiol groups at the surface may induce encapsulation by the CD molecules (Scheme 1). The cavity diameters of α-, β-, and γ-CDs are 0.57, 0.78, and 0.95 nm, respectively.45 The diameter of the L-cysteine coated Au NCs is 1-2 nm as obtained from the mass spectroscopic, microscopic, and light scattering studies. Thus, depending on the CD cavity size they will surround the Au NCs. Fig. 5 shows the probable structural motif of aggregation of the CDs around the L-cysteine coated Au NCs. Formation of spherical aggregates with α-CD and cubical aggregates with γ-CD were observed through atomic force microscopy (AFM) and transmission electron microscopy (TEM) (Fig. 6). Cavity size of β-CD probably cannot generate any regular morphology like the other two. The spherical aggregates with α-CD tend to form cylindrical arrangements, which is a natural tendency as has been shown long back through mathematical modelling.46,47 This sort of cylindrical arrangements by spheres happens through “parastichies” depending on the spherical contacts. Such type of aggregations could be found in various biological systems, such as actin, salmonella, tobacco mosaic virus, etc.47 Size of γ-CD tentatively allows six molecules to surround one L-cysteine coated Au NC and generates a cubical morphology when the unit structure spreads in three dimensions through hydrogen bonding. Lack of symmetric arrangement of β-CD does not show such regular structural motif. | ||
| Fig. 5 Representative cartoon showing the formation of the regular structural morphologies of the aggregates formed by α- and γ-CDs around the L-cysteine coated Au NCs. The golden sphere represents the L-cysteine coated Au NCs. | ||
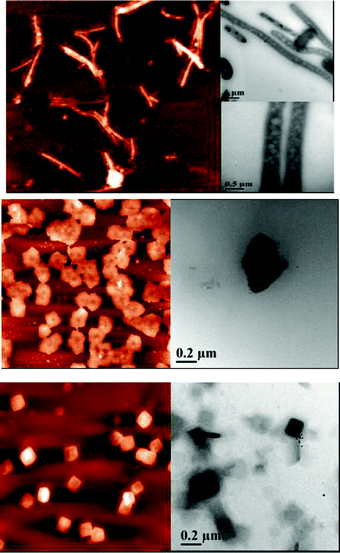 | ||
| Fig. 6 AFM (left panels) and TEM (right panels) micrographs of (a) α-CD, (b) β-CD, and (c) γ-CD aggregates surrounding the L-cysteine coated Au NCs. Elongated and regular cuboid type arrangements are observed for α- and γ-CD aggregates, respectively. | ||
The aggregation of the CD surrounded L-cysteine coated Au NCs are proven using spectroscopy and the morphology produced could be explained from the different sizes of the CDs and the thiol surrounded surface of the Au NCs. To further prove that the Au NCs are really embedded inside the CD aggregates, we performed confocal microscopy imaging with the aggregates. As shown in Fig. 7, we could observe blue emissions from the CD-Au NC aggregates. We checked the stability of the different CD aggregates of the L-cysteine coated Au NCs by thermogravimetric analysis (TGA) (see Supporting Information, Fig. S1 and S2†) and found that the aggregates made by α-CDs are the most stable ones.
 | ||
| Fig. 7 Confocal microscopy images of (a) α-CD, (b) β-CD, and (c) γ-CD aggregates surrounding the L-cysteine coated Au NCs. | ||
In one of the previous attempts to synthesise α-CD protected Au NPs, a sequence of thermally treated and room temperature yielded Au species were used to produce ill-structured flaky materials with CD and Au salts.48 These suprastructures were developed through aggregation of α-CD on Au seeds. In another attempt to produce β-CD capped artificial nanoenzyme, the workers have developed protected Au NPs of ∼4 nm diameter.31 However, this size range of Au NPs are within the reported toxic limits. The present work has demonstrated a unique and intellectual method of synthesis of biocompatible Au NCs within 1–2 nm diameter surrounded by CDs of different sizes. This unique size based distribution of the CDs around the Au NCs results into formation different superstructures ranging from spheres to cubes. The result yields a vast area of application of the thus proposed CD-surrounded L-cysteine coated Au NCs in fields of nanomedicine, targeted drug delivery, sensors, nano-reactors, etc.
Conclusions
L-cysteine coated blue emitting Au NCs have been prepared and the thiol groups on the surface of the bilayer formed by L-cysteine on the nanoclusters were used in host–guest chemistry with CDs of different cavity sizes. It is observed that due to hydrophobic nature of the thiol groups, CDs can encapsulate them and depending on the cavity size can aggregate around the Au NCs. The smallest of the three CDs, α-CD, surrounds the Au NC spherically leading to the formation of spherical microaggregates that naturally get converted to a cylindrical bunch of spheres in the form of a necklace. Whereas, six of the γ-CD molecules (the biggest among the naturally occurring CDs) can accumulate around one Au NC leading to the formation of a cuboidal structure. The intermediate size of β-CD cannot give any regular geometrical shape to the aggregate. As per the structural morphology, the reported aggregates contain nanochannels of different dimensions inside the formed aggregates and thus can be used as bionanotechnological storage devices for ions and gas molecules. The fluorescent and non-toxic nature of the aggregates will also help to track them in biological systems.Acknowledgements
This work is supported by Department of Science, Government of India (SR/S1/PC-35/2011). T.D., P.G., A.M., and S.M. acknowledge Council of Scientific and Industrial Research and University Grants Commission for their fellowships. The authors show acknowledgement to Central Research Facility of IIT Kharagpur for the transmission electron microscopy studies and Indian Institute of Chemical Biology for mass spectrometry.References
- W. Jiang, S. Mardyani, H. Fischer and W. C. W. Chan, Chem. Mater., 2006, 18, 872 CrossRef CAS.
- C. W. Lai, Y. H. Wang, Y. C. Chen, C. C. Hsieh, B. P. Uttam, J. K. Hsiao, C. C. Hsu and P. T. Chou, J. Mater. Chem., 2009, 19, 8314 RSC.
- S. Chen, R. S. Ingram, M. J. Hostetler, J. J. Pietron, R. W. Murray, T. G. Schaaff, J. T. Khoury, M. M. Alvarez and R. L. Whetten, Science, 1998, 280, 2098 CrossRef CAS.
- R. S. Ingram, M. J. Hostetler, J. J. Pietron, R. W. Murray, T. G. Schaaff, J. T. Khoury, R. L. Whetten, T. P. Bigioni, D. K. Guthrie and P. N. First, J. Am. Chem. Soc., 1997, 119, 9279 CrossRef CAS.
- F. R. F. Fan and A. J. Bard, Science, 1997, 277, 1791 CrossRef CAS.
- J. D. Roth, G. J. Lewis, L. K. Stafford, X. Jiang, L. R. Dahl and M. J. Weaver, J. Am. Chem. Soc., 1992, 114, 6159 CrossRef CAS.
- L. A. Peyser, A. E. Vinson, A. P. Bartko and R. M. Dickson, Science, 2001, 291, 103 CrossRef CAS.
- Y. Negishi, Y. Takasugi, S. Sato, H. Yao, K. Kimura and T. Tsukuda, J. Am. Chem. Soc., 2004, 126, 6518 CrossRef CAS.
- C. A. J. Lin, T. Y. Yang, C. H. Lee, S. H. Huang, R. A. Sperling, M. Zanella, J. K. Li, J. L. Shen, H. H. Wang, H. I. Yeh, W. J. Parak and W. H. Chang, ACS Nano, 2009, 3, 395 CrossRef CAS.
- Z. Wu and R. Jin, Nano Lett., 2010, 10, 2568 CrossRef CAS.
- C. L. Liu, M. L. Ho, Y. C. Chen, C. C. Hsieh, Y. C. Lin, Y. H. Wang, M. J. Yang, H. S. Duan, B. S. Chen, J. F. Lee, J. K. Hsiao and P. T. Chou, J. Phys. Chem. C, 2009, 113, 21082 CAS.
- X. L. Guével, B. Hötzer, G. Jung, K. Hollemeyer, V. Trouillet and M. Schneider, J. Phys. Chem. C, 2011, 115, 10955 Search PubMed.
- M. A. H. Muhammed, P. K. Verma, S. K. Pal, A. Retnakumari, M. Koyakutty, S. Nair and T. Pradeep, Chem.–Eur. J., 2010, 16, 10103 CrossRef.
- J. Xie, Y. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888 CrossRef CAS.
- C. L. Liu, H. T. Wu, Y. H. Hsiao, C. W. Lai, C. W. Shih, Y. K. Peng, K. C. Tang, H. W. Chang, Y. C. Chien, J. K. Hsiao, J. T. Cheng and P. T. Chou, Angew. Chem., Int. Ed., 2011, 50, 7056 CrossRef CAS.
- S. Roy, G. Palui and A. Banerjee, Nanoscale, 2012, 4, 2734 RSC.
- S. N. Sarangi, A. M. P. Hussain and S. N. Sahu, Appl. Phys. Lett., 2009, 95, 073109 CrossRef.
- A. M. P. Hussain, S. N. Sarangi, J. A. Kesarwani and S. N. Sahu, Biosens. Bioelectron., 2011, 29, 60 CrossRef CAS.
- G. Dodero, L. De Micieli, O. Cavalleri, R. Rolandi, L. Oliveri, A. Daccà and R. Parodi, Colloids Surf., A, 2000, 175, 121 CrossRef CAS.
- K. Uvdal, P. Bodö and B. Leidberg, J. Colloid Interface Sci., 1992, 149, 162 CrossRef CAS.
- A. Abraham, E. Mihaliuk, B. Kumar, J. Legleiter and T. Gullion, J. Phys. Chem. C, 2010, 114, 18109 CAS.
- A. Abraham, A. J. Ilott, J. Miller and T. Gullion, J. Phys. Chem. B, 2012, 116, 7771 CrossRef CAS.
- E. S. Shibu and T. Pradeep, Chem. Mater., 2011, 23, 989 CrossRef CAS.
- T. Huang, F. Meng and L. Qi, J. Phys. Chem. C, 2009, 113, 13636 CAS.
- C. Park, H. Youn, H. Kim, T. Noh, Y. H. Kook, E. T. Oh, H. J. Park and C. Kim, J. Mater. Chem., 2009, 19, 2310 RSC.
- T. Ogoshi and A. Harada, Sensors, 2008, 8, 4961 CrossRef CAS.
- J. Liu, S. Mendoza, E. Román, M. J. Lynn, R. Xu and A. E. Kaifer, J. Am. Chem. Soc., 1999, 121, 4304 CrossRef CAS.
- J. Liu, W. Ong, E. Román, M. J. Lynn and A. E. Kaifer, Langmuir, 2000, 16, 3000 CrossRef CAS.
- J. Liu, J. Alvarez, W. Ong and A. E. Kaifer, Nano Lett., 2001, 1, 57 CrossRef CAS.
- M. C. Paau, C. K. Lo, X. Yang and M. M. F. Choi, J. Phys. Chem. C, 2010, 114, 15995 CAS.
- X. Li, Z. Qi, K. Liang, X. Bai, J. Xu, J. Liu and J. Shen, Catal. Lett., 2008, 124, 413 CrossRef CAS.
- Y. Liu, Y. L. Zhao, Y. Chen and M. Wang, Macromol. Rapid Commun., 2005, 26, 401 CrossRef CAS.
- T. Huang, F. Meng and L. Qi, J. Phys. Chem. C, 2009, 113, 13636 CAS.
- R. I. Masel, Principles of Adsorption and Reaction on Solid Surfaces, John Wiley and Sons, New York, 1996 Search PubMed.
- K. B. Stavens, S. V. Pusztay, S. Zou, R. P. Andres and A. Wei, Langmuir, 1999, 15, 8337 CrossRef CAS.
- A. Wei, K. B. Stavens, S. V. Pusztay and R. P. Andres, Mater. Res. Soc. Symp. Proc. Ser., 1999, 581, 59 CrossRef.
- R. Balasubramainan, B. Kim, S. L. Tripp, X. Wang, M. Lieberman and A. Wei, Langmuir, 2002, 18, 3676 CrossRef.
- T. Loftsson and M. E. Brewster, J. Pharm. Sci., 1996, 85, 1017 CrossRef CAS.
- T. Loftsson, D. Hreinsdóttir and M. Másson, Int. J. Pharmaceut., 2005, 302, 18 CrossRef CAS.
- C. M. Goodman, C. D. McCusker, T. Yilmaz and V. M. Rotello, Bioconjugate Chem., 2004, 15, 897 CrossRef CAS.
- Y. Pan, A. Leifert, D. Ruau, S. Neuss, J. Bornemann, G. Schmid, W. Brandau, U. Simon and W. Jahnen-Dechent, Small, 2009, 5, 2067 CrossRef CAS.
- M. Turner, V. B. Golovko, O. P. Vaughan, P. Abdulkin, A. Berenguer-Murcia, M. S. Tikhov, B. F. Johnson and R. M. Lambert, Nature, 2008, 454, 981 CrossRef CAS.
- X. Le Guével, N. Daum and M. Schneider, Nanotechnology, 2011, 22, 275103 CrossRef.
- T. A. C. Kennedy, J. L. MacLean and J. Liu, Chem. Commun., 2012, 48, 6845 RSC.
- J. Szejtli, Chem. Rev., 1998, 98, 1743 CrossRef CAS.
- G. van Iterson, Mathematische und Mikroskopisch-Anatomische Studien uber Blattstellungen, Fischer, Jena, 1970 Search PubMed.
- R. O. Erickson, Science, 1973, 181, 705 CAS.
- J. W. Chung, Y. Guo, R. D. Priestly and S. Y. Kwak, Nanoscale, 2011, 3, 1766 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Thermogravimetric analysis detail for the CD encapsulated L-cysteine coated Au NCs. See DOI: 10.1039/c2ra21896h |
| This journal is © The Royal Society of Chemistry 2012 |
