Binder strategy towards improving the rate performance of nanosheet-assembled SnO2 hollow microspheres†
Xinyu
Zhao
ab,
Minhua
Cao
*a and
Changwen
Hu
a
aKey Laboratory of Cluster Science, Ministry of Education of China, Department of Chemistry, Beijing Institute of Technology, Beijing, 100081, P.R. China. E-mail: caomh@bit.edu.cn; Fax: +86-10-68912631; Tel: +86-10-68918648
bSchool of Petrochemical Engineering, Shenyang University of Technology, Liaoyang, 111003, P.R. China
First published on 25th September 2012
Abstract
How to improve SnO2-based anode lithium-storage performance at high rate is a very important issue. Herein, a binder strategy on improving the high rate performance of SnO2 has been proposed. First of all, well-designed nanosheet-assembled SnO2 hollow microspheres were synthesized via a facile template-free hydrothermal route and then Na-alginate as a binder, instead of the commonly used polyvinylidene difluoride (PVDF), was first used with the SnO2 hollow microspheres as an anode material for Li ion batteries. The result shows that the SnO2 hollow microspheres exhibited a remarkable capacity of 690 and 570 mA h g−1 after 50 cycles at a rate of 1 and 2 C, respectively, which is much better than those of other cases, such as the SnO2 hollow microspheres with PVDF as a binder and SnO2 nanoparticles with Na-alginate. This work demonstrates that the binder strategy indeed is an effective means to improve cycling performance and rate capability for high-capacity electrode materials with large volume variations.
1. Introduction
Rechargeable Li-ion batteries (LIBs), as one of the most important energy storage devices for portable electronics, have also gained enormous interest recently for large-scale applications, such as electric vehicles (EVs), hybrid electric vehicles (HEVs), and stationary energy storage.1–7 Tin dioxide (SnO2), as a promising anode material in LIBs, offers a high theoretical gravimetric Li-storage capacity of 790 mA h g−1, which is more than twice that of the currently commercialized graphite (370 mA h g−1).8–11 However, like many other alloying anode materials, a large volume change during electrochemical alloy formation can lead to rapid deterioration of the electrode, thus greatly limiting the practical use of the SnO2 anode materials. To relieve this problem, tremendous efforts have been dedicated to nanostructural engineering to improve SnO2 anode performance, including reducing particle size,12 constructing hollow or porous structures,8,11,13–15 and coating with carbon.16,17 These strategies, especially hollow structures, indeed could buffer the volume change to some extent, but generally at lower rates (0.1 or 0.5 C), whereas at higher rates capacity and cycling life are not satisfactory. The reason is probably due to a drastic volume change of the active materials resulting from the rapid Li uptake and release precesses.18 Development of an electrode material with high capacity and good cyclability at a high charge/discharge rate has become an important issue for present battery technology, because this performance is the essential factor in the design and development of electrode materials that meet the requirement of advanced LIBs for EVs.Up to now, the reported research, to the best of our knowledge, mainly focused on the nanostructural engineering of SnO2 with improved properties and almost no attention was devoted to the advancement of the electrically inactive components of battery electrodes, such as the binder. Yet, most excitingly, a few recent reports have shown that the binder's properties also have an important influence on many important battery characteristics, including stability and irreversible capacity losses, and appropriate binder characteristics could effectively buffer the substantial volume changes so that a markedly improved electrode performance could be obtained.19–22 For example, some binders that contain carboxy groups, such as polyacrylic acid (PAA), carboxymethyl cellulose (CMC), and Na-alginate, have been documented to exhibit better characteristics as binders for Si-based anodes than commonly used polyvinylidene difluoride (PVDF). Especially, Na-alginate, a high-modulus natural polysaccharide extracted from brown algae, has been considered to be a potential binder due to its lower cost and environmental benignancy.19 Therefore, the binder should also be considered in improving the electrode performance besides the active materials.
Inspired by the above idea, we report here for the first time the binder strategy on improvement of SnO2 lithium-storage high rate performance. First, the nanosheet-assembled SnO2 hollow microspheres were prepared by one step hydrothermal reaction with template-free approaches. The synthesis method is simple and easily scaled up. The nanosheet-assembled SnO2 hollow microspheres as active materials were then made into a working electrode by choosing Na-alginate as a binder instead of commonly used PVDF. When evaluated as anode materials, they exhibit good rate performance with a capacity of 690 mA h g−1 after 50 cycles at a rate of 1 C. Even cycled at rates as high as 2 C, they still deliver more than 72% of the theoretical capacity. The good rate performance can be attributed to the hollow structure of the active materials and the high-modulus characteristic of the binder.
2. Experimental
Materials synthesis
All chemicals used were analytical grade without further purification. In a typical procedure to prepare nanosheet-assembled SnO2 hollow microspheres, 10 mmol SnCl2·2H2O were added to deionized water. The mixture was stirred at room temperature for 30 min before an appropriate amount NH4F was added. After being stirred for another 30 min, a clear transparent solution was formed, which was transferred into a 100 mL Teflon-lined stainless steel autoclave. The autoclave was sealed and maintained at 180 °C for 24 h. After the solution was cooled down to room temperature naturally, the as-prepared dark yellow precipitation was rinsed extensively with water and absolute alcohol, and finally dried in air at room temperature for further characterization.Materials characterization
The composition and phase purity of the as-synthesized samples were analyzed by X-ray diffractometry (XRD) with monochromatized Cu-Kα (λ = 1.54178 Å) incident radiation by a Shimadzu XRD-6000 instrument operating at 40 kV voltage and 50 mA current. XRD patterns were recorded from 10° to 80° (2θ) with a scanning step of 0.02°. The size distribution and morphologies of the samples were characterized by a field-emission scanning electron microscopy (FESEM, S-4800). A JEM-2010 transmission electron microscope (TEM) operating at a 200 kV accelerating voltage was used for TEM, high-resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SAED) analyses. The specific surface area was estimated using the Brunauer-Emmett-Teller (BET) equation based on the nitrogen adsorption isotherm obtained with a Belsorp-max. The pore size distribution was determined with the Barrett–Joyner–Halenda (BJH) method applied to the desorption branch of adsorption–desorption isotherm. X-ray photoelectron spectroscopy (XPS) analyses were carried out using a KRATOS 165 Ultra photoelectron spectrometer with a monochromatic Al-Kα (hν = 1486.6 eV) radiation source.Electrochemical measurements
Galvanostatic cycling performances of the as-prepared nanosheet-assembled SnO2 hollow microspheres were measured by using CR2025 coin cells at room temperature on a multi-channel battery testing system (LAND CT2001A) with a cutoff voltage of 1.2–0.01 V versus Li/Li+. For the preparation of the working electrode, a mixture of active material (nanosheet-assembled SnO2 hollow microspheres), Super P carbon black, and Na-alginate in the weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 was ground in a mortar with appropriate amount of deionized water as the solvent to make a slurry, and the resultant slurry was then uniformly pasted on a Cu foil current collector. The typical electrode was dried at 105 °C for 24 h under vacuum before being assembled into a coin cell in an argon-filled glovebox. A lithium foil was used as the counter electrode and a solution of 1 M LiPF6 in ethylene carbonate (EC)/dimethyl carbonate (DMC)/diethyl carbonate (DEC) (1
10 was ground in a mortar with appropriate amount of deionized water as the solvent to make a slurry, and the resultant slurry was then uniformly pasted on a Cu foil current collector. The typical electrode was dried at 105 °C for 24 h under vacuum before being assembled into a coin cell in an argon-filled glovebox. A lithium foil was used as the counter electrode and a solution of 1 M LiPF6 in ethylene carbonate (EC)/dimethyl carbonate (DMC)/diethyl carbonate (DEC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) was used as electrolyte. Cyclic voltammetry (CV) curves were recorded on a CHI-660D potentiostat at a scanning rate of 0.1 mV s−1 at room temperature.
1 in volume) was used as electrolyte. Cyclic voltammetry (CV) curves were recorded on a CHI-660D potentiostat at a scanning rate of 0.1 mV s−1 at room temperature.
3. Results and discussion
Fig. 1a shows the X-ray diffraction (XRD) pattern of the as-synthesized product, in which all of the diffraction peaks can be indexed to a tetragonal rutile structure of SnO2 (JCPDS Card no. 41-1445). No other diffraction peaks corresponding to crystalline byproducts are detected, indicating the high purity of the as-synthesized sample. To further determine the valence state of Sn in the as-synthesized SnO2 sample, X-ray photoelectron spectroscopy (XPS) measurement was carried out, as shown in Fig. 1b. Two strong peaks centered at 486.5 and 495.1 eV could be attributed to Sn 3d5/2 and 3d3/2 states, respectively, indicating that tin is in the SnIV state.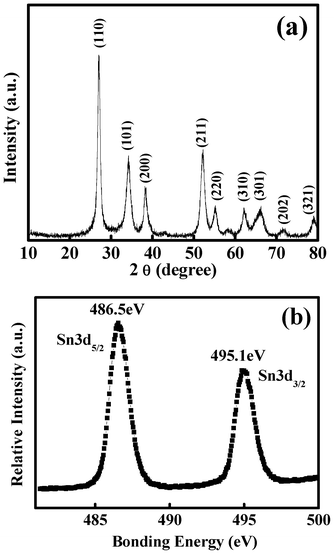 | ||
| Fig. 1 (a) XRD pattern and (b) XPS patterns of nanosheet-assembled SnO2 hollow microspheres. | ||
The morphology and size of the SnO2 sample were first characterized by field-emission scanning electron microscopy (FE-SEM). A survey view at low magnification (Fig. 2a,b) reveals that the sample is composed of uniform flower-like microspheres with diameters of ∼500 nm and a relatively narrow size distribution. No other morphologies could be detected except these three-dimensional (3D) nanostructures, indicating the morphological homogeneousness of the sample. A high magnification FE-SEM image of a broken microsphere (Fig. 2c) clearly presents a hollow interior of the flower-like SnO2 microspheres. By closer observation it was found that the shell of the SnO2 hollow microsphere is constructed from nanosheets with a thickness 10 nm and a width of 100–200 nm (Fig. 2d), and numerous exposed nanosheets can be clearly observed. The surface of the nanosheets is smooth, and the nanosheets are staggered with respect to each other.
 | ||
| Fig. 2 FE-SEM images of the nanosheet-assembled SnO2 hollow microspheres. | ||
To further provide insight into the hollow structure and microstructure of the SnO2 microspheres, transmission electron microscopy (TEM) and selected area electron diffraction (SAED) were carried out. The obvious contrast between the dark edge and the pale center confirms the hollow structure (Fig. 3a,b) and the nanosheet structure of the shell of the SnO2 hollow microspheres also could be clearly seen by a high-magnification TEM image (Fig. 3c). The morphology and size of the sample obtained from the TEM observation are in good agreement with those from the SEM images. Furthermore, a high resolution TEM (HRTEM) image taken from the marked region in Fig. 3c provides more detailed crystallographic information of the SnO2 hollow microspheres. As shown in Fig. 3d, perfect atomic lattice fringes can be clearly seen and the lattice fringe spacings, determined to be 0.336 nm, correspond to the (110) lattice planes of SnO2 (JCPDS no. 41-1445). The inset of Fig. 3d is the SAED pattern of a single hollow SnO2 microsphere, which clearly demonstrates that the microspheres are polycrystalline.
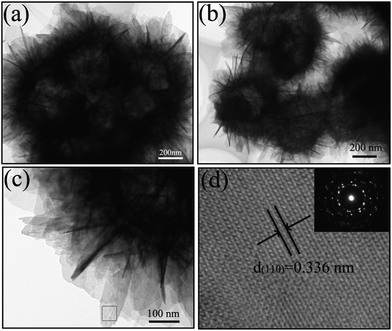 | ||
| Fig. 3 TEM characterization of the nanosheet-assembled SnO2 hollow microspheres: (a–c) low-magnification TEM image; (d) HRTEM images of the nanosheet-assembled SnO2 hollow microspheres. The inset shows that these nanosheet-assembled SnO2 hollow microspheres are polycrystalline. | ||
It is worth mentioning that the nanosheet-assembled SnO2 hollow microspheres were synthesized in the presence of NH4F. In contrast, only SnO2 nanoparticles were obtained if no NH4F was used (Fig. S1†). The formation of the NH4F-assisted SnO2 hollow nanostructures resembles the fluoride-mediated self-transformation mechanism recently proposed by Yu et al.23–25 According to their viewpoint, F− has a higher electronegativity and therefore can reduce the chemical reactivity of the precursor. Thus, in our case, F− could effectively reduce the hydrolysis and condensation rates of Sn2+ by complexation. As a result, sufficient nutrients are left to sustain crystal growth during the subsequent hydrothermal treatment. In addition, it has been proved that crystal growth involves a dissolution-recrystallization process. The added fluoride will be helpful for dissolution of the precursor, taking into account the etching effect of F−. For the reasons given above, the added fluoride anion helps both the dissolution of the interior and the subsequent mass transfer from the interior to the surface. During this ripening process, SnO2 hollow nanostructures were obtained.23–25
To examine the porous structure of the 3D nanosheet-assembled SnO2 hollow microspheres, full nitrogen sorption isotherms were measured, as shown in Fig. 4. A type IV isotherm with a type H3 hysteresis loop at the relative pressure of 0.45–0.9 is observed, implying the presence of a mesoporous structure in SnO2 hollow microspheres. The size of the pores based on desorption data mainly centered at 2 nm with a relatively narrow distribution (inset in Fig. 4). Combining the size of the pores with sorption isotherms, the nature of the pores could be considered to belong in the mesoporous range. Such a porous structure results in a relatively high Brunauer–Emmett–Teller (BET) specific surface area of 85.61 m2 g−1.
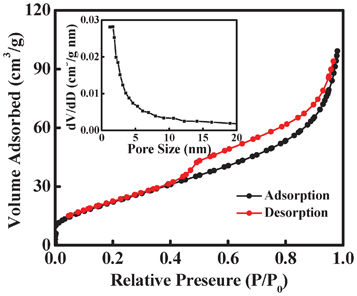 | ||
| Fig. 4 N2 adsorption-desorption isotherms of the nanosheet-assembled SnO2 hollow microspheres. The inset shows the pore size distribution calculated by BJH method from the desorption curve. | ||
SnO2 has been widely studied as an anode material due to high theoretical reversible capacity.8–17 However, one major challenge of Sn-based anode materials is the pulverization of electrodes due to the large volume change during the alloying/dealloying processes, leading to the poor cycling performance. Nanostructure electrode materials hold the key to overcome the limits, especially the rate performance and lifetime.26 In recent years, numerous types of morphology have been designed and synthesized to investigate and improve the Li-storage properties based on SnO2 nanostructures.8–17 Among them, nanosheet-assembled SnO2 micro-/nanostruture can take both the advantages of large specific surface areas and the porous nanostructure due to these nanostructures composed by the adjacent building blocks, which have been shown to exhibit potential Li-storage capabilities.10,15 The unique structure of the present nanosheet-assembled SnO2 hollow microspheres and suitable binder could partly overcome such a problem. Thus, a series of electrochemical measurements were performed to study the lithium storage properties.
Fig. 5a shows the representative cyclic voltammograms (CVs) of the nanosheet-assembled SnO2 hollow microspheres. As can be clearly seen, two reduction peaks are presented around 0.84 and 0.09 V, which can be ascribed to the irreversible decomposition of SnO2 to Sn and the reversible alloying reaction between Sn and Li, respectively. One distinct oxide peak centers at 0.58 V, which derives from the reversible dealloying reaction of Sn and Li. An inconspicuous oxidation peak at 1.27 V can be found, which may be due to the partly reversible reaction of the formation of Li2O.16,27,28 This CV behavior is consistent with those reported previously, indicating a similar electrochemical pathway.13–16,27,28
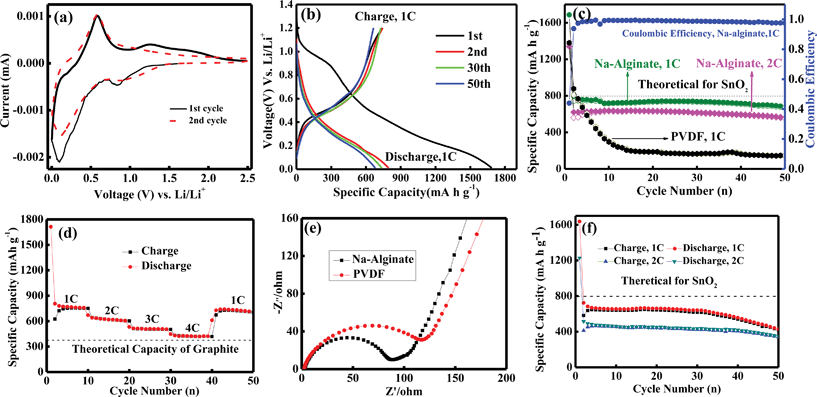 | ||
| Fig. 5 (a) Cyclic voltammograms (CVs) of nanosheet-assembled SnO2 hollow microspheres. (b) Discharge/charge voltage profile between 0.01–1.2 V at a rate of 1 C of nanosheet-assemble SnO2 hollow microspheres. (c) Cycling performance (left) and coulombic efficiency (right) of nanosheet-assemble SnO2 hollow microspheres. (d) Cycling performance of nanosheet-assemble SnO2 hollow microspheres at different rates (1–4 C). (e) Electrochemical impedance spectroscopy (EIS) of nanosheet-assemble SnO2 hollow microspheres. (f) Cycling performance of SnO2 nanoparticles. | ||
The as-prepared nanosheet-assembled SnO2 hollow microspheres were then tested as an anode material in lithium ion batteries. Fig. 5b shows representative discharge/charge voltage profiles of the SnO2 hollow microsphere electrode cycled at a rate of 1 C over the voltage range of 0.1–1.2 V vs. Li+/Li. The sample delivers initial discharge and charge specific capacities of 1686.1 and 741.3 mA h g−1 at a rate of 1 C. The large initial Li-storage capacity might be associated with the unique structure of the nanosheet-assembled hollow microspheres, which probably helps to store more lithium, and such features have also been observed in many other SnO2 hollow nanostructures.8–17 In addition, the Na-alginate binder with a high-modulus may also indirectly contribute to this issue. Common to most of SnO2-based electrode materials, a large initial irreversible capacity is also observed in our case, which may be mainly attributed to the irreversible reduction of SnO2 to Sn or Li2O formation and other possible irreversible processes, such as the formation of solid electrolyte interface (SEI).8–17 Nonetheless, a perfect reversibility of the capacity was still obtained, with an average coulombic efficiency of higher than 99% for up to 50 cycles after the second cycle (Fig. 5c).
For comparison, nanosheet-assembled SnO2 hollow microspheres with PVDF as a binder were also tested. Fig. 5c shows the comparative cycling performance of the nanosheet-assembled SnO2 hollow microsphere electrodes with Na-alginate and PVDF as binders, respectively. For comparison, nanosheet-assembled SnO2 hollow microspheres with PVDF as a binder and SnO2 nanoparticles with Na-alginate as a binder were also tested. Fig. 5c shows the comparative cycling performance of the nanosheet-assembled SnO2 hollow microsphere electrodes with Na-alginate and PVDF as binders. When Na-alginate was used as a binder, the as-prepared electrode exhibits excellent cycling capacity retention, with a highly stable capacity of ∼700 mA h g−1 at a rate of 1 C and after 50 cycles, a reversible capacity of as high as 690 mA h g−1 can still be retained, whereas for PVDF binder only 147.6 mA h g−1 was retained after 50 cycles at the same rate due to its much faster capacity fading. Even at a high rate of 2 C, the nanosheet-assembled SnO2 hollow microspheres electrodes with Na-alginate binder still keeps a discharge capacity of 571 mA h g−1 after 50 cycles, which is much higher than the theoretical capacity of graphite (372 mA h g−1). It is very pronounced that the as-prepared nanosheet-assembled SnO2 hollow microsphere electrode with Na-alginate binder exhibits much better lithium storage properties compared with PVDF binder, in the light of both storage capability and cycling capacity retention. This result undoubtedly confirmed the fact that Na-alginate binder could remarkably improve performance of SnO2 anodes, in good agreement with that reported recently.19
The rate capability is another outstanding aspect for the nanosheet-assembled SnO2 hollow microsphere electrode with the Na-alginate binder. As shown in Fig. 5d, the nanosheet-assembled SnO2 hollow microspheres show an excellent rate capability under all tested rates. Even at a rate 4 C, a specific capacity of as high as 425 mA h g−1 is retained, which is still higher than the theoretical capacity of graphite. More importantly, the specific capacity of the electrode could recover to the initial reversible value after the high-rate measurements, indicating their good reversibility. In addition, the nanosheet-assembled SnO2 hollow microsphere electrode with the Na-alginate binder shows enhanced electrical conductivity compared to that with PVDF, which was confirmed by electrochemical impedance spectroscopy (EIS) (Fig. 5e). This result indicates that the nanosheet-assembled SnO2 hollow microsphere electrode possesses a high electrical conductivity, leading to better rate capability and higher reversible capacity in comparison to that with PVDF. In Na-alginate, carboxylic groups are naturally present and evenly distributed in the polymer chain. And the higher concentration and more uniform distribution of the carboxylic polar units can ensure better interfacial interaction between the polymer binder and the SnO2 nanostructures, as well as stronger adhesion between the electrode layer and Cu substrate. Moreover, the high viscosity of Na-alginate solution in water could prevent SnO2 nanomaterials and Super P carbon black from sedimentation and aggregation, and after water is evaporated results in the electrode formation of high uniformity.19 Hence, the uniform distribution of electrical conductivity networks and stronger adhesion between current collector and binders both ensure the good electrical contact of the electrode during cycling, which provides more diffusion paths for ionic transport and electronic conduction.
In order to determine whether the hollow structure also contributes to the performance improvement, the electrochemical performance of SnO2 nanoparticle electrode also with Na-alginate as a binder was carried out, as shown in Fig. 5f (The SnO2 nanoparticles were synthesized in the absence of NH4F while other reaction conditions were kept the same, and the SEM image is shown in Fig. S1, ESI†). As expected, the SnO2 nanoparticle electrodes also give a satisfying result at higher charge/discharge rates, which retain a high reversible capacity of 430 and 350 mA h g−1 at a rate of 1 C and 2 C after 50 cycles, which are much better than most of the reported results on SnO2. But this result is inferior to that of the nanosheet-assembled SnO2 hollow microspheres. The observation is indeed in support of the fact that both the hollow nanostructural engineering and Na-alginate binder are responsible for enhanced electrochemical properties of anode materials.
From above results, we can see that the superior performance of the nanosheet-assembled SnO2 hollow microspheres could be ascribed to the binder and the nanostructure of the anode materials. On the one hand, the nanosheet building blocks provide short distances for Li+ flux across the interface, and the structural strain and the volume change associated with the repeated Li+ insertion/extraction processes could be buffered effectively by the porosity in the wall and hollow cavity.10,15 On the other hand, the Na-alginate binder possesses a much higher modulus than the commercial PVDF binder used in Li-ion battery electrodes, in a dry or wet (immersed into the electrolyte solvent) environment.19 This property contributes to a negligibly small swellability of the Na-alginate and at the same time it also may prevent undesirable access of the electrolyte liquid to the binder/anode active material interface. Thus, a very weak binder–electrolyte interaction was observed, which is needed for the long-term anode stability. This behavior of the Na-alginate has been well studied with Si anodes.19 To the best of our knowledge, this is the first report on utilizing Na-alginate as binder in the SnO2-based LIBs. However, this strategy could be applied to not only hollow microsphere anode materials, but also other micro- and nanostructures. For example, the nanoflaky MnO2–graphene hybrids showed a excellent high-rate and long-cycling electrochemical performance as cathode materials by using Na-alginate as binder.29 The strategy can aid the exploration and development of new battery electrodes for advanced LIBs.
4. Conclusions
In summary, uniform nanosheet-assembled SnO2 hollow microspheres have been synthesized by a facile template-free hydrothermal method. With the Na-alginate as a binder, the as-synthesized SnO2 hollow microspheres as an anode material for Li-ion batteries exhibit excellent cycling performance and rate capability in comparison with the commonly used PVDF, which could be attributed to the binder and the unique structure of the anode materials. This strategy that focuses on the corporate improvement of the anode active component and the inactive binder could be extended to other high-capacity electrode materials with large volume variations to improve their cyclability and rate capability.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China 21173021, 21231002, 21276026, 21271023, 91022006, and 20973023, the 111 Project (B07012), the Program of Cooperation of the Beijing Education Commission (20091739006), and specialized research fund for the doctoral program of higher education (SRFDP. No. 20101101110031).References
- J. Kim and A. Manthiram, Nature, 1997, 390, 265 CrossRef CAS.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS.
- J. Maier, Nat. Mater., 2005, 4, 805 CrossRef CAS.
- C. K. Chan, H. L. Peng, G. Liu, K. McIlwrath, X. F. Zhang, R. A. Huggins and Y. Cui, Nat. Nanotechnol., 2008, 3, 31 CrossRef CAS.
- B. Kang and G. Ceder, Nature, 2009, 458, 190 CrossRef CAS.
- B. Scrosati, J. Hassoun and Y. K. Sun, Energy Environ. Sci., 2011, 4, 3287 CAS.
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243 CAS.
- X. W. Lou, Y. Wang, C. L. Yuan, J. Y. Lee and L. A. Archer, Adv. Mater., 2006, 18, 2325 CrossRef CAS.
- M. S. Park, G. X. Wang, Y. M. Kang, D. Wexler, S. X. Dou and H. K. Liu, Angew. Chem., Int. Ed., 2007, 46, 750 CrossRef CAS.
- C. Wang, Y. Zhou, M. Y. Ge, X. B. Xu, Z. L. Zhang and J. Z. Jiang, J. Am. Chem. Soc., 2010, 132, 46 CrossRef CAS.
- R. Demir-Cakan, Y. S. Hu, M. Antonietti, J. Maier and M. M. Titirici, Chem. Mater., 2008, 20, 1227 CrossRef CAS.
- S. H. Ng, D. I. Dos Santos, S. Y. Chew, D. Wexler, J. Wang, S. X. Dou and H. K. Liu, Electrochem. Commun., 2007, 9, 915 CrossRef CAS.
- S. J. Ding, J. S. Chen, G. G. Qi, X. N. Duan, Z. Y. Wang, E. P. Giannelis, L. A. Archer and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 21 CrossRef CAS.
- Z. Y. Wang, D. Y. Luan, F. Y. C. Boey and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 4738 CrossRef CAS.
- S. J. Ding and X. W. Lou, Nanoscale, 2011, 3, 3586 RSC.
- X. W. Lou, C. M. Li and L. A. Archer, Adv. Mater., 2009, 21, 2536 CrossRef CAS.
- X. W. Lou, J. S. Chen, P. Chen and L. A. Archer, Chem. Mater., 2009, 21, 2868 CrossRef CAS.
- L. W. Ji, Z. Lin, M. Alcoutlabi and X. W. Zhang, Energy Environ. Sci., 2011, 4, 2682 CAS.
- I. Kovalenko, B. Zdyrko, A. Magasinski, B. Hertzberg, Z. Milicev, R. Burtovyy, I. Luzinov and G. Yushin, Science, 2011, 334, 75 CrossRef CAS.
- J. S. Bridel, T. Azais, M. Morcrette, J. M. Tarascon and D. Larcher, Chem. Mater., 2010, 22, 1229 CrossRef CAS.
- D. Mazouzi, B. Lestriez, L. Roue and D. Guyomard, Electrochem. Solid-State Lett., 2009, 12, A215 CrossRef CAS.
- A. Magasinski, B. Zdyrko, I. Kovalenko, B. Hertzberg, R. Burtovyy, C. F. Huebner, T. F. Fuller, I. Luzinov and G. Yushin, ACS Appl. Mater. Interfaces, 2010, 2, 3004 CAS.
- J. G. Yu, H. T. Guo, S. A. Davis and S. Mann, Adv. Funct. Mater., 2006, 16, 2035 CrossRef CAS.
- J. G. Yu, S. W. Liu and H. G. Yu, J. Catal., 2007, 249, 59 CrossRef CAS.
- S. W. Liu, J. G. Yu and M. Jaroniec, J. Am. Chem. Soc., 2010, 132, 11914 CrossRef CAS.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930 CrossRef CAS.
- H. Wang, Q. Q. Liang, W. J. Wang, Y. R. An, J. H. Li and L. Guo, Cryst. Growth Des., 2011, 11, 2942 CAS.
- H. Wang, Y. M. Wu, Y. S. Bai, W. Zhou, Y. R. An, J. H. Li and L. Guo, J. Mater. Chem., 2011, 21, 10189 RSC.
- J. Li, Y. Zhao, N. Wang, Y. H. Ding and L. Guan, J. Mater. Chem., 2012, 22, 13002 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available: SEM of nanoparticles. See DOI: 10.1039/c2ra21867d |
| This journal is © The Royal Society of Chemistry 2012 |
