Reversible solid-state hydration and dehydration process involving anion transfer in a self-assembled Cu2 system†
T.
Washizaki
a,
R.
Ishikawa
b,
K.
Yoneda
c,
S.
Kitagawa
d,
S.
Kaizaki
e,
A.
Fuyuhiro
e and
S.
Kawata
*a
aDepartment of Chemistry, Faculty of Science, Fukuoka University, Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan. E-mail: kawata@fukuoka-u.ac.jp; Fax: +81- 92-865-6030
bDepartment of Chemistry, Graduate School of Science, Tohoku University, 6-3 Aramaki-Aza-Aoba, Aoba-ku, Sendai, Miyagi 980-8578, Japan
cDepartment of Chemistry and Applied Chemistry, Faculty of Science and Engineering, Saga University, Saga, Japan
dDepartment of Synthetic and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Kyoto 615-8510, Japan
eDepartment of Chemistry, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan
First published on 19th October 2012
Abstract
Supramolecular assembly [CuII2(μ-bpypz)2Br1.25(H2O)0.75]Br0.75·2.25H2O (1) was prepared by using 3,5-di(2-pyridyl)pyrazole (Hbpypz). Assembly 1 consists of supramolecular dimers of dinuclear species based on doubly bpypz− bridged dinuclear building blocks. The supramolecular dimer is flexible as a result of π⋯π interactions. Compound 1 undergoes reversible structural transformation in the solid state involving reversible apical-ligand substitution at the copper centers upon hydration–dehydration.
Supramolecular coordination compounds such as polynuclear metal clusters and coordination polymers have shown interesting functional properties, for innovative technological applications as functional crystalline materials in a number of different fields: gas storage,1 molecular sieves,2 sensors,3 magnetic response,4 electroconductivity5 and so on. Considerable efforts have been made to create supramolecular coordination compounds composed of a variety of structures6 with flexible and dynamic properties, and have been highlighted as a new class of solid-state materials chemistry.7
To date, we have reported several types of building blocks obtained by the self-assembly reaction of the short and rigid π-conjugated aromatic ligand, Hbpypz (3,5-di(2-pyridyl)pyrazole) and some metal ions.6,7 This approach offers an easy and efficient way to tune the crystal packing and to add various functional properties.6,7
To explore new supramolecular routes toward the construction of functional materials, we report herein a new supramolecular system assembly [CuII2(μ-bpypz)2Br1.25(H2O)0.75]Br0.75·2.25H2O (1) which was designed by using the Hbpypz ligand. Compound 1 is a flexible supramolecular dimer of a dinuclear species based on the doubly bpypz− bridged dinuclear building blocks connected by π⋯π stacking interactions. Reaction of Hbpypz with CuBr2 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio under aerobic conditions in H2O–MeOH produced single crystals of 1. An ORTEP view of the dinuclear units of 1 is presented in Fig. 1.
1 ratio under aerobic conditions in H2O–MeOH produced single crystals of 1. An ORTEP view of the dinuclear units of 1 is presented in Fig. 1.
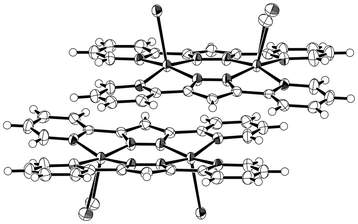 | ||
| Fig. 1 An ORTEP drawing of the supramolecular dimer of 1 (30% probability thermal ellipsoids). O(1) and Br(1) atoms are disordered and occupy the same site. | ||
The supramolecular dimers have been self-assembled to a three-dimensional structure by π⋯π stacking interaction. In addition, compound 1 shows flexible structural transformation in the supramolecular dimers and apical-ligand substitution at the copper center upon the dehydration–hydration processes between 1, partially dehydrated form 1a, and anhydrous form 1b (Scheme 1).
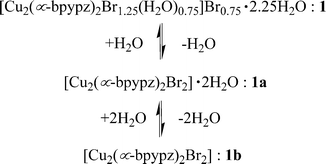 | ||
| Scheme 1 Dehydration–hydration processes of 1. | ||
Compound 1 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Z = 2).11 In the dimer unit each copper atom is bound to two pyridine and two pyrazole nitrogen atoms of bpypz− on the basal plane (average bond distances are Cu–Npyridine = 2.089 Å and Cu–Npyrazolate = 1.954 Å). One apical position is occupied by a Br− anion, while the other axial position is occupied by a disordered water molecule with an occupancy of 0.75 and a Br− anion with an occupancy of 0.25, forming square-pyramidal environments (Cu(1)–Br(1) = 2.730(2) Å, Cu(2)–Br(2) = 2.6733(4) Å, Cu(1)–O(1) = 2.201(6) Å). The trigonal distortion parameter (τ) is 0.018 for Cu(1) and 0.040 for Cu(2), respectively.9 Both of the axial ligands jut out to the same side of the dimer, that is, the two copper–axial ligand bonds adopt a syn configuration.8 The adjacent dimer complexes form a supramolecular dimer unit through a π⋯π stacking interaction (C(12)–C(17) = 3.328(6) Å) (Fig. 2a). Each supramolecular dimer is arranged in a parallel form to make an one-dimensional column along the crystallographic a-axis, and the column is supported by offset and slipped π⋯π stacking interactions between the bpypz− ligands: i.e., a pyrazolate ring of one supramolecular dimer core and a pyridyl ring of the other neighboring one. It is interesting to note the outward appearance of the supramolecular dimer induced by the stacking interaction. The water molecules are captured between the apical-ligands and form a hydrogen-bonding network among other interstitial water molecules and disordered non-coordinated Br− anions in the one-dimensional channels (Fig. 2b and S2, ESI†).
(Z = 2).11 In the dimer unit each copper atom is bound to two pyridine and two pyrazole nitrogen atoms of bpypz− on the basal plane (average bond distances are Cu–Npyridine = 2.089 Å and Cu–Npyrazolate = 1.954 Å). One apical position is occupied by a Br− anion, while the other axial position is occupied by a disordered water molecule with an occupancy of 0.75 and a Br− anion with an occupancy of 0.25, forming square-pyramidal environments (Cu(1)–Br(1) = 2.730(2) Å, Cu(2)–Br(2) = 2.6733(4) Å, Cu(1)–O(1) = 2.201(6) Å). The trigonal distortion parameter (τ) is 0.018 for Cu(1) and 0.040 for Cu(2), respectively.9 Both of the axial ligands jut out to the same side of the dimer, that is, the two copper–axial ligand bonds adopt a syn configuration.8 The adjacent dimer complexes form a supramolecular dimer unit through a π⋯π stacking interaction (C(12)–C(17) = 3.328(6) Å) (Fig. 2a). Each supramolecular dimer is arranged in a parallel form to make an one-dimensional column along the crystallographic a-axis, and the column is supported by offset and slipped π⋯π stacking interactions between the bpypz− ligands: i.e., a pyrazolate ring of one supramolecular dimer core and a pyridyl ring of the other neighboring one. It is interesting to note the outward appearance of the supramolecular dimer induced by the stacking interaction. The water molecules are captured between the apical-ligands and form a hydrogen-bonding network among other interstitial water molecules and disordered non-coordinated Br− anions in the one-dimensional channels (Fig. 2b and S2, ESI†).
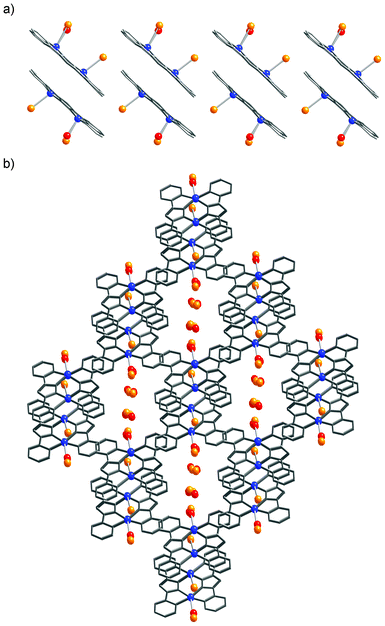 | ||
| Fig. 2 The one-dimensional columnar structure (a) and the assembled structure (b) of 1. Atoms: Cu (blue), Br (orange), C (gray), N (gray), O (red). | ||
Single crystals of the partially dehydrated form 1a and the anhydrous form 1b were obtained by drying single crystals of 1. On the other hand, by using the same starting materials for the synthesis of 1 and only methanol as the reaction solvent, an anhydrous anti form compound, anti-[Cu2(μ-bpypz)2Br2] (2) was selectivity isolated (Fig. S1, ESI†).8,11
In order to explain the structural change from 1 to 1b upon the dehydration process, direct single crystal-to-single crystal transformation of 1 to 1b was performed.
Compound 1a also crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Z = 2).11 In the dimer unit each copper atom is bound to two pyridine and two pyrazole nitrogen atoms of bpypz− in the basal plane (average bond distances are Cu–Npyridine = 2.089 Å and Cu–Npyrazolate = 1.954 Å). Both of the apical positions are occupied by ordered Br− anions, forming a square-pyramidal environment (Cu(1)–Br(1) = 2.730(2) Å, Cu(2)–Br(2) = 2.6733(4) Å), different from that of 1, where one of the axial ligands is occupied by the disordered water and a Br− anion. The apical Br− anions jut out to the same side of the dimer and the two apical Cu–Br bonds adopt a syn configuration.8 The trigonal distortion parameter (τ) is 0.018 for Cu(1) and 0.028 for Cu(2), respectively (Fig. S3, ESI†).9
(Z = 2).11 In the dimer unit each copper atom is bound to two pyridine and two pyrazole nitrogen atoms of bpypz− in the basal plane (average bond distances are Cu–Npyridine = 2.089 Å and Cu–Npyrazolate = 1.954 Å). Both of the apical positions are occupied by ordered Br− anions, forming a square-pyramidal environment (Cu(1)–Br(1) = 2.730(2) Å, Cu(2)–Br(2) = 2.6733(4) Å), different from that of 1, where one of the axial ligands is occupied by the disordered water and a Br− anion. The apical Br− anions jut out to the same side of the dimer and the two apical Cu–Br bonds adopt a syn configuration.8 The trigonal distortion parameter (τ) is 0.018 for Cu(1) and 0.028 for Cu(2), respectively (Fig. S3, ESI†).9
The adjacent dimer complexes form a tetramer unit through π⋯π stacking interactions the feature of which is similar to that of 1. Each tetramer is arranged in a parallel form to make a one-dimensional column along the crystallographic a-axis, and the column is supported by offset and slipped π⋯π stacking interactions (C(7)–C(17) = 3.161(4) Å) between the bpypz− ligands. Moreover, one of the water molecules as the solvent of crystallization is captured between the coordinated Br− anions on the dimer and the other disordered interstitial water molecules form a hydrogen-bonding network with the captured water in the one-dimensional channel (Fig. S3, ESI†). These structural features of 1a suggest that one water molecule per dimer unit is extruded from the crystal and then the axial position is substituted by the Br− anion completely, that is, the Br− anions transfer from the one-dimensional channels to the copper centers in the dehydration process (Scheme 2).
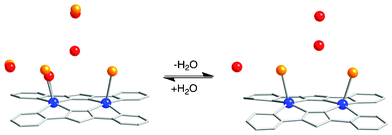 | ||
| Scheme 2 Dehydration–hydration processes between 1 (left) and dehydrated form 1a (right). Atoms: Cu (blue), Br (orange), C (gray), N (gray), O (red). | ||
Compound 1b also crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Z = 2),11 while the lattice parameters are different from those of 1 and 1a. Although the single crystallinity of 1b was poor, we were able to determine the cell lattice and successfully integrate the diffraction image data (Fig. S4, ESI†). Therefore, we discuss an outline-view of the supramolecular dimer structure. The same supramolecular dimer unit of 1 is maintained in 1b. The dimer structure is also confirmed by the thermal dependence of χMT (Fig. 3) and ESR (Fig. S5, ESI†).
(Z = 2),11 while the lattice parameters are different from those of 1 and 1a. Although the single crystallinity of 1b was poor, we were able to determine the cell lattice and successfully integrate the diffraction image data (Fig. S4, ESI†). Therefore, we discuss an outline-view of the supramolecular dimer structure. The same supramolecular dimer unit of 1 is maintained in 1b. The dimer structure is also confirmed by the thermal dependence of χMT (Fig. 3) and ESR (Fig. S5, ESI†).
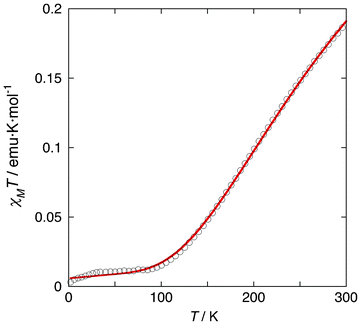 | ||
| Fig. 3 Temperature dependence of the χMT product of 1. A solid red line is the best fit of the experimental data using by the Hamiltonian H = −2JS1·S2. The best-fit parameters are J = −390 cm−1, g = 2.12 and ρ (paramagnetic impurities) = 0.018 with TIP = 60 × 10−6 cm3 mol−1. The J value is close to those of doubly bpypz− bridged copper(II) complexes with the same structural features. | ||
Interestingly, the assembled structure of compound 1 shows that the stacking mode in the supramolecular dimers changes greatly in the dehydration processes from 1 to 1b (Scheme 3). These features could be driven by the breakage of the hydrogen-bonding network in the channels upon dehydration.
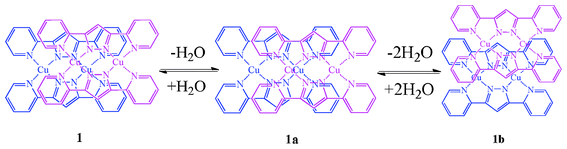 | ||
| Scheme 3 Schematic representation of the stacking modes in the supramolecular dimers for 1, 1a and 1b. | ||
Fig. 3 shows the water adsorption and desorption isotherms of 1 at 298 K. In compound 1, the starting material at P/P0 = 0 corresponds the completely anhydrous form 1b, and the amount resorbed is three water molecules per Cu2 unit at P/P0 ∼ 0.8 to return the fully original form 1 (Fig. 4). The adsorption profile of 1 consists of two parts. The adsorption isotherm increases steeply with pressure in the first region (P/P0 = 0–0.05), then it rises in the next region (P/P0 = 0.05–0.9). These features indicate that stepwise adsorption occurs and then adsorption of water: at first two water molecules are introduced into the interstitial space resulting in the formation of [CuII2(μ-bpypz)2Br2]·2H2O (1a) and then the remaining one water molecule is introduced to the resulting 1D channel by the coordinating and hydrogen-bonding interactions. Alternatively, TG results show that the numbers of water molecules are consistent with the single-crystal analyses (Fig. S6, ESI†).
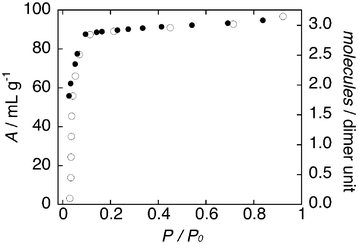 | ||
| Fig. 4 Water sorption isotherms for 1 at 298 K. Adsorption and desorption are indicated by open and filled circles. | ||
The electronic diffuse reflectance spectra of 1 and 1b are shown in Fig. S4 (ESI†). The original form 1 has a main band at about 630 nm, which is a typical d–d band of Cu(II) complexes. The d–d band for 1b is shifted to about 700 nm. (Fig. 5a). Based on the observed structural and isothermal changes, a simple model is proposed to explain the vapochromic behavior (Fig. 5b).10
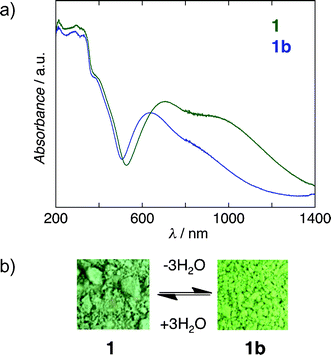 | ||
| Fig. 5 (a) UV/Vis absorption spectra of the original form 1 and the anhydrous form 1b. (b) The vapochromic change from the original form 1 to the anhydrous form 1b. | ||
The dinuclear [CuII2(μ-bpypz)2] unit of 1 is maintained upon the dehydration and rehydration processes. Stepwise dehydration corresponds to (1) the breakage of hydrogen-bonding network and the substitution of the apical ligand for 1 to 1a, and (2) the disappearance of hydrogen-bonding network for 1a to 1b: each structure of 1a and 1b shows that the one and two water molecules per dimer unit are extruded from the crystal and then the axial position is substituted by the Br− anion completely, that is, the Br− anions move from the one-dimensional channels to the copper center in the dehydration process. Furthermore, the stacking mode in the supramolecular dimer for 1 changes greatly in the dehydration process. These features may be driven by the disruption of the H-bonded network. Upon exposure of 1b to air at room temperature, it changed back to the initial hydrated form 1 (Fig. S7, ESI†). This reversible apical-ligand substitution at the copper center is associated with the change in the absorption bands described above.
In summary, the results of this work reveal the flexible structural transformation process originated in change of stacking mode in the supramolecular dimers and the reversible anion transfer caused by the apical-ligand substitution at the copper ion in compound 1. Future work will attempt to improve the new framework construction by using the varying metal sources or reaction solvents and adding functional substituents for the ligand moiety such as hydrogen-bonding sites. We believe that the synthetic strategy and properties could offer new possibilities and important key factors for the construction of supramolecular assemblies with novel functionality and the elucidation of a molecular-scale dynamic mechanism.
Acknowledgements
This work was supported by funds (No. 101501) from the Central Research Institute of Fukuoka University and Grant-in-Aids for Science Research (No.22550067) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.References
- (a) O. M. Yaghi, G. Li and H. Li, Nature, 1995, 378, 703 CrossRef CAS; (b) H. Li, M. Eddaoudi, T. L. Groy and O. M. Yaghi, J. Am. Chem. Soc., 1998, 120, 8571 CrossRef CAS; (c) N. Yanai, K. Kitayama, Y. Hijikata, H. Sato, R. Matsuda, Y. Kubota, M. Takata, M. Mizuno, T. Uemura and S. Kitagawa, Nat. Mater., 2011, 10, 787 CrossRef CAS; (d) H. Sato, R. Matsuda, M. H. Mir and S. Kitagawa, Chem. Commun., 2012, 48, 7919 RSC.
- (a) S. M. Kuznicki, V. A. Bell, S. Nair, H. W. Hillhouse, R. M. Jacubinas, C. M. Braunbarth, B. H. Toby and M. Tsapatsis, Nature, 2001, 412, 720 CrossRef CAS; (b) S. R. Venna and M. A. Carreon, J. Am. Chem. Soc., 2010, 132, 76 CrossRef CAS.
- (a) B. Xiao, P. J. Byrne, P. S. Wheatley, D. S. Wragg, X. B. Zhao, A. J. Fletcher, K. M. Thomas, L. Peters, J. S. O. Evans, J. E. Warren, W. Z. Zhou and R. E. Morris, Nat. Chem., 2009, 1, 289 CrossRef CAS; (b) Z. G. Xie, L. Q. Ma, K. E. deKrafft, A. Jin and W. B. Lin, J. Am. Chem. Soc., 2010, 132, 922 CrossRef CAS.
- F. J. Muñoz Lara, A. B. Gaspar, D. Aravena, E. Ruiz, M. C. Muñoz, M. Ohba, R. Ohtani, S. Kitagawa and J. A. Real, Chem. Commun., 2012, 48, 4686 RSC.
- (a) S. Takaishi, M. Hosoda, T. Kajiwara, H. Miyasaka, M. Yamashita, Y. Nakanishi, Y. Kitagawa, K. Yamaguchi, A. Kobayashi and H. Kitagawa, Inorg. Chem., 2009, 48, 9048 CrossRef CAS; (b) Y. Kobayashi, B. Jacobs, M. D. Allendorf and J. R. Long, Chem. Mater., 2010, 22, 4120 CrossRef CAS.
- (a) K. Yoneda, K. Adachi, S. Hayami, Y. Maeda, M. Katada, A. Fuyuhiro, S. Kawata and S. Kaizaki, Chem. Commun., 2006, 45 RSC; (b) R. Ishikawa, K. Nishio, A. Fuyuhiro, K. Yoneda, H. Sakamoto, S. Kitagawa and S. Kawata, Inorg. Chim. Acta, 2012, 386, 122 CrossRef CAS; (c) K. Yoneda, K. Adachi, S. Hayami, Y. Maeda, M. Katada, A. Fuyuhiro, S. Kawata and S. Kaizaki, Chem. Commun., 2006, 45 RSC; (d) R. Ishikawa, A. Fuyuhiro, S. Hayami, K. Inoue and S. Kawata, J. Mol. Struct., 2008, 892, 220 CrossRef CAS.
- (a) M. Viciano-Chumillas, S. Tanase, L. J. de Jongh and J. Reedijk, Eur. J. Inorg. Chem., 2010, 3403 CrossRef CAS; (b) J. Klingele, S. Dechert and F. Meyer, Coord. Chem. Rev., 2009, 253, 2698 CrossRef CAS.
- Careful diffusion of an equimolar solution of Hbpypz and CuBr2 in methanol yielded single crystals (3). 3 is composed of [Cu2(m-bpypz)2Br2], but the structure is quite different from 2. The two copper-axial ligand bonds in 3 adopt an anti configuration. Another research group reported the synthesis of [Cu2(μ-bpypz)2Br2], but the crystal structure was not determined: J. Pons, X. Lopez, J. Casabo, F. Teixidor, A. Caubet, J. Rius and C. Miravitlles, Inorg. Chim. Acta, 1992, 195, 61 CrossRef CAS.
- (a) B. J. Hathaway, Struct. Bonding, 1973, 14, 49 CrossRef CAS; (b) A. W. Addison, T. Nageswara, J. Reedijk, J. van Rijn and G. C. Verehoor, J. Chem. Soc., Dalton Trans., 1984, 1349 RSC.
- (a) S. Kawata, S. R. Breeze, S. Wang, J. E. Greedan and N. P. Raju, Chem. Commun., 1997, 717 RSC; (b) K. Yamada, S. Yagishita, H. Tanaka, K. Adachi, K. Tohyama, H. Kumagai, K. Inoue, S. Kaizaki, R. Kitaura, H.-C. Chang, S. Kitagawa and S. Kawata, Chem.–Eur. J., 2004, 10, 2647 CrossRef CAS; (c) K. Yamada, H. Tanaka, S. Yagishita, K. Adachi, T. Uemura, S. Kitagawa and S. Kawata, Inorg. Chem., 2006, 45, 4322 CrossRef CAS; (d) L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105 CrossRef CAS.
- G. M. Sheldrick, SHELXL-97, Programs for Crystal Structure Analysis, University of Göttingen, Germany, 1997 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data, magnetic properties, X-ray fluorescence analysis and thermogravimetric analysis. CCDC reference numbers 704681 and 704683–704685. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra21865h |
| This journal is © The Royal Society of Chemistry 2012 |
