Preparation of dispersible graphene through organic functionalization of graphene using a zwitterion intermediate cycloaddition approach†
Xiaoyan
Zhang
ab,
Wesley R.
Browne
*a and
Ben L.
Feringa
*ab
aStratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands. E-mail: w.r.browne@rug.nl; b.l.feringa@rug.nl; Fax: +31-050-363-4296
bZernike Institute for Advanced Materials, University of Groningen, The Netherlands
First published on 19th October 2012
Abstract
Highly functionalized graphene were obtained through a zwitterion intermediate cycloaddition onto exfoliated graphene flakes under new reaction conditions. The functionalized graphene obtained formed stable dispersions in common solvents, including dimethylformamide (DMF), CHCl3 and water. Its dispersion in water is especially useful in a wide range of areas, such as composites, devices and biological applications.
In the last decade, graphene, a single-layer of graphite with a two-dimensional honeycomb lattice, has emerged as one of the most promising future materials due to its unique physical, mechanical and thermal properties.1 Potential applications of graphene in Li-ion batteries, supercapacitors, transparent electrode, sensors and composites have already been demonstrated.2 However, a key challenge in handling graphene based materials is in how to obtain a large amount of dispersible graphene flakes in either organic solvents or water for certain uses, such as in composites, devices and biological applications.3 Several chemical methods have been developed, including reduction of graphene oxide,4 bottom-up organic synthesis5 and dispersion of graphite in certain solvents.6–8 Methods employing the reduction of graphene oxide requires harsh oxidation conditions to prepare graphene oxide and a subsequent chemical and/or thermal reduction step. Graphene prepared by the organic synthesis route is impeded by limitations to size. Of these approaches, direct sonication of graphite in solvents promises to be the simplest method to obtain dispersible and relatively defect-free graphene flakes. However, the graphene flakes suspended in solvents (such as in N-methyl pyrrolidone,6ortho-dichlorobenzene7a and ethanol)8a tend to aggregate due to the strong π–π interactions between the individual flakes, which seriously limits their application. In order to solve this problem and further improve their dispersibility, stability and processability, considerable efforts have been devoted to functionalize graphene flakes by noncovalent9 or covalent methods.10 In contrast to noncovalent methods, covalent methods can provide for more stable and robust materials. Covalently attached functional groups on graphene can improve the dispersibility of graphene, but also increase compatibility with various interfaces and matrixes. Furthermore, covalently functionalized graphene can be applied in subsequent chemical processes that are usually unsuitable for non-covalent/physisorbed functionalized graphene. More importantly, covalently functionalized graphene can yield graphene samples that show long-term stability when dispersed in solvents, which is necessary for application. However, covalent functionalization may affect the electronic properties of graphene. Thus, controlling the degree of functionalization of graphene is essential. Although the reactivity of graphene is less than those of fullerenes and carbon nanotubes, the wrinkled and folded structure and defects present in graphene can increase its reactivity towards organic reagents.11 To date, a number of chemical reactions on graphene have been reported, including 1,3-dipolar cycloaddition,10 diazonium chemistry,12 nitrene addition,13 radical addition14 and click chemistry.15 Through these reactions, a range of functional groups can be introduced and also the degree of functionalization of graphene can be tuned. However, chemical modification of graphene has yet to be fully studied and more facile and mild methods are highly desirable.
Here, we take a zwitterion intermediate cycloaddition functionalization approach, which was previously applied to fullerenes and carbon nanotubes,16 to functionalize graphene obtained using the solvent dispersion and exchange method.10a The functionalized graphene formed stable dispersions in common solvents (most remarkably in water) and was characterized by several spectroscopic and microscopic techniques.
Functionalization of graphene through the zwitterion approach is shown in Scheme 1 (see Scheme S1† for the proposed mechanism).16 4-dimethylaminopyridine (DMAP) reacts with the triple bond of acetylene dicarboxylates to form a zwitterionic intermediate, which then reacts with the double bond of graphene followed by reaction with the carbonyl group. Finally, the positively charged DMAP moiety is substituted by an alkoxy group, yielding the functionalized graphene product. Graphene in ODCB was prepared by the solvent dispersion method,10a followed by a solvent exchange process developed by our group to transfer graphene into dry toluene (see supporting information†).8a The graphene dispersed in dry toluene was reacted with DMAP, and acetylenedicarboxylates 1 or 2 at 85 °C under Ar atmosphere for 3 d. The functionalized graphene was purified by multiple filtration/redispersion cycles (see supporting information). Notably, the functionalized graphene is no longer dispersible in ODCB or toluene but 1 functionalized graphene shows good dispersion in both DMF (∼0.28 mg mL−1) and CHCl3 (∼0.19 mg mL−1), while 2 functionalized graphene shows good dispersibility in water (∼0.06 mg mL−1) (Fig. S1†).17 UV/Vis absorption spectrometry can be used to investigate the stability of graphene samples in the solvents by measuring the changes in apparent absorption (in fact the changes in scattering of light by graphene) with time.6,8a In the present case, 1 functionalized graphene was stable in DMF and CHCl3 for at least two months, while 2 functionalized graphene dispersed in water was stable for at least one month. The stability is comparable with the 1,3-dipolar cycloaddition functionalized graphene dispersed in ethanol (at least 30 days).10c
 | ||
| Scheme 1 Preparation of functionalized graphene through the zwitterion approach. | ||
The Fourier transform infrared spectroscopy (FTIR) spectra of graphene, 1 and 2 functionalized graphene, and the control samples are shown in Fig. 1. The FTIR spectrum of graphene itself is almost featureless, indicative of a low content of defects in the graphene. For both 1 and 2 functionalized graphene, the absorption bands at 1715 and 1240 cm−1 correspond to carbonyl and ether groups, respectively. The spectra of the control samples (Control 1: graphene reacted with 1 only; Control 2 : graphene reacted with 2 only; Control 3: graphene reacted with DMAP only), did not show the above mentioned absorptions. This strongly supports that the modification is covalent in the two functionalized graphene samples and not physisorption.
 | ||
| Fig. 1 FTIR spectra of graphene, 1 and 2 functionalized graphene, and control samples. | ||
The presence of organic functional groups in the functionalized graphene products is further confirmed by thermal gravimetric analysis (TGA). Fig. 2 shows the TGA curves of graphene, 1 and 2 functionalized graphene. The weight loss of graphene is about 5% between 200 °C and 450 °C, which is due to the defects caused by sonication, and also residual solvents. In the same temperature range, 1 and 2 functionalized graphene show about 54% and 36% weight loss, respectively. This weight loss is attributed to the decomposition of organic functional groups attached onto graphene. The degree of functionalization was estimated to be one functional group per 10 carbon atoms for 1 functionalized graphene, and per 50 carbon atoms for 2 functionalized graphene.
Raman spectroscopy is widely used to study the structural and electronic properties of graphitic materials.18 The typical Raman bands for graphitic materials are: a disorder-induced D band at ∼1350 cm−1, a doubly degenerate zone centre E2g mode at about 1580 cm−1 (G band, indicative to sp2 carbon bonds), and a two phonon double resonance Raman process at ∼2700 cm−1 (2D band).18 The intensity ratio ID/IG between the D band and G band is often used to quantify the defects in graphitic materials. Graphene shows a small D band and a strong G band, with a ID/IG ratio of 0.3 (Fig. 3). For 1 and 2 functionalized graphene, an increased ID/IG ratio (0.4 and 0.54, respectively) is observed compared with graphene. Attachment of organic functional groups changes some carbon atoms from sp2 to sp3 and therefore results in an increased ID/IG ratio, which indicates successful covalent functionalization.10,19 Due to the relatively small size of the graphene flakes and aggregation when deposited onto the substrate, it is difficult to distinguish single-layer graphene by Raman spectroscopy in the present case. However, the positions and shapes of the spectra of graphene and functionalized graphene indicates the graphene flakes are a mixture of single- and few-layer graphene.6,8a,10
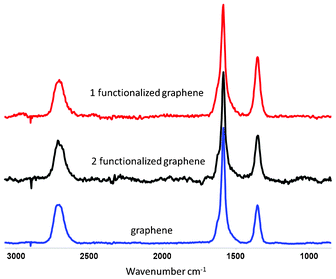 | ||
| Fig. 3 Raman spectra of graphene, 1 and 2 functionalized graphene (λexc = 532 nm). | ||
The morphologies of graphene before and after functionalization were studied by transmission electron microscopy (TEM) analysis. The presence of single-layer graphene flakes is shown in Fig. 4a, which is confirmed by its electron diffraction pattern (Fig. S2†, the intensity of inner spots is greater than the outer spots).6,8a,10a A large number of few-layer graphene flakes are also present in the graphene samples used for functionalization (Fig. 4b). 1 functionalized graphene shows good dispersion behaviour both in DMF and CHCl3 (Fig. 4c and Fig. 4d), while 2 functionalized graphene disperse well in water (Fig. 4e, Fig. 4f and Fig. S3†). After functionalization, little change in the morphology of graphene is observed, and importantly, the size of the graphene flakes is not decreased. This is an advantage compared with the harsh oxidation methods used to prepare graphene oxide, in which the flake size is normally significantly reduced.
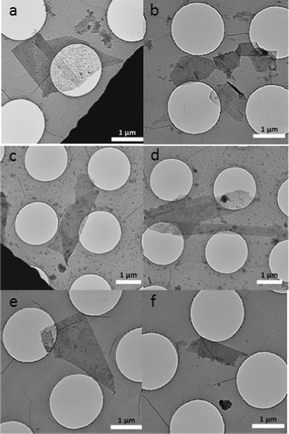 | ||
| Fig. 4 TEM images of graphene in ODCB (a, b), 1 functionalized graphene in DMF (c) and CHCl3 (d) and 2 functionalized graphene in H2O (e, f). The samples were prepared by dropcasting the dispersion onto a holey carbon grid. For HRTEM images, see Fig. S4† in the supporting information. | ||
The conductivity of films of graphene, 1 and 2 functionalized graphene was also determined using the standard four-point probe method. The films were prepared by vacuum filtration of the corresponding dispersions. The graphene film shows a conductivity of 3269 ± 132 S m−1, whereas for 1 and 2 functionalized graphene films a conductivity of 6.88 ± 0.36 and 665 ± 20 S m−1 was determined, respectively (see Table S1† for details). The reduced conductivity of the two functionalized graphene films compared to graphene itself is due to the covalent modification of the graphene. However, it should be noted that the two conductivity values are still much higher than that of graphene oxide (10−8–10−5 S m−1).20
In conclusion, functionalized graphene has been successfully prepared using a zwitterion intermediate cycloaddition approach under new reaction conditions, and was characterized by FTIR, Raman spectroscopy and by additional control experiments. The degree of functionalization on graphene was determined by TGA and more interestingly it was relevant to the acetylenedicarboxylates uses. TEM images show that the functionalized graphene has good dispersibility in common solvents, such as DMF, CHCl3 and especially water. The functionalized graphene films show conductivity several orders of magnitude better than graphene oxide. We believe this novel zwitterion functionalization method will broaden the chemistry of graphene substantially and that these functionalized graphene materials could be useful in composites, devices and biological applications (sensing, drug loading and delivery).3
Acknowledgements
We acknowledge the financial support from The Zernike Institute for Advanced Materials (X.Y.Z.), NWO-Vidi (W.R.B.) and the ERC advanced investigator grant (no. 227897, B.L.F). We thank María Jesús Ortiz Iniesta for the assistance with TGA measurements and Dr Marc Stuart for assistance in obtaining high resolution TEM images.References
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183 CrossRef CAS.
- (a) Y. Zhu, S. Murali, W. Cai, X. Li, J. Suk, J. Potts and R. Ruoff, Adv. Mater., 2010, 22, 3906 CrossRef CAS; (b) S. J. Guo and S. J. Dong, Chem. Soc. Rev., 2011, 40, 2644 RSC; (c) C. N. R. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj, Angew. Chem., Int. Ed., 2009, 48, 7752 CrossRef CAS; (d) M. Pumera, Chem. Soc. Rev., 2010, 39, 4146 RSC.
- X. Huang, Z. Y. Yin, S. X. Wu, X. Y. Qi, Q. Y. He, Q. C. Zhang, Q. Y. Yan, F. Boey and H. Zhang, Small, 2011, 7, 1876 CrossRef CAS.
- G. Eda, G. Fanchini and M. Chhowalla, Nat. Nanotechnol., 2008, 3, 270 CrossRef CAS.
- J. Wu, W. Pisula and K. Müllen, Chem. Rev., 2007, 107, 718 CrossRef CAS.
- Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Sun, S. De, I. T. McGovern, B. Holland, M. Byrne, Y. K. Gun'ko, J. J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, Nat. Nanotechnol., 2008, 3, 563 CrossRef CAS.
- (a) C. E. Hamilton, J. R. Lomeda, Z. Z. Sun, J. M. Tour and A. R. Barron, Nano Lett., 2009, 9, 3460 CrossRef CAS; (b) A. Catheline, C. Valles, C. Drummond, L. Ortolani, V. Morandi, M. Marcaccio, M. Iurlo, F. Paolucci and A. Pénicaud, Chem. Commun., 2011, 47, 5470 RSC.
- (a) X. Y. Zhang, A. C. Coleman, N. Katsonis, W. R. Browne, B. J. van Wees and B. L. Feringa, Chem. Commun., 2010, 46, 7539 RSC; (b) W. F. van Dorp, X. Y. Zhang, B. L. Feringa, J. B. Wagner, T. W. Hansen and J. Th. M. De Hosson, Nanotechnology, 2011, 22, 505303 CrossRef CAS.
- (a) Y. Xu, H. Bai, G. Lu, C. Li and G. Shi, J. Am. Chem. Soc., 2008, 130, 5856 CrossRef CAS; (b) D.-W. Lee, T. Kim and M. Lee, Chem. Commun., 2011, 47, 8259 RSC; (c) J. M. Englert, J. Röhrl, C. D. Schmidt, R. Graupner, M. Hundhausen, F. Hauke and A. Hirsch, Adv. Mater., 2009, 21, 4265 CrossRef CAS; (d) B. G. Choi, W. H. Hong, Y. M. Jung and H. S. Park, Chem. Commun., 2011, 47, 10293 RSC; (e) R. Hao, W. Qian, L. Zhang and Y. L. Hou, Chem. Commun., 2008, 6576 RSC; (f) J. Malig, N. Jux, D. Kiessling, J.-J. Cid, P. Vázquez, T. Torres and D. M. Guldi, Angew. Chem., Int. Ed., 2011, 50, 3561 CrossRef CAS; (g) Y. W. Hu, F. H. Li, X. X. Bai, D. Li, S. C. Hua, K. K. Wang and L. Niu, Chem. Commun., 2011, 47, 1743 RSC; (h) M. Castelain, H. J. Salavagione, R. Gomez and J. Luis Segura, Chem. Commun., 2011, 47, 7677 RSC; (i) J. Geng, B.-S. Kong, S. B. Yang and H.-T. Jung, Chem. Commun., 2010, 46, 5091 RSC.
- (a) X. Y. Zhang, L. L. Hou, A. Cnossen, A. C. Coleman, O. Ivashenko, P. Rudolf, B. J. van Wees, W. R. Browne and B. L. Feringa, Chem.–Eur. J., 2011, 17, 8957 CrossRef CAS; (b) M. Quintana, K. Spyrou, M. Grzelczak, W. R. Browne, P. Rudolf and M. Prato, ACS Nano, 2010, 4, 3527 CrossRef CAS; (c) V. Georgakilas, A. B. Bourlinos, R. Zboril, T. A. Steriotis, P. Dallas, A. K. Stubos and C. Trapalis, Chem. Commun., 2010, 46, 1766 RSC.
- (a) K. Loh, Q. Bao, P. Ang and J. Yang, J. Mater. Chem., 2010, 20, 2277 RSC; (b) L. Yan, Y. B. Zheng, F. Zhao, S. J. Li, X. F. Gao, B. Q. Xu, P. S. Weiss and Y. L. Zhao, Chem. Soc. Rev., 2012, 41, 97 RSC.
- E. Bekyarova, M. E. Itkis, P. Ramesh, C. Berger, M. Sprinkle, W. A. de Heer and R. C. Haddon, J. Am. Chem. Soc., 2009, 131, 1336 CrossRef CAS.
- (a) T. A. Strom, E. P. Dillon, C. E. Hamilton and A. R. Barron, Chem. Commun., 2010, 46, 4097 RSC; (b) X. Xu, W. Lv, J. Huang, J. Li, R. Tang, J. Yan, Q. Yang, J. Qin and Z. Li, RSC Adv., 2012, 2, 7042 RSC.
- A. Mukherjee, J. Kang, O. Kuznetsov, Y. Sun, R. Thaner, A. Bratt, J. Lomeda, K. Kelly and W. E. Billups, Chem. Mater., 2011, 23, 9 CrossRef CAS.
- (a) H.-X. Wang, K.-G. Zhou, Y.-L. Xie, J. Zeng, N.-N. Chai, J. Li and H.-L. Zhang, Chem. Commun., 2011, 47, 5747 RSC; (b) Z. Jin, T. P. McNicholas, C. Shih, Q. Wang, G. L. C. Paulus and M. S. Strano, Chem. Mater., 2011, 23, 3362 CrossRef CAS; (c) M. Castelaín, G. Martínez, P. Merino, J. Á. Martín-Gago, J. L. Segura, G. Ellis and H. J. Salavagione, Chem.–Eur. J., 2012, 18, 4965 CrossRef.
- (a) W. Zhang and T. M. Swager, J. Am. Chem. Soc., 2007, 129, 7714 CrossRef CAS; (b) W. Zhang, J. K. Sprafke, M. Ma, E. Y. Tsui, S. A. Sydlik, G. C. Rutledge and T. M. Swager, J. Am. Chem. Soc., 2009, 131, 8446 CrossRef CAS.
- 13C NMR spectroscopy of the two functionalized graphene samples were attempted without success, possibly due to slow relaxation due to limited rotation rates of the large graphene flakes and the limited concentrations achievable.
- (a) A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K.Geim, Phys. Rev. Lett., 2006, 97, 187401 CrossRef CAS; (b) M. A. Pimenta, G. Dresselhaus, M. S. Dresselhaus, L. G. Cancado, A. Jorio and R. Saito, Phys. Chem. Chem. Phys., 2007, 9, 1276 RSC.
- (a) X. Zhong, J. Jin, S. W. Li, Z. Y. Niu, W. Q. Hu, R. Li and J. T. Ma, Chem. Commun., 2010, 46, 7340 RSC; (b) M. Quintana, A. Montellano, A. E. del Rio Castillo, G. V. Tendeloo, C. Bittencourt and M. Prato, Chem. Commun., 2011, 47, 9330 RSC.
- S. Mao, H. H. Pu and J. H. Chen, RSC Adv., 2012, 2, 2643 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available: Methods, TEM and optical images, NMR data. See DOI: 10.1039/c2ra22440b |
| This journal is © The Royal Society of Chemistry 2012 |

